- Epoxy Networks Cured with α,ω-Diamino Polyoxytetramethylene in the Presence of Polyoxytetramethylene-coated Silica Nanoparticles
School of Chemistry, Damghan University, 36715-364, Damghan, Iran
- 폴리옥시테트라메틸렌으로 코팅된 실리카 나노입자의 존재하에 α,ω-다이아미노 폴리옥시테트라메틸렌으로 경화되는 에폭시 네트워크
In this study, α,ω-diamino polyoxytetramethylene (APOTM) was used as a new polyoxyalkylene hardener of diglycidyl ether of bisphenol A (DGEBA) epoxy resin. To reinforce the thermo-mechanical behavior of the corresponding epoxy networks, the curing processes were performed in the presence of amine-functionalized silica nanoparticles (AFSNP's). Three different contents of AFSNP's, i.e. 5.0, 7.5 and 10.0 wt%, were employed for covalent embedding into the resultant DGEBA/APOTM epoxy networks. Therefore, three epoxy-silica films labeled by EN/AFSNP′s-5, EN/ AFSNP′s-7.5 and EN/AFSNP′s-10 were fabricated. The AFSNP′s-loaded DGEBA/APOTM epoxies showed superior thermo-stability in comparison with the neat epoxy counterpart. According to the results obtained from DMTA measurements, the storage moduli (E' values) of the epoxies were significantly improved by adding the organically modified silica nanofiller.
Keywords: polyoxytetramethylene, epoxy network, silica nanoparticles, heat stability, storage modulus
The authors wish to express their gratitude to the School of Chemistry and Research Council of Damghan University for financial support of this work.
Due to their outstanding chemical and mechanical properties, epoxy–amine networks based on diglycidyl ether of biphenyl A (DGEBA) and aliphatic/aromatic diamines are widely used in different industrial applications such as coatings, adhesives and composite materials.1-4 One of the most important features of these networks is the tuning of their architecture by varying the crosslink density and/or the flexibility of the chains between crosslinks.5,6 Indeed, the decrease of crosslink density of epoxy–amine networks, for example by employing macrodiamines, is one effective approach to toughen brittle epoxy networks.7 To attain this purpose, a variety of diamino terminated polyoxyalkylenes with different molecular weights such as α,ω-diamino homopolyoxypropylene (POP) and α,ω-diamino polyoxypropylene-polyoxyethylene- polyoxypropylene (POP-POE-POP) block terpolymer have been reported yet. Diamino terminated POP chains have been extensively used as efficient curing agents of various kinds of epoxy resins, and are known as Jeffamine D series.8-11 In some cases, diamino terminated POP-POE-POP amphiphilic block terpolymers have been also utilized. Patil et al.12 employed diamino terminated POP and POP-POE-POP chains, each with two molecular weights, for preparing DGEBA-based rubbery and glassy epoxy–amine networks. The structural relaxations at segmental (α relaxation) and local (β relaxation) levels were investigated by modulated differential scanning calorimetry (MDSC) and dynamic mechanical analysis (DMA). Moreover, it has been found that the networks obtained from the curing processes of structurally flexible epoxy resins such as diglycidyl ether of POE with the aforementioned diamino terminated polyoxyalkylenes show a significant water swelling behavior. Krakovsky et al.13 prepared a series of hydrophilic epoxy networks by reaction of diglycidyl ether of POP epoxy resin with diamino terminated POP-POE-POP curing agent. If necessary, by employing hydrophobic polyoxyalkylene curing agents such as those of containing more than three methylene groups between oxygen atoms the hydrophilicity of the resulting epoxies can be significantly reduced.
On the other hand, it has been demonstrated that nanosilicafilled epoxy systems offer many new and greatly improved properties over pristine epoxies, which cannot be matched by filled systems of micron-sized particles.14-16 Undoubtedly, the magnitude of improvement strongly depends on the state of dispersion of the nanosilica particles in the epoxy network. One approach to reach an epoxy-silica nanocomposite with homogeneous composition is the surface modification of silica nanoparticles (SNP´s) with organic moieties possessing amine functional groups.17-20 Amine-functionalization of SNP´s makes them capable of participating in the curing process of epoxy resins. In such a situation, in addition to the main curing an agent of the process, NH2-functionalized silica nanoparticles (AFSNP´s) play the role of a second curing agent. Recently, we investigated the effects of AFSNP′s on the thermal and mechanical behaviors of some DGEBA-based epoxy networks cured by isophorone diamine (IPD).21 The nanoparticles were surface modified by (3-aminopropyl) triethoxysilane prior to use. Obviously, the glassy-like networks obtained were thermally and mechanically reinforced by adding SNP´s. In the present work, however, a diamino functionalized polyoxyalkylene with hydrophobic nature, i.e., α,ω-diamino terminated polyoxytetramethylene (APOTM), was used as the new curing the agent of DGEBA epoxy resin. The use of APOTM macrodiamine instead of IPD and other microdiamines strongly decreases crosslink density, which can lead to a significant decrease in rigidity and brittleness of the resulting networks. An α,ω-diisocyanato terminated telechelic of polyoxytetramethylene (encoded with IPOTM) was also used as the coupling agent of silica nanofiller for preparing the corresponding silica-containing epoxy networks. The thermal stabilities of the resulting AFSNP′s-filled DGEBA/APOTM epoxies as well as their degradation phase transition temperatures were investigated. Furthermore, the storage moduli (E' values) and the temperatures associated with α relaxations (Tg values) were measured and compared with each other.
Materials. Diglycidyl ether of bisphenol A (DGEBA) epoxy resin (D.E.R. 332) with epoxide equivalent weight (EEW) of 170.20 g/eq was supplied from Sigma-Aldrich Chemical Co. Polyoxytetramethylene (POTM) with average Mn=1000 g/mol were obtained from Merk Chemical Co. Hexamethylene diisocyanate (HDI) was supplied from Across Chemical Co. Both diisocyanate HDI and polyether POTM were used as received. Dibutyltin dilaurate (DBTDL; ≥96% purity) as a catalyst obtained from Sigma-Aldrich Chemical Co. was also used without further purification. Nano-SiO2 powder with particle size of 10-20 nm (BET), 99.5% trace metals basis was purchased from Sigma-Aldrich Chemical Co. and dried in a vacuum oven at 120 ℃ for 1 h prior to use. Dimethylformamide (DMF), toluene, dichloromethane (DCM), and diethyl ether (DEE) were fully dried prior to use by distillation over calcium hydride drying agent and then stored over 4 Å molecular sieves.
Synthesis of APOTM Curing Agent. α,ω-diamino polyoxytetramethylene (APOTM) was synthesized starting from the corresponding hydroxyl-terminated polyoxytetramethylene (POTM) through a three-step pathway. The synthetic details were thoroughly reported in our previous work.22
Amine-functionalization of SNP′s Using α,ω-Diisocyanato Terminated Telechelic of Polyoxytetramethylene (IPOTM). In a three-necked round-bottomed flask equipped with a nitrogen gas inlet tube, a magnetic stirring bar, and a reflux condenser, hydroxyl-terminated POTM (6.000 g, 6 mmol) and diisocyanate HDI (2.018 g, 12 mmol) were reacted with each other in the presence of DBTDL catalyst (0.043 g, 0.5 wt%) in CaH2-dried DMF (100 mL) at 70 ℃ for 1 h under nitrogen flow, while stirring. Next, in a 50 mL beaker, SNP’s (2.640 g) were homogeneously suspended in dried DMF (15 mL) by sonication in an ultrasonic bath for 1 h at 40 ℃. The prepared SNP′s/DMF suspension was then added to the former mixture of the reaction flask in one portion. The final mixture was vigorously stirred at 70 ℃ for additional 3 h under nitrogen atmosphere. After cooling, the mixture was filtered in a vacuum, and then in order to eliminate the unreacted HDI and all the substances physically adsorbed on the surface of SNP’s, the precipitate was fully washed with DMF. To hydrolyze the remaining isocyanate end groups, the product obtained was allowed to be stirred in distilled water (75 mL) for 4 h. The nanoparticles coated by amine-functionalized POTM (AFSNP′s) were thoroughly dried in vacuum oven at 60 ℃ for 12 h.
Fabrication of AFSNP's-loaded DGEBA/APOTM Epoxy Sheets. A representative DGEBA/APOTM epoxy film loaded by 5 wt% of AFSNP′s (EN/AFSNP′s-5) was fabricated as follows: DGEBA resin (0.340 g, 2 meq) was poured in a 10 mL round-bottomed flask, and heated to 40 ℃ for 15 min. AFSNP′s filler (0.042 g) was gradually added to the heat-softened resin under ultrasonication at the same temperature for additional 20 min. APOTM curing agent (0.500 g, 2 meq) was then added to the reaction flask, and the mixture was mechanically stirred at room temperature for 20 min. The colorless and transparent viscose fluid obtained was poured into a Teflon-coated mold. The hardening process was carried out in a vacuum oven at 80 ℃ for 4 h, and then at 120 ℃ for 1 h. The bubble-free transparent film with dimensions of 30×10×2 mm was finally brought out from the mold. Other rectangular specimens including EN/AFSNP′s-7.5 and EN/AFSNP′s-10 were also fabricated in a similar manner. In addition, a neat epoxy film was fabricated without any addition of AFSNP′s to the DGEBA resin.
Characterization. Fourier transform infra-red (FTIR) spectra of the samples were performed on a PERKIN ELMER RX I FTIR infrared spectrophotometer using potassium bromide pellets. Wide-angle X-ray diffraction (XRD) patterns were recorded at room temperatures with film specimens on a Bruker Advance D8 X-ray diffractometer with Ni-filtered Cu/Kα radiation (30 kV, 25 mA). The patterns were collected in the range of angles 2θ from 10o to 80°. MIRA3 LM TESCAN FE scanning electron microscope was used for FE-SEM analysis. Prior to the capturing, the samples were attached to double-sided carbon tape, and coated with <10 nm gold using a DC sputtering coater. Thermo-gravimetric analysis (TGA) and differential thermal analysis (DTA) were performed by a BAHR-thermoanalyse-simultaneous thermal analyzer STA 503 under argon inert atmosphere. The samples were heated from 25 to 800 ℃ at a heating rate of 10 ℃/min with Al2O3 as a reference. TT DMA, Triton Technology was used for dynamic mechanical thermal analysis (DMTA). Rectangular specimens with dimensions of 30 mm×10 mm×2 mm were used for the study. Temperature sweeps were operated at a heating rate of 3 ℃/min using a frequency of 1 Hz, at a temperature range from -80 to 80 ℃.
Preparation of Epoxy-silica Nanocomposites. As Figure 1(A) shows, α,ω-diisocyanato polyoxytetramethylene (IPOTM) was synthesized through the reaction between hydroxyl-terminated POTM and two molar equivalents of HDI in DMF. The flexible chains of IPOTM could be readily grafted to the surface of silica nanoparticles (SNP’s) through the reaction between isocyanate groups of the polyoxytetramethylene and silanol groups of the nanoparticles, leading to urethane linkages. Since IPOTM was used in large excess amounts, naturally it is expected that many isocyanate groups remain unreacted. To obtain amine-functionalized SNP′s (AFSNP′s), these unreacted isocyanate groups were then thoroughly hydrolyzed. On the other hand, according to our previous report, α,ω-diamino terminated polyoxytetramethylene (APOTM) was synthesized through a three-step reaction starting from hydroxylterminated POTM.22 Although the ammonium quaternary salt of APOTM was previously used for exfoliation of clay nanolayers, applying this amine-functionalized telechelic polymer in the field of epoxy systems is reported for the first time. Herein, as a new amine hardener belonging to polyoxyalkylene family, APOTM was utilized in the curing process of DGEBA resin. For this purpose, three homogeneous mixtures including 5, 7.5, and 10 wt% of AFSNP′s in DGEBA resin were prepared prior to the addition of APOTM hardener. The curing process of DGEBA resin was accomplished by the end-linking reaction of the resin with APOTM curing agent. Schematic representation for preparing AFSNP′s-containing DGEBA/APOTM epoxy networks is shown in Figure 1(B). Regardless the amine end groups of the filler, all the flexible epoxy films were prepared stoichiometrically, i.e., initial molar ratio of DGEBA resin and APOTM hardener was 2.0, consequently, the stoichiometric ratio, r = 2[NH2]0/[E]0 = 1.0.
Figure 2 shows FTIR spectra of the IPOTM and AFSNP's. The spectra of initial materials including of POTM and the SNP's also displayed for comparison purposes. The broad band at 3200-3500 cm-1 associated with the OH vibrations of HOterminated POTM disappeared in IPOTM. Instead, a weak peak related to the vibration of urethane N-H groups appeared near the same region. Moreover, the vibration of isocyanate groups appeared at about 2275 cm-1. This peak disappeared in the spectra of AFSNP′s, indicating the full removal of unreacted IPOTM from the sample as well as full hydrolysis of the second -NCO group at the water treatment stage. The peak at ~800 cm-1 is assigned to the Si-O bond bending vibration absorption, and the peak at ~1100 cm-1 is attributed to the Si-O asymmetric stretching vibration absorption. In the spectrum of AFSNP′s, besides of the peaks belonged to the SNP’s, there are short-height peaks of ~2950 cm-1 assigned to -CH2- groups appeared. Also, the peaks attributed to the NH2 end groups of the organic moiety as well as the remaining OH groups of the nanoparticle surface overlaps with each other at above 3300 cm-1. The additional experimental evidence for the formation of Si-O-C bond is based on an elemental analysis. The elemental analysis of AFSNP′s clearly showed the presence of carbon (found: 52.10%) and nitrogen (found: 8.69%) elements in the sample. Therefore, In addition to the FTIR results, the results of elemental analysis can confirm indirectly the formation of O-C bonds.
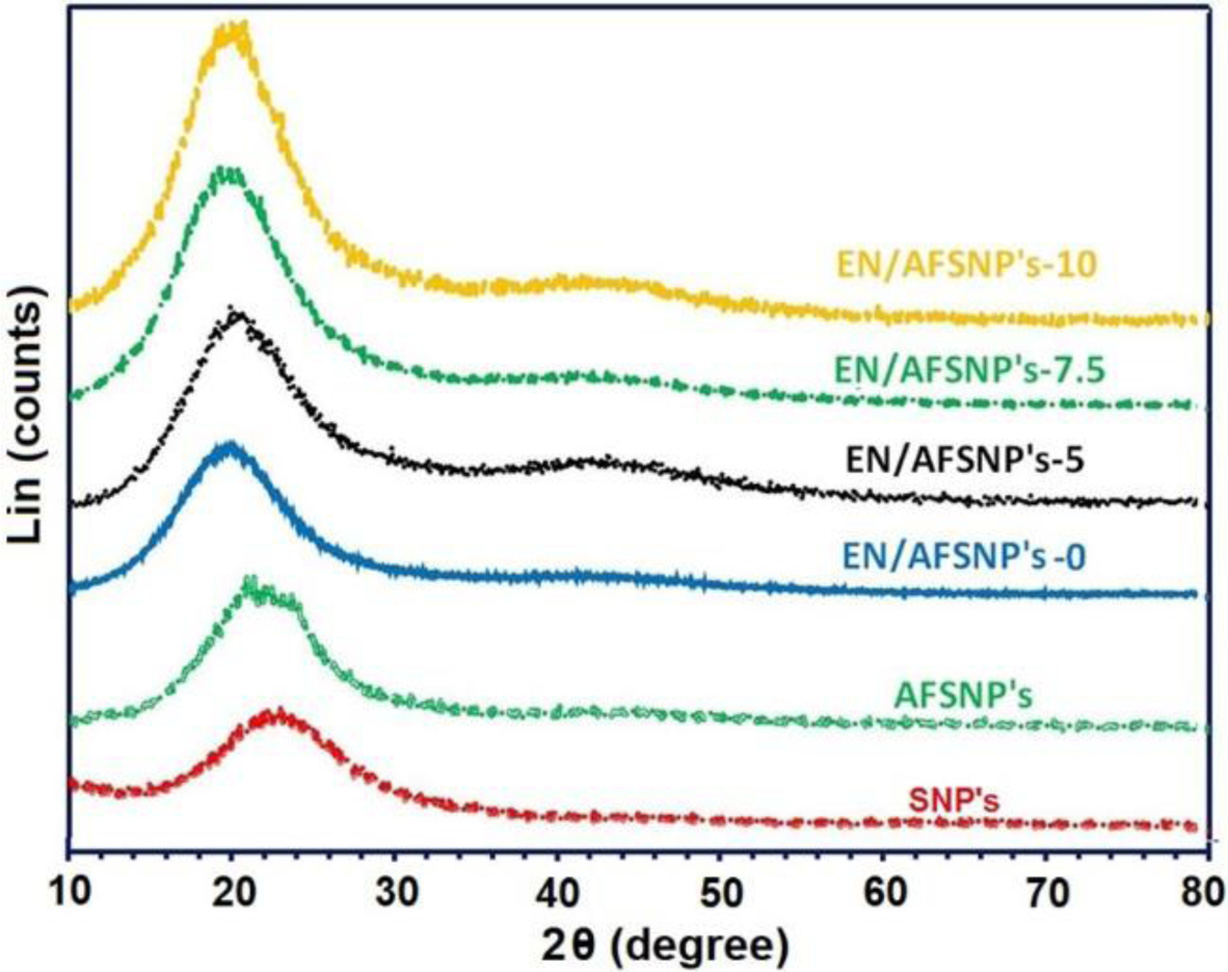
Figure 3 shows the FTIR spectra of the resulting DGEBAAPOTM epoxy networks including EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5, and EN/AFSNP′s-10. The broad peak appeared at the range of 3200-3600 cm-1 is related to the stretching vibration of N-H and O-H bonds. The fulfillment of the curing process of DGEBA resin could be clearly confirmed with the formation of this peak. In addition, elimination of the characteristic peak of the epoxide end groups indicates that the initial resin was fully consumed. On the other hand, the spectra of silica-loaded nanocomposites are equivalent to that of neat epoxy network (EN/AFSNP′s-0), except the peak appeared at about 1675 cm-1. This distinguishing peak is attributed to the stretching vibration of carbonyl groups of the urethane linkages present at the surface of the nanoparticles. Figure 4 displays FE-SEM micrographs of SNP’s, AFSNP′s and epoxysilica films with the same magnification. Although no significant change in the micrograph of SNP’s was observed after organo-modification process. Contrary to the micrograph of the unloaded epoxy film (EN/AFSNP′s-0), in the micrographs of EN/AFSNP′s-5, EN/AFSNP′s-7.5, and EN/AFSNP′s-10 the presence of the organo-silica clusters is obviously detectable. X-ray diffraction patterns of the SNP’s, AFSNP′s and the resulting DGEBA-APOTM epoxy films are presented in Figure 5. In agreement with most X-ray patterns of DGEBAbased epoxy networks,23 all the diffractograms obtained show a characteristic broad peak appeared at the range of 15° < 2θ < 30°, indicating the amorphousness of the epoxy networks. In addition to this characteristic peak, in EN/AFSNP′s composites a short and broad peak appeared near 42° due to nanofillerepoxy matrix interactions. As can be seen, almost no significant difference is observed in their X-ray patterns after embedding AFSNP′s filler into the epoxy networks.
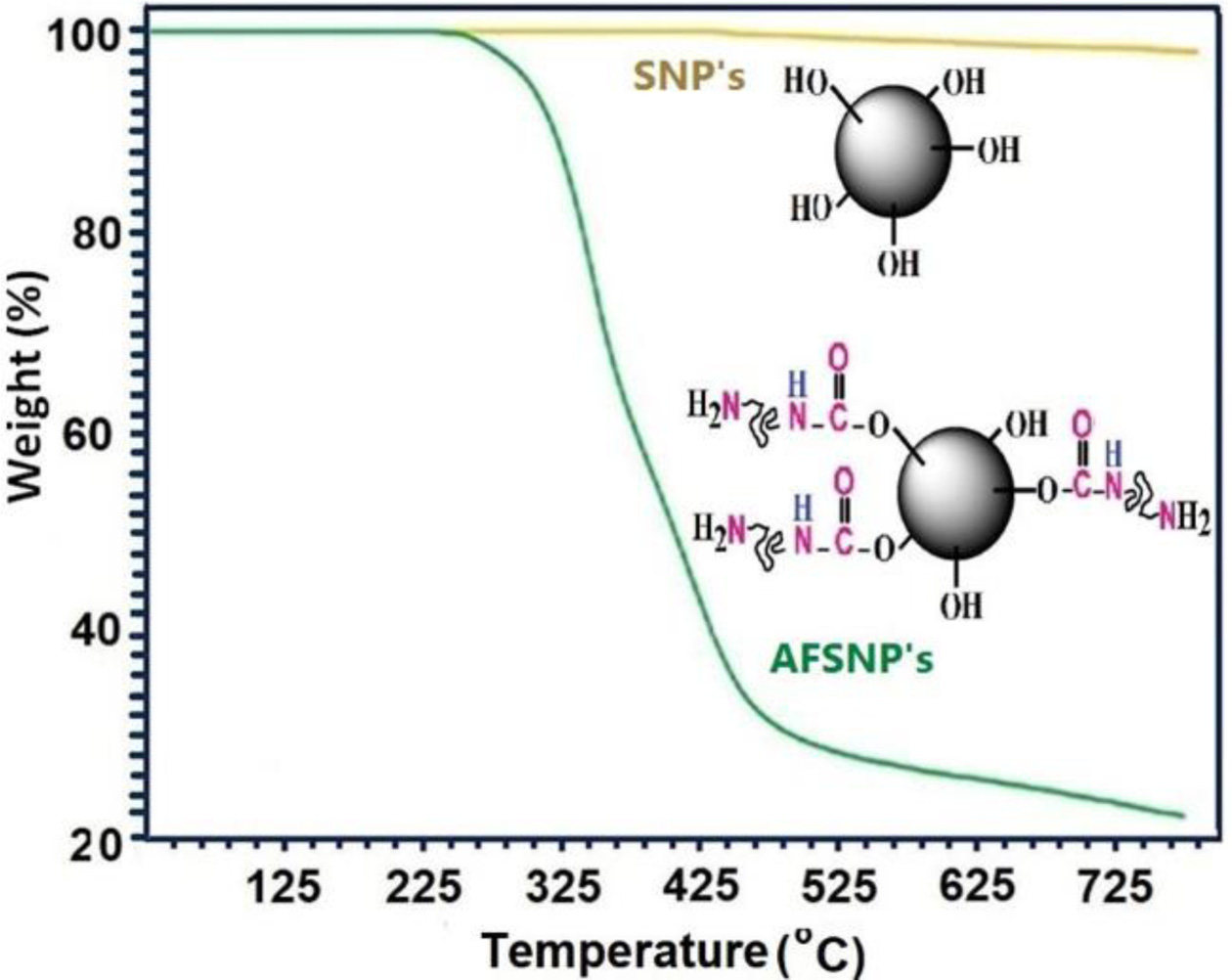
Heat Stability and Thermal Phase Transitions. In Figure 6, the thermal behavior of the SNP’s and AFSNP's were investigated by their TGA thermogram. According to the TGA curves, about 72% of the surface silanol groups of the SNP's were treated by POTM modifier via urethane links. Residue weights (char yield at 750 ℃) of SNP’s and AFSNP′s were found to be 93% and 25%, respectively. This was somewhat predictable due to the long size of the crossed macrochains.
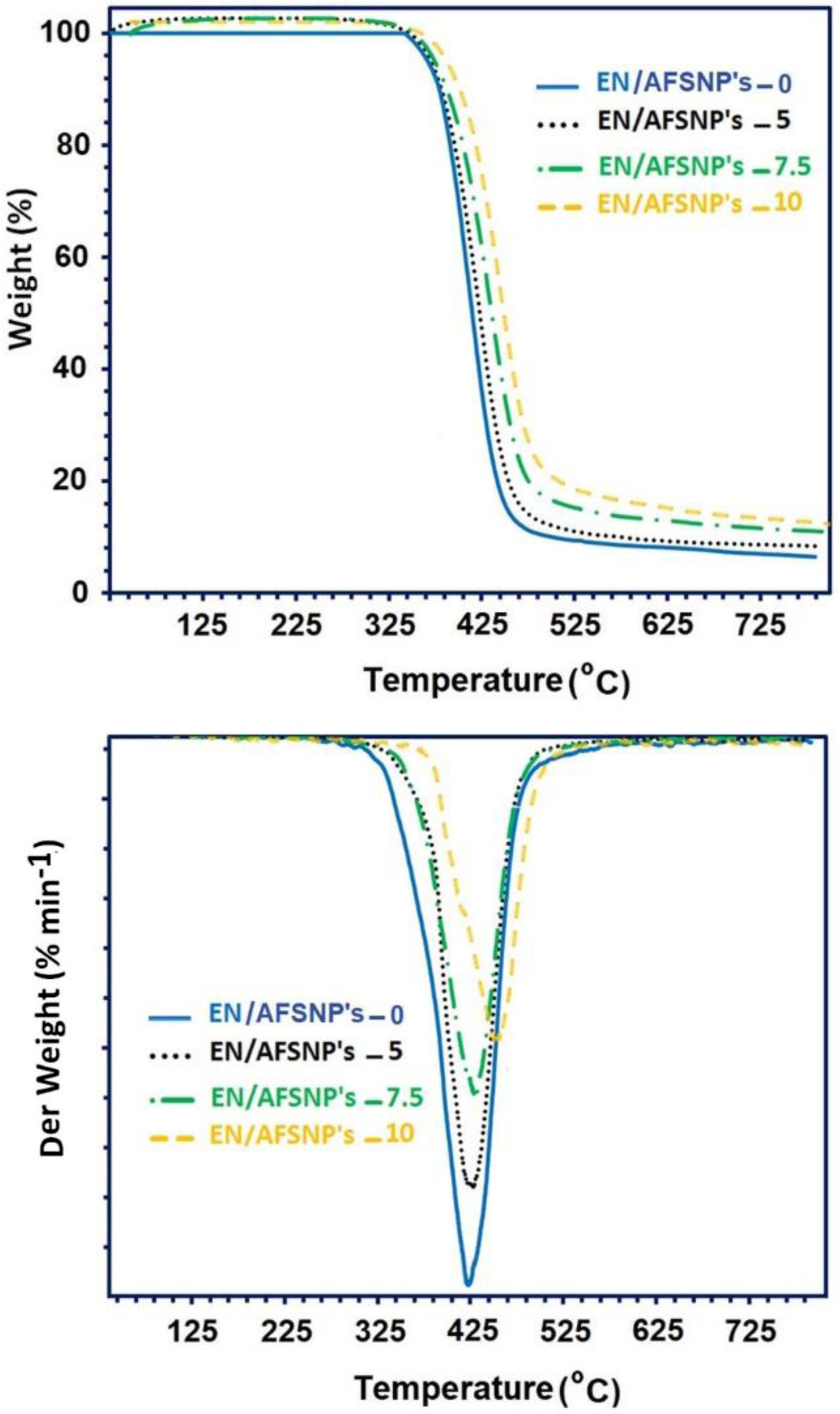
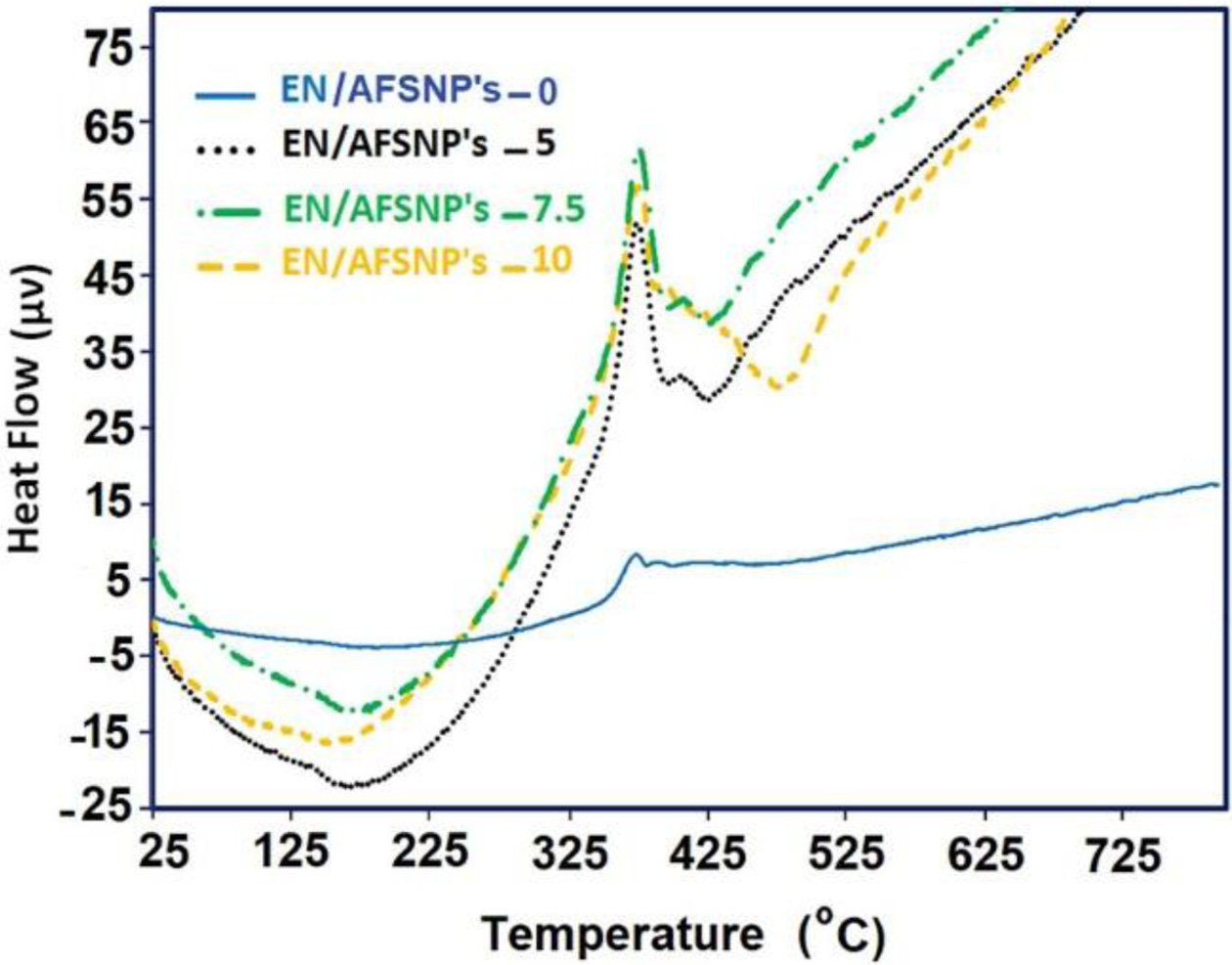
Undoubtedly, the chemical structure of curing agents can affect the thermal behavior of the resulting epoxy systems. In this work, APOTM was used as a new amine curing agent belonging to polyoxyalkylene family. In addition, three different contents of SiO2 nanoparticles (5, 7.5, and 10 wt%) chemically coated with amine-functionalized POTM were present during the curing process of the resin. Both the curing agent used and the nanofiller present in the medium impress the thermostability of the networks obtained. Figure 7 displays TGA/DTG thermograms of EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5, and EN/AFSNP′s-10. Some results obtained from these measurements are also listed in Table 1. In order to evaluate the effect of APOTM on the heat stability of the resulting epoxy networks, thermal data of EN/AFSNP′s-0 have been compared with those of obtained from DGEBA-Jeffamine D400 epoxy system.8 As the thermograms show, all epoxy networks are fully stable towards heat up to 340 ˚C. The presence of AFSNP′s in the curing process of DGEBA resin clearly caused an appreciable delay in the thermodegradation of APOTM-cured epoxy networks. All the thermostability parameters given in Table 1 including the onset temperature of decomposition (Tonset), the temperature at which 10% weight loss of the samples occurs (Td10%), the temperature of maximum decomposition (Tdmax), and the char yields measured at 700 ℃ are improved by AFSNP′s filler. Moreover, the heat stability of the networks increases with increasing the filler content. Hence, among the epoxy networks obtained, EN/ AFSNP′s-10 showed the best heat stability. Indeed, this enhancement in the heat stability of the epoxy networks originates from the nature of the filler. Only a strong filler-epoxy interaction can improve the thermal behavior of the resulting network. Here, the organic shell grafted with SiO2 nanoparticles is an amine-functionalized POTM, which allow the nanoparticles to participate effectively in the curing process. The thermal phase transitions regarding the thermodegradation of DGEBA/APOTM epoxy networks are shown in Figure 8. Since the peaks associated with the phase transitions in the DTA thermograms appeared at the thermal decomposition region of the epoxy networks (350-450 ℃), these endothermic phase transitions are reasonably attributed to the thermodegradation of the resulting networks. As the profiles show, the intensity of the degradation phase transition for each AFSNP′scontaining DGEBA/APOTM epoxy network is greater than that of the neat network. It seems that the presence of AFSNP′s filler into the epoxy matrix causes a tremendous change in its texture with heat, leading to a significant change in the epoxy phase. As Figure 6 shows, a great weight loss is observed in the thermogram of AFSNP′s by disconnection of the organic moiety. The removal of the organic moieties from the silica nanoparticle significantly change the phase of the networks containing these nanoparticles, leading to a phase transition peak with more intensity in comparison with the neat network.
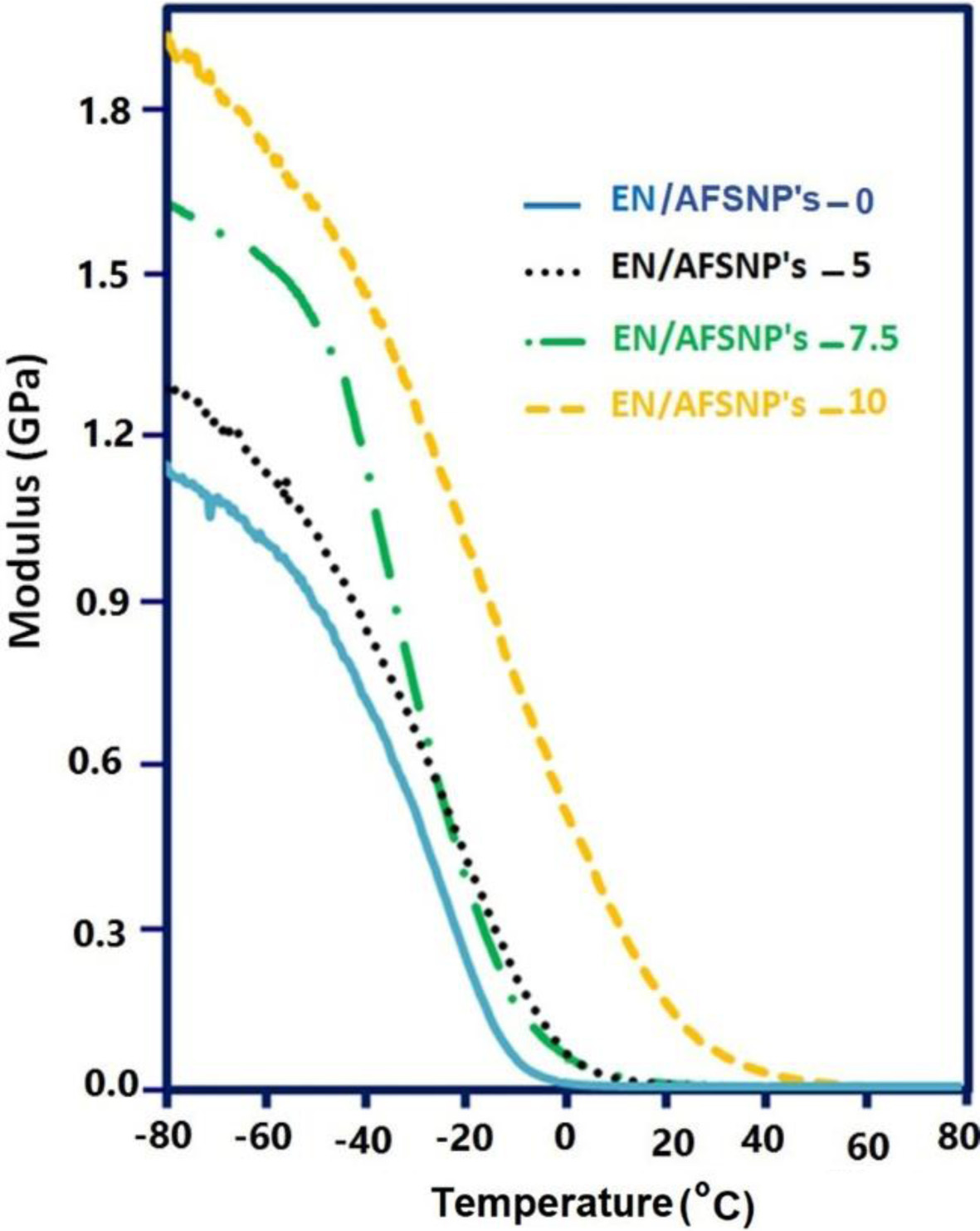
DMTA Measurements. The stiffness properties of the resulting epoxy networks could be estimated by measuring their storage (elastic) moduli (E' values) using DMTA technique. The E'-T profiles of EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5, and EN/AFSNP′s-10 are illustrated in Figure 9. According to the results obtained, it seems that the magnitude of the effect of AFSNP′s filler on the stiffness of the resulted networks is even greater than the magnitude of its effect on the thermal behaviors. In addition, the profiles clearly show that the modulus of elasticity increases with increasing AFSNP′s content at all temperatures. For example, at -20 ℃, a significant enhancement (up to 0.8 GPa) in the E' value of EN/AFSNP′s-0 could be observed after 10 wt% adding of the filler. The increase in the storage modulus of epoxy networks in consequence of the addition of inorganic fillers has also been reported by others.7 Herein, this favorable effect arises from the homogeneous dispersion of silica within the epoxy networks. Amine-functionalization of POTM organic shell allowed the nanoparticles to be chemically linked with the polymer matrix, leading to a stronger epoxy-filler interaction, and consequently a better dispersion of the filler.
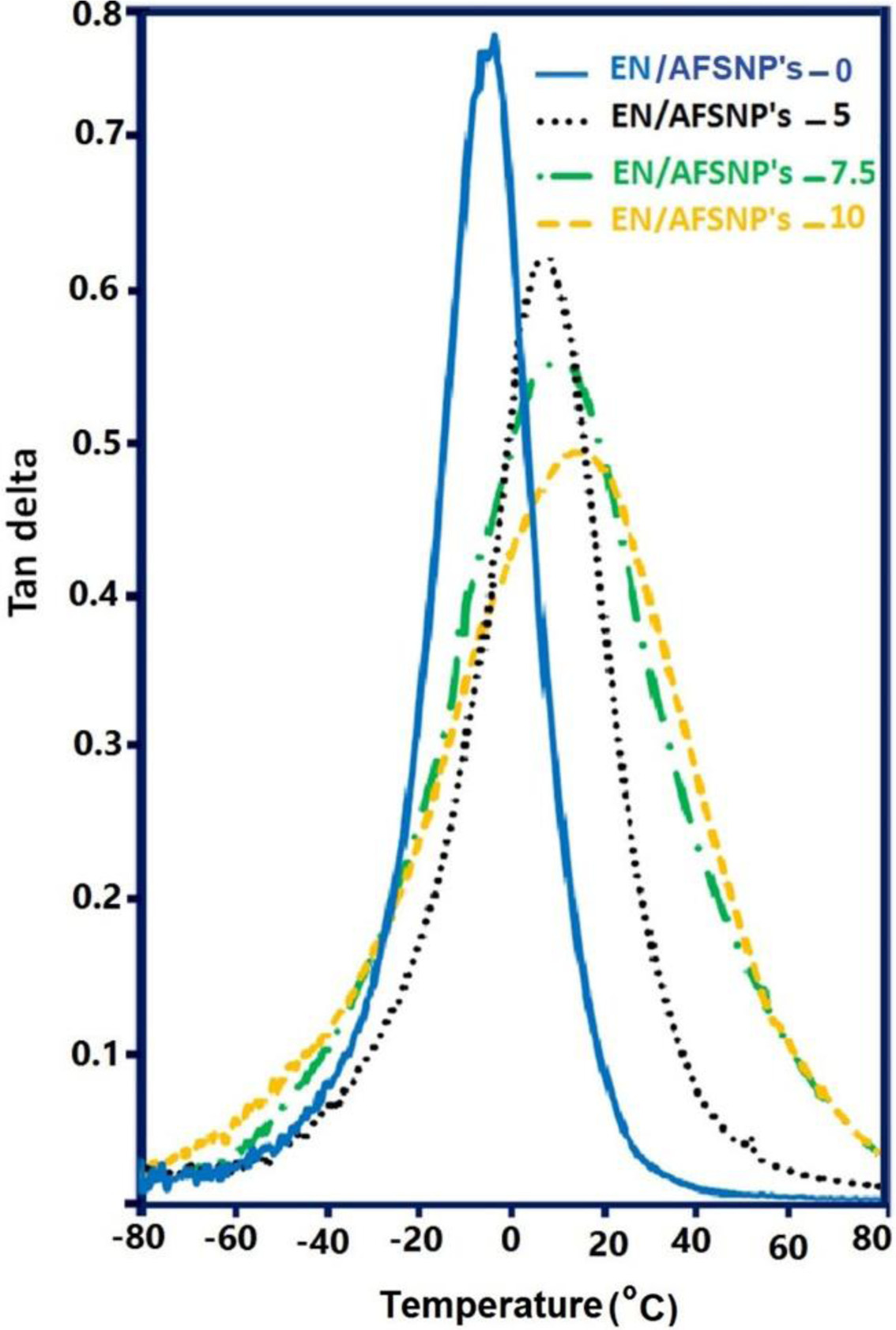
In addition to measuring the storage moduli, the loss factors (damping coefficients, tan δ), which are the ratio of the loss modulus (E′′) to the storage modulus (E′) were investigated for the resulting DGEBA-APOTM epoxy films (Figure 10). The peak appeared in the tan δ-T profile of each epoxy network is related to the α-transition of the chains. This transition appears when the chains in the amorphous regions begin to coordinate large-scale motions.24 In these profiles, the temperature at which α-transition occurs is the same the glass transition tem-perature (Tg) of the corresponding epoxy network. As the filler content increases, the peaks become shorter and wider, and slightly shift towards higher temperatures. Accordingly, EN/AFSNP′s-10 nanocomposite showed the maximum amount of Tg value, almost 25 ℃ greater than that of the neat epoxy network. This result indicates that there is a strong interaction between AFSNP′s filler and DGEBA-APOTM epoxy matrix. This strong filler-epoxy interaction could restrict the largescale motions of the polymer chains in their amorphous regions. The silanol (Si-OH) groups of the nanoparticles and the ether hinges of the curing agent can link with each other via formation of H-bonding. Moreover, amine-functionalization of the organically modified silica filler allow it to participate in the curing process of DGEBA resin via the formation of covalent bonds. It should be noted that the low Tg values of these DGEBA-APOTM epoxy networks originate from the ether-containing macromolecular size of APOTM curing agent. As stated earlier, utilization of this polyoxyalkylene decreases the crosslink density in the resulting network. While the Tg value of DGEBA-IPD epoxy network was found to be above 130 ℃,21 it is only -5 ℃ for the neat DGEBAAPOTM epoxy (EN/AFSNP′s-0). As a result, the fabricated epoxy nanocomposites showed the high degree of strength because of DGEBA resin and AFSNP′s filler as well as high degree of flexibility because of APOTM curing agent.
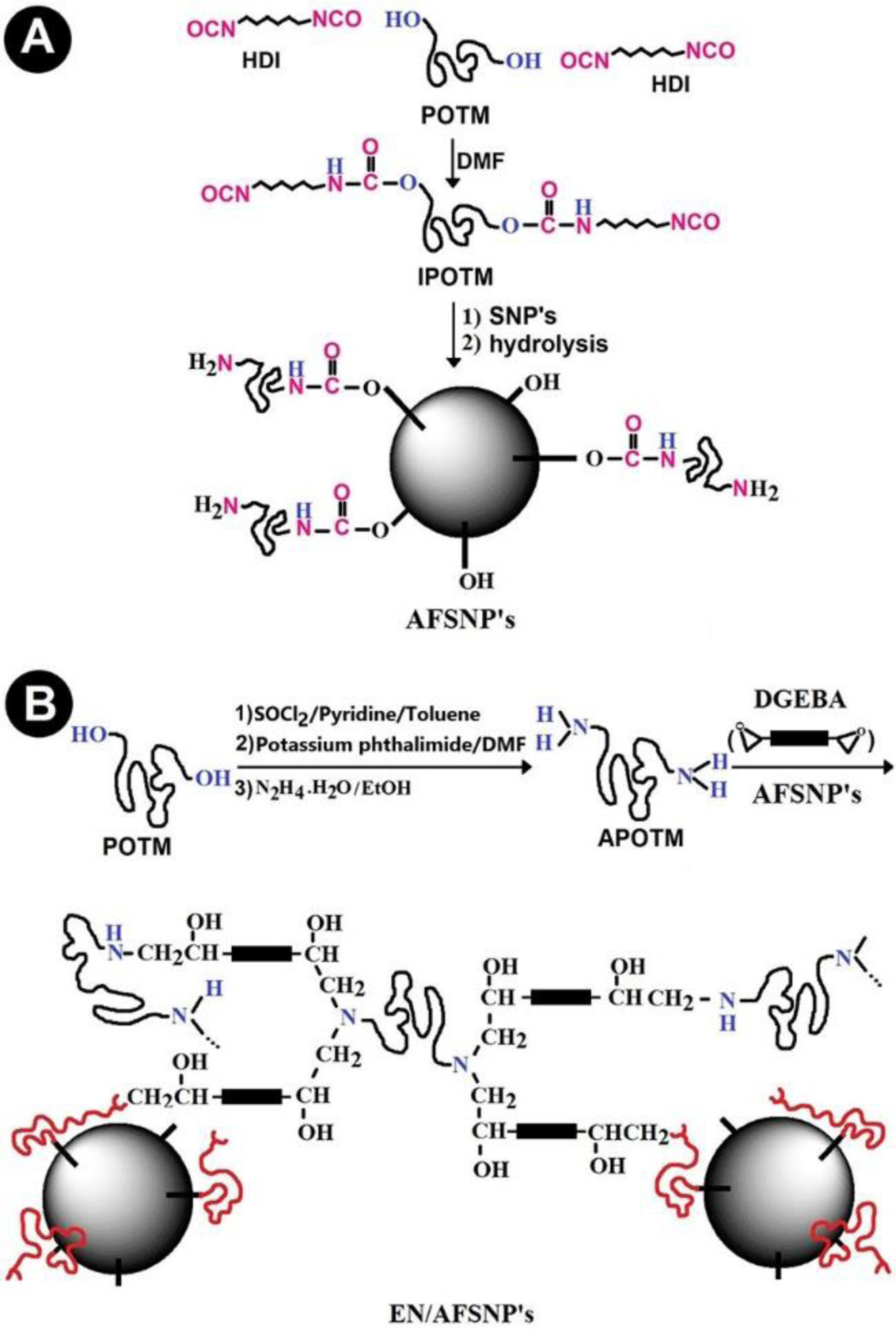
|
Figure 1 (A) Preparation of polyoxytetramethylene-coated silica nanoparticles (AFSNP′s); (B) Synthesis of DGEBA-APOTM epoxy networks possessing AFSNP's filler. |
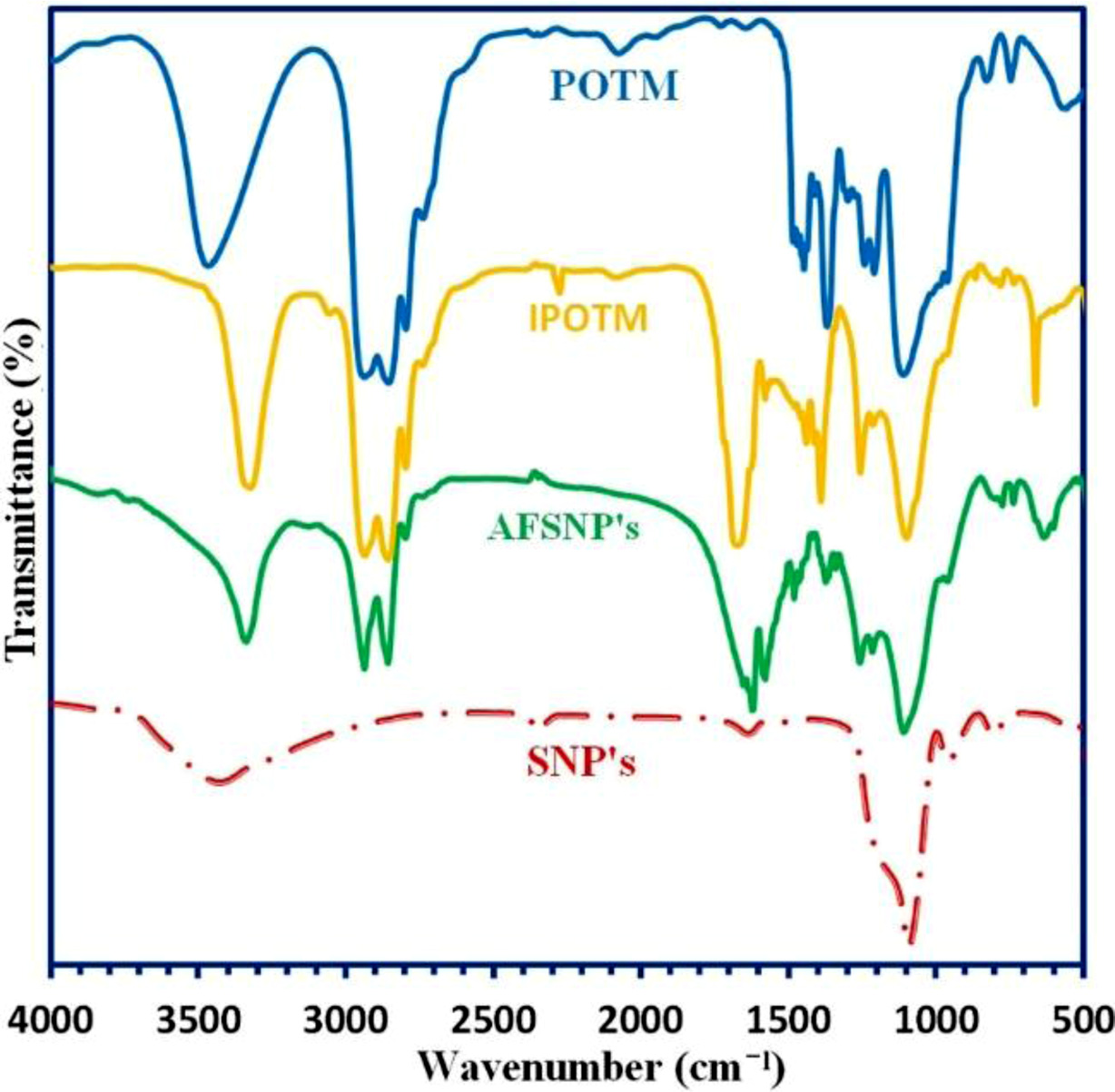
|
Figure 2 FTIR spectra of the IPOTM and AFSNP's. |
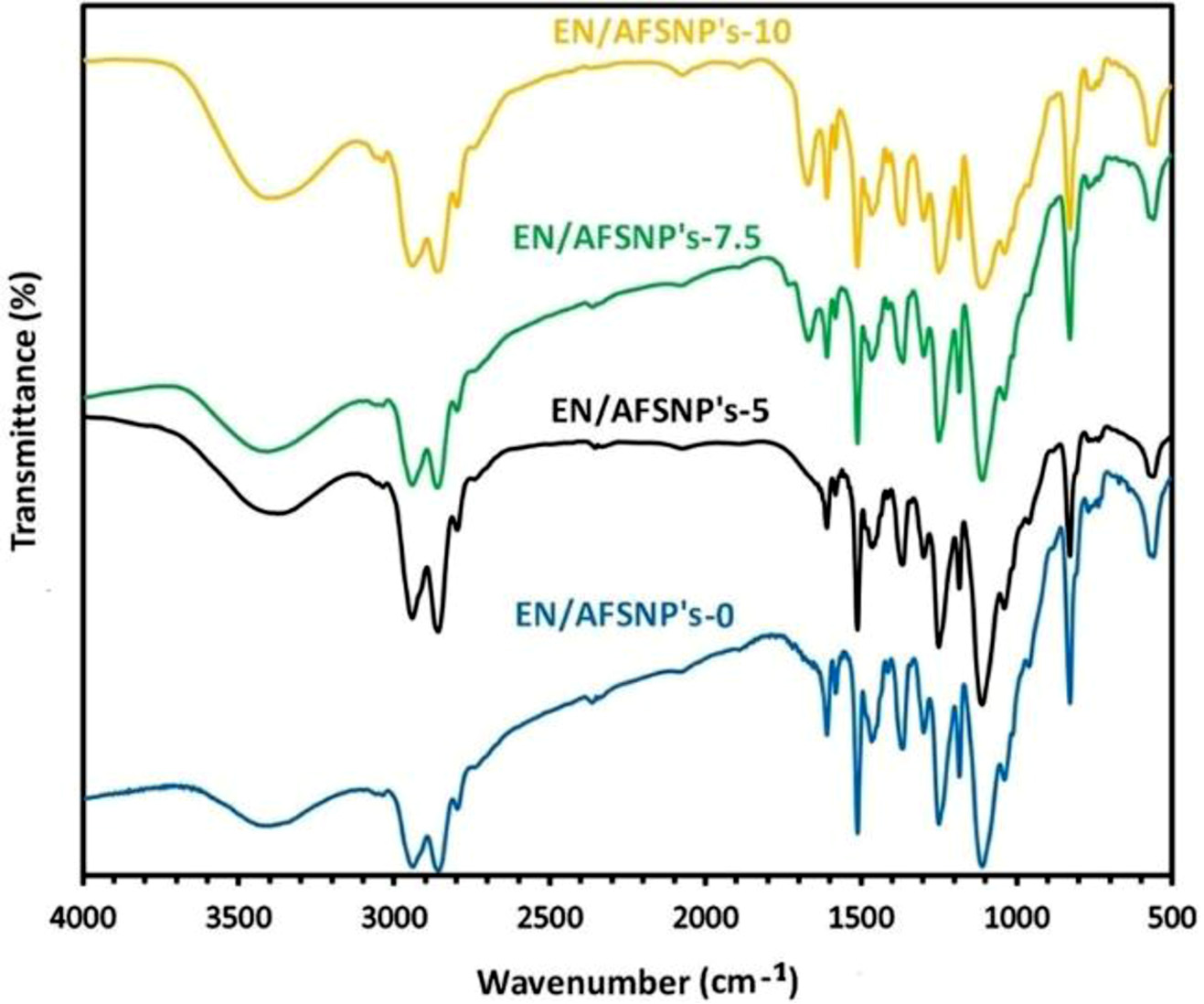
|
Figure 3 FTIR spectra of DGEBA-APOTM epoxy networks including EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5 and EN/AFSNP′s-10. |
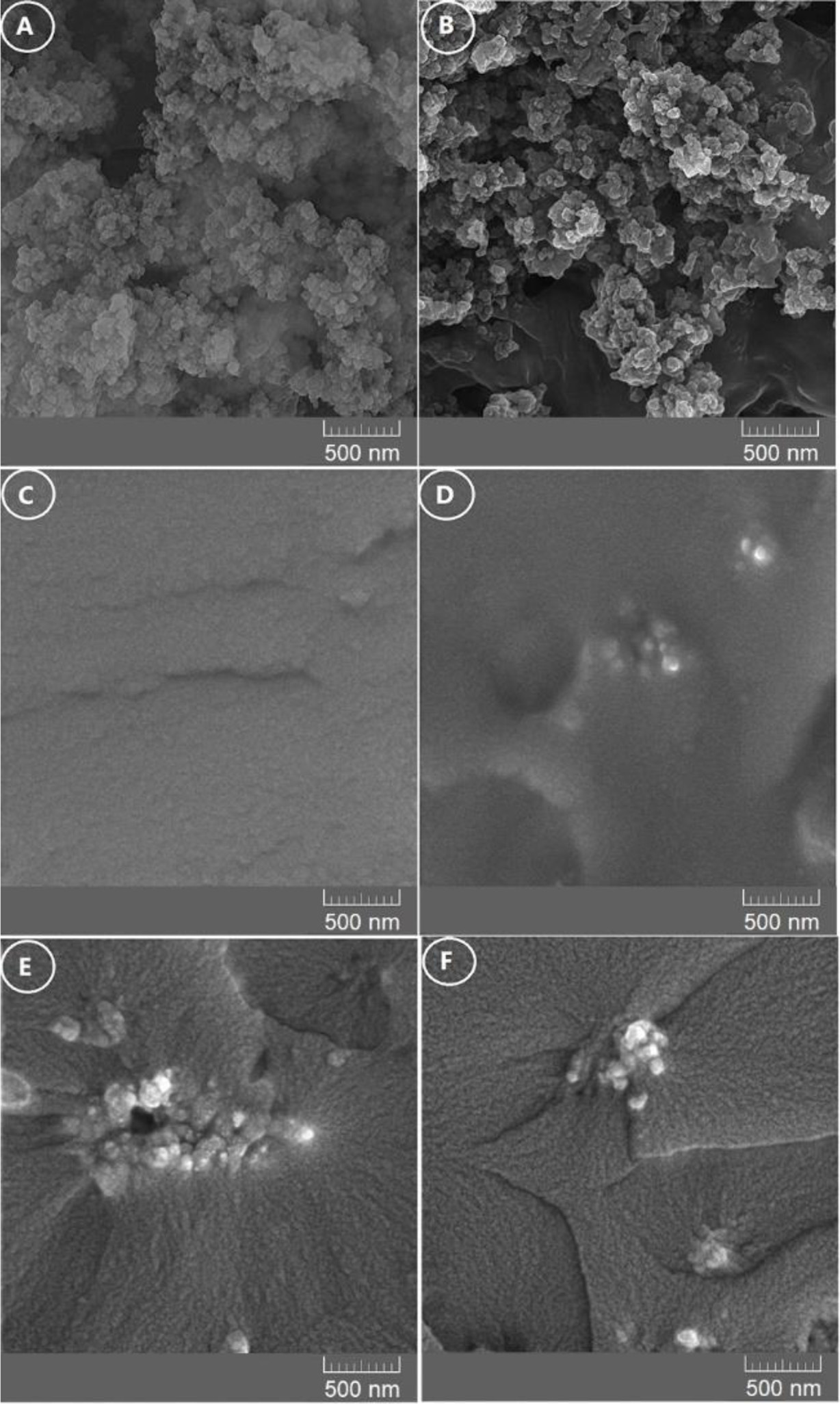
|
Figure 4 FE-SEM micrographs of the SNP's (A); AFSNP's (B); EN/AFSNP′s-0 wr% (C); EN/AFSNP′s-5 wt% (D); EN/AFSNP′s-7.5 wt% (E); EN/AFSNP′s-10 wt%; (F) nanocomposites. |
|
Figure 5 X-ray diffraction patterns of the SNP's, AFSNP's, and the resulting DGEBA-APOTM epoxy films. |
|
Figure 6 TGA thermogram of the SNP's and AFSNP's. |
|
Figure 7 TGA/DTG thermograms of EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5, and EN/AFSNP′s-10. |
|
Figure 8 Thermal phase transitions regarding to the thermodegradation of DGEBA-APOTM epoxy networks. |
|
Figure 9 E′-T profiles of EN/AFSNP′s-0, EN/AFSNP′s-5, EN/AFSNP′s-7.5 and EN/AFSNP′s-10. |
|
Figure 10 The loss factors (tan δ) related to the α-transition of the chains for the resulting DGEBA-APOTM epoxy films. |
|
Table 1 Some Results Obtained from TGA-DTG Measurements for EN/AFSNP's Series |
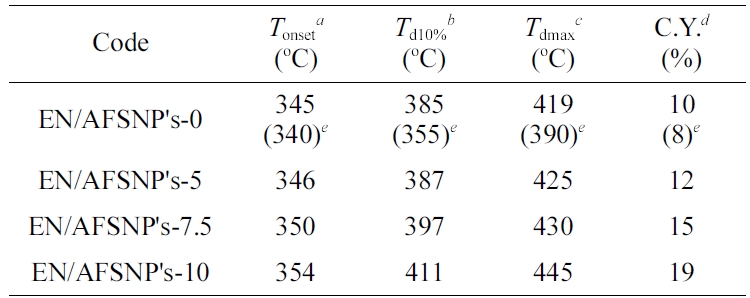
aOnset temperature of decomposition. bThe temperature at which 10% weight loss of the samples occurs; obtained from TGA thermograms. cThe temperature of maximum decomposition; obtained from DTG thermograms. dChar yields; measured at 700 ℃. eData in parentheses are related to DGEBA resin cured with Jeffamine D400 (Soares et al.).8 |
DGEBA-based epoxy resin was cured with a new α,ω- diamino polyoxyalkylene, i.e., APOTM in the presence of three different quantities of SNP′s. Prior to use, the inorganic nanoparticles were organically modified and amine-functionalized with a polyoxyalkylene structurally similar to that of the curing agent. It was found that the DGEBA-APOTM epoxy films were stabilized toward heat after charging with aminated SNP′s filler (AFSNP′s). Moreover, the storage moduli (E′ values) of the resulted epoxy networks significantly increased with increasing the filler content. This indicates that AFSNP′s filler enhances the stiffness of the epoxy-based nanocomposites obtained. The macromolecular size of APOTM curing agent along with its ether hinges led to some epoxy networks with a high degree of flexibility. Unlike conventional amine hardeners such as IPD, APOTM-cured DGEBA resins gave epoxy-based films with Tg values up to -5 ℃. The presence of AFSNP′s filler, however, enhanced these α-transitions due to strong matrix-filler interactions. In summary, the use of APOTM curing agent alongside AFSNP′s filler resulted in the fabrication of some new flexible DGEBA-based epoxy films with reinforced thermal behavior and enhanced storage moduli.
- 1. R. Ren, X. Xiong, X. Ma, S. Liu, J. Wang, P. Chen, and Y. Zeng, Thermochim. Acta, 623, 15 (2016).
-

- 2. I. Karimzadeh, M. Sabzi, B. Safibonab, I. Hasanzadeh, M. Farhangmehr, and A. Arman, Polym. Korea, 41, 98 (2017).
-

- 3. S. E. Lee, M. S. Park, E. Jeong, M. Y. Lee, M. K. Lee, and Y. S. Lee, Polym. Korea, 39, 426 (2015).
-

- 4. W. Hu, X. Luo, and Z. Yan, Polym. Korea, 41, 944 (2017).
-

- 5. Y. G. Hsu, K. H. Lin, T. Y. Lin, Y. L. Fang, S. C. Chen, and Y. C. Sung, Mater. Chem. Phys., 132, 688 (2012).
-

- 6. Z. Wang, Z. Wang, H. Yu, L. Zhao, and J. Jing, RSC Adv., 2, 2759 (2012).
-

- 7. S. B. Jagtap, V. S. Rao, S. Barman, and D. Ranta, Polymer, 63, 41 (2015).
-

- 8. B. G. Soares, A. A. Silva, S. Livi, J. Duchet-Rumeau, and J. F. Gerard, J. Appl. Polym. Sci., 131, 39834 (2014).
-

- 9. G. Yang, S. Y. Fu, and J. P. Yang, Polymer, 48, 302 (2007).
-

- 10. J. Macan, I. Brnardic, M. Ivankovic, and H. J. Mencer, J. Therm. Anal. Calorim., 81, 369 (2005).
-

- 11. A. R. Cedeno and C. S. P. Sung, Polymer, 46, 9378 (2005).
-

- 12. P. N. Patil, S. K. Rath, S. K. Sharma, K. Sudarshan, P. Maheshwari, M. Patri, S. Praveen, P. Khandelwal, and P. K. Pujari, Soft Matter, 9, 3589 (2013).
-

- 13. I. Krakovsky, J. Plestil, and L. Almasy, Polymer, 47, 218 (2006).
-

- 14. C. Zhou, A. Gu, G. Liang, and Y. Xia, Ind. Eng. Chem. Res., 54, 7102 (2015).
-

- 15. A. J. Kinloch, R. D. Mohammed, and A.C. Taylor, Mater. Sci., 40, 5083 (2005).
-

- 16. K. Leelachai, P. Kongkachuichay, and P. Dittanet, J. Polym. Res., 24, Article 41 (2017).
-

- 17. A. Legrand and C. Soulie-Ziakovic, Macromolecules, 49, 5893 (2016).
-

- 18. S. Sprenger, J. Mater. Sci., 49, 2391 (2014).
-

- 19. S. Liu, X. Fan, and C. He, Compos. Sci. Technol., 125, 132 (2016).
-

- 20. Z. Ahmad and F. Al-Sagheer, Prog. Org. Coat., 80, 65 (2015).
-

- 21. H. Behniafar and M. K. Nazemi, J. Polym. Bull., 74, 3739 (2017).
-

- 22. H. Behniafar, A. Ahmadi-khaneghah, and M. Yazdi, J. Polym. Res., 23, Article 202 (2016).
-

- 23. Q. Lian, K. Li, S. A. A. Shah, J. Cheng, and J. Zhang, J. Mater. Chem. A, 5, 14562 (2017).
-

- 24. J. Chandradass and D. S. Bae, Mater. Manuf. Process., 23, 116 (2008).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(1): 52-59
Published online Jan 25, 2019
- 10.7317/pk.2019.43.1.52
- Received on Jul 19, 2018
- Revised on Oct 10, 2018
- Accepted on Oct 30, 2018
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Hossein Behniafar
-
School of Chemistry, Damghan University, 36715-364, Damghan, Iran
- E-mail: h_behniafar@du.ac.ir
- ORCID:
0000-0001-6418-7490










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.