- Effect of pH on the Accelerated Aging Test of Gastrointestinal Absorption Inhibiting Stent Materials
Bong Seok Jang*,**, Ja Young Cheon*, Min Young Jung**,†
 , Oh Hyeong Kwon***, and Won Ho Park*,†
, Oh Hyeong Kwon***, and Won Ho Park*,† 
*Department of Advanced Organic Materials and Textile System Engineering, Chungnam National University, Daejeon 34134, Korea
**R&D Institute of Intervention, M. I. Tech Co., Ltd., Pyeongtaek 17711, Korea
***Departement of Polymer Science and Engineering, Kumoh National Institute of Technology, Gumi 39177 Korea- 위장관 흡수 억제용 스텐트 재료의 가속 노화시험에 대한 pH의 영향
*충남대학교 유기소재섬유시스템공학과, **㈜엠아이텍, ***금오공과대학교 고분자공학과
The stability of silicone and fluoropolymer materials, such as expanded polytetrafluoroethylene (e-PTFE) and fluorinated ethylene propylene copolymer (FEP), was assessed as bypass liners of an absorption inhibition stent to treat obesity. The stability was evaluated by conducting an accelerated aging test in a pH condition similar to a stomach acid and bile acid environments in contact with the actual stent bypass liner. The changes in the morphology and properties (chemical and physical) after accelerated aging were analyzed via SEM, FTIR and UTM. In addition, a bile acid permeation experiment was carried out using e-PTFE to confirm that in vitro testing could be used as a bypass liner of the stent to treat obesity.
연신 polytetrafluoroethylene(e-PTFE) 및 fluorinated ethylene propylene copolymer(FEP)와 같은 불소계 고분자와 실리콘 재료의 안정성을 비만 치료용 흡수 억제 스텐트의 바이패스 라이너로 평가하였다. 안정성 평가는 실제 스텐트의 바이패스 라이너가 접촉하는 위산과 담즙산 환경과 유사한 pH 조건에서 가속 노화실험을 통해 이루어졌다. 가속 노화실험 전후의 형태학적, 물리·화학적 변화는 주사전자현미경, 적외선분광분석, 인장시험 등을 통해서 확인하였다. 또한 담즙산 투과실험은 in vitro 시험이 비만 치료용 스텐트의 바이패스 라이너로 유용한지를 확인하기 위해 e-PTFE 시료를 사용하여 수행되었다.
Keywords: bypass liner stent, silicone, expanded poly(tetrafluoroethylene), fluorinated ethylene propylene copolymer, accelerated aging test
This study was financially supported by the National Research Foundation of Korea (2018R1A2A2A 05021100).
Obesity is rapidly increasing worldwide, and it has increased by more than twice over the past two decades. In general, people are considered to be obese when their body mass index (BMI), a value obtained by dividing a person’s weight by the square of the person’s height, is greater than 30 kg/m2, and a BMI in a range of 25-30 kg/m2 is defined as overweight.1 Obesity is a phenomenon where excess energy accumulates into fat in the body. An abnormal increase in body fat leads to metabolic abnormalities that are presumed by neuroendocrine causes, drugs, physical inactivity, metabolic defects, endocrine disorders and genetic disease.2-5
Methods treating obesity include diet, exercise, behavioral therapy, medication, and surgical treatment.6-10 In some cases, a combination of methods are also used. Of these, surgical treatment including restrictive surgery and malabsorptive surgery are administered to patients with a high BMI of over 40 kg/m2.4,5,10,11 Treatment using such surgical procedures is associated with a sustained weight loss, but complications or side effects are frequent. Recently, laparoscopic treatment techniques have been emphasized to remedy these problems.12
A stent is a medical device that temporarily or permanently expands the narrow lumen of a lesion with an interventional minimally invasive technique, and not a surgical procedure.13 Modern stents to treat obesity are inserted into the duodenum or small intestine after the insertion of the endoscope.14 Stent insertion using the duodenal-jejunal bypass liner is an endoscopic method that prevents the absorption and digestion of nutrients in food. The materials used in these bypass liners should have chemical resistance and mechanical durability that do not degrade at least within 6 months in the human body.15
Hydrophobic silicone and fluoropolymers, such as expanded polytetrafluoroethylene (e-PTFE) and fluorinated ethylene-propylene copolymer (FEP)16 are the most commonly used non-biodegradable materials for medical applications. These are also used as medical materials, such as for artificial blood vessels and stent grafts, due to their abrasion resistance.
Park et al. reported that silicone-coated stents were more stable than urethane-coated ones in a gastric environment.17 In another study, the FEP was used as a bypass liner of the stent to treat obesity, and its function was confirmed through preclinical testing.18,19 In this study, the stability of silicone, e-PTFE and FEP in the gastric environment was evaluated using an accelerated aging test.20,21
Materials. The silicone, e-PTFE and FEP used to cover the stent were purchased from NuSil Inc., ZEUS Inc., and Dupont Inc., respectively. These polymers are non-biodegradable in the human body. The FEP and PTFE were sheet type, and silicone was dispersion type (20 wt% in xylene).
To apply to the stent membrane, the silicone dispersion was first diluted to 13 wt% through the addition of xylene. The diluted silicone dispersion was then poured into a PTFE-based square mold (20 cm (w)×20 cm (d)×3 cm (h)) and was dried in the vacuum oven. The vacuum drying was performed in 3 steps, at 35, 75, and 150 ℃ for 30, 60, and 180 min, respectively.
Accelerated Aging Test. The accelerated aging test is a stability test designed to speed up the chemical or physical degeneration of medical devices, such as under severe temperature conditions, for a short period of time relative to the actual shelf life of the product.22 Accelerated aging of polymers is generally carried out in accordance with Ministry of Food & Drug Safety (MFDS) notification. This test should involve maintaining the material at a test temperature of 60 ℃ for 40 days to accelerate aging (12 months).
An accelerated aging factor (AAF) estimate was calculated using the following Arrhenius equation:23

where Q10 equaling to 2 is a common and conservative means of calculating the aging factor. TAA was the accelerated aging temperature (℃), and TRT was the ambient temperature. The accelerated aging time (AAT) needed for the equivalence to real time aging was determined by dividing the required shelf time by the AAF.23
Accelerated aging time (AAT) = Required shelf time/AAF (2)
In this experiment, the stent to treat obesity was placed in the human body for 6 months. Therefore, the required shelf life for the accelerated aging test at 60 ℃ using the above equation was calculated to be 20 days. As a result, all samples were immersed and stored at 60 ℃ for 20 days and the test was carried out at different pH conditions (pH 2 and 7 buffer solutions). This creates an environment similar to digestive enzymes, such as gastric acid and bile acid, produced in the human digestive organs.
Characterization. The surface morphology and thickness of the silicone, e-PTFE, and FEP were observed via scanning electron microscopy (SEM) (S2400, Hitachi Instruments Inc, San Jose, CA, USA) before and after the accelerated aging test at different pH conditions. The specimens were peeled off, cut into 1×1 cm squares, and subjected to gold coating for SEM observation with a total magnification of 1000x to 2000x. In addition, the surface of the materials was observed after the in vitro experiment. Also, the mechanical strength of the membrane materials with the accelerated aging test was analyzed using an universal testing machine (UTM) (LS series, Lloyd Instruments Ltd, West Sussex, UK). The tensile test was conducted according to ASTM D882-12, and the specimen was prepared as 26×100 mm. Initial gauge length of 50 mm was fixed to both grip and pulled both directions to break in speed rate of 500 mm/min. To confirm the chemical resistance, the chemical structure of the membranes subjected to the accelerated aging test at different pH conditions was confirmed via Fourier-transform infrared spectroscopy (FTIR) (NicoletTM iSTM 50, Thermo Fisher Scientific, Boston, MA, USA). Sample of 10 mg was thoroughly mixed with 100 mg of KBr. And then an appropriate pressure was exerted to obtain a transparent pellet. FTIR spectrum was recorded between the range from 4000 to 400 cm-1. After the in vitro test, the atomic compositions on the membrane surface were investigated via energy dispersive X-ray spectroscopy (EDS) (S2400, Hitachi Instruments Inc, San Jose, CA, USA) to check the presence of passage of bile acid.
In Vitro Test. The tube-type e-PTFE blocks the movement of the substances using porcine bile, similar to human bile. The materials used in the in vitro experiment were tubular e-PTFE. The porcine bile solution was prepared by dissolving the porcine bile powder (Sigma Aldrich, Bile extract porcine, St. Louis, MO, USA) at a concentration of 10% in distilled water. The porcine bile solution (200 mL) was put into an e-PTFE tube, and both ends were tied together to form a balloon. Then, the porcine bile-loaded e-PTFE tube was immersed in distilled water with stirring at 300 rpm for 72 h. After the porcine bile was removed from the e-PTFE tube and dried, the inside and outside structure of e-PTFE tube were analyzed via SEM and EDS.
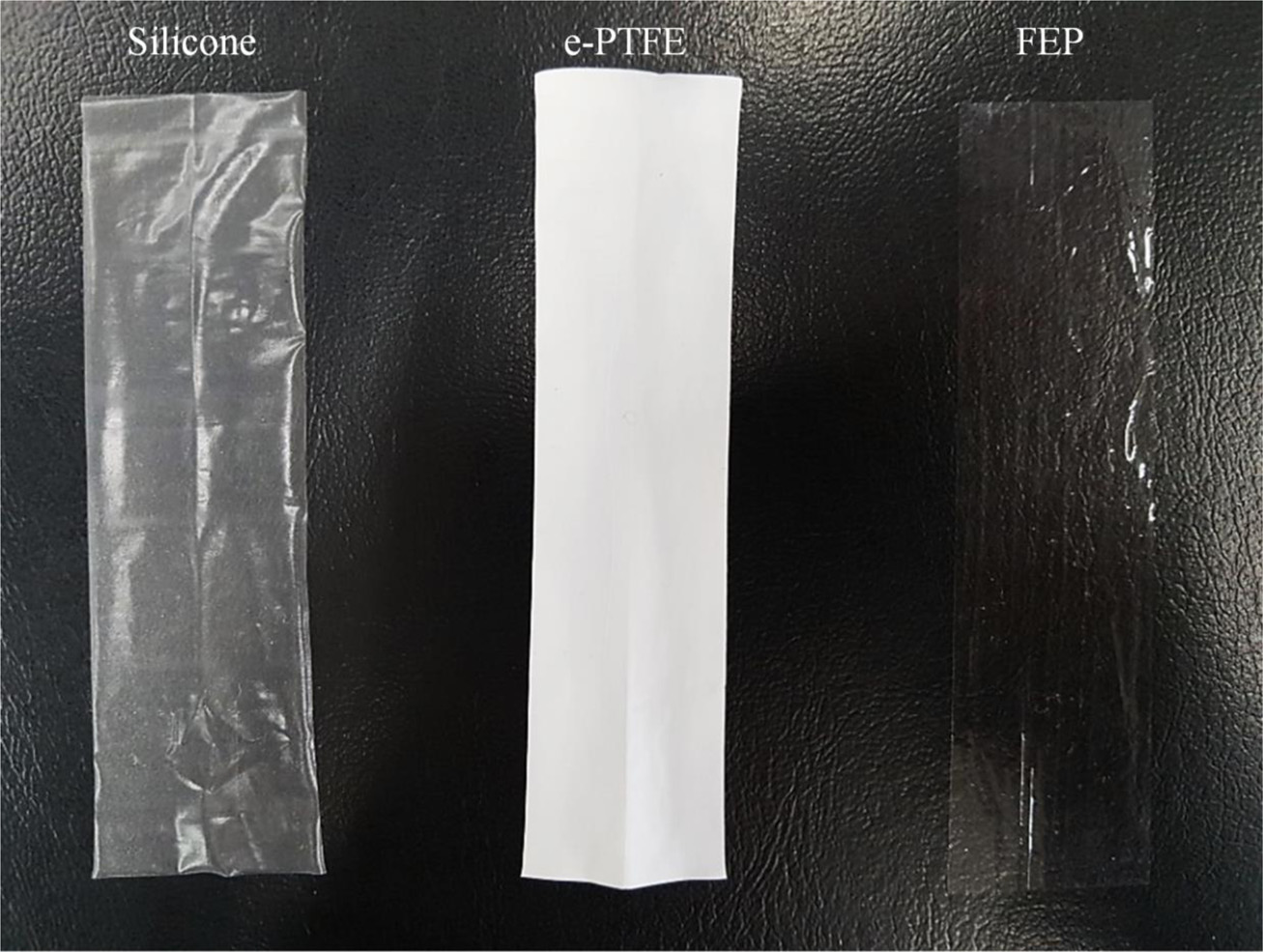
Stability Evaluation by Accelerated Aging Test. The photographic images of each membrane sample are shown in Figure 1. The sample showed a distinct difference in color, i.e., the silicone is translucent, e-PTFE is white and FEP is transparent.
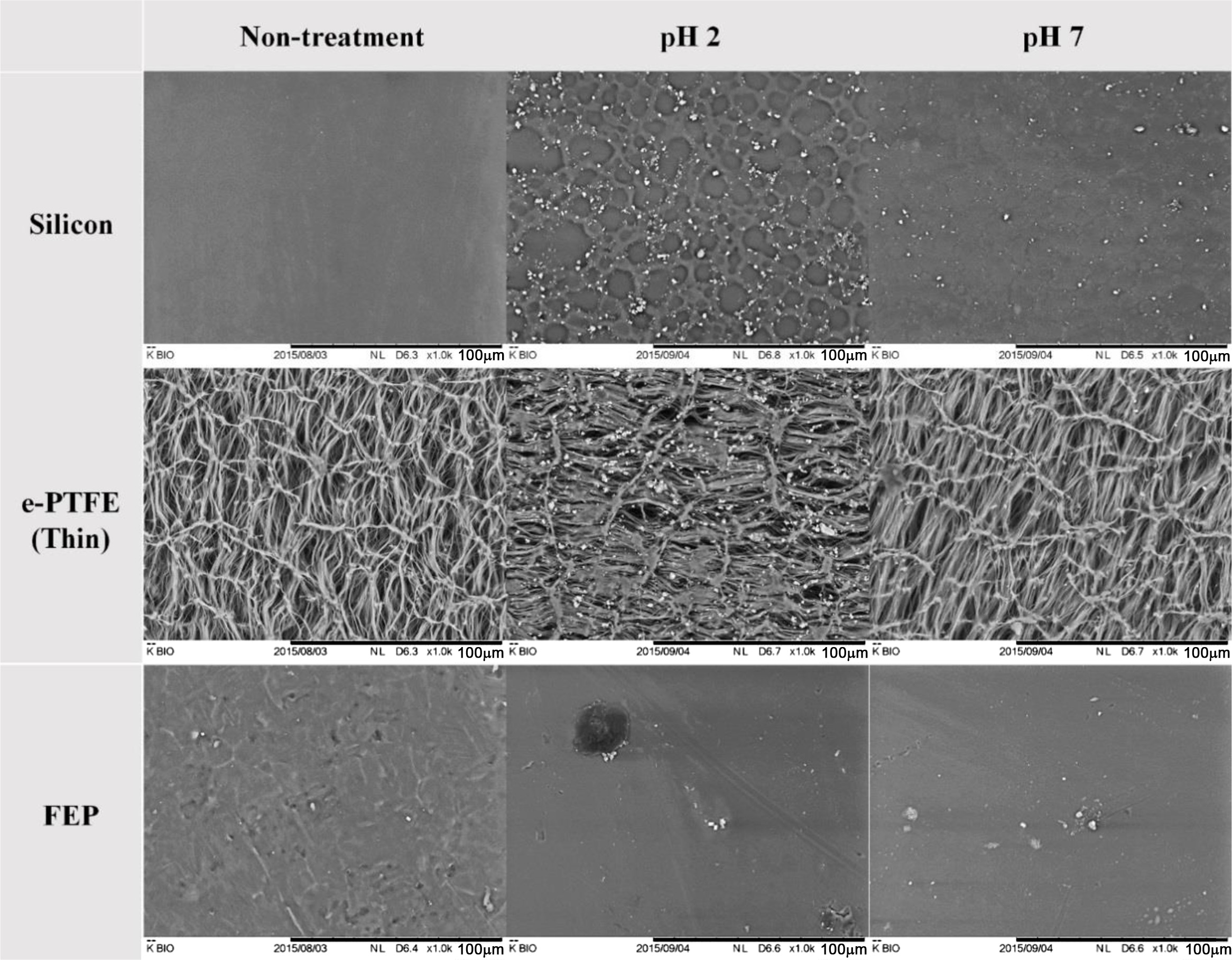
Figure 2 shows SEM images of the surface microstructure after the accelerated aging test in different buffer solutions (pH 2 and pH 7). In the case of silicone, the surface deformation and debris were observed at both pH 2 and pH 7. In particular, at pH 2, the aging proceeded only with the shelf life test, and thus the physical properties were likely to be weak. On the other hand, in the case of e-PTFE and FEP, the surface was hardly deformed, because the two materials consist of fluorine-based polymers and are thus highly resistant to chemicals.24
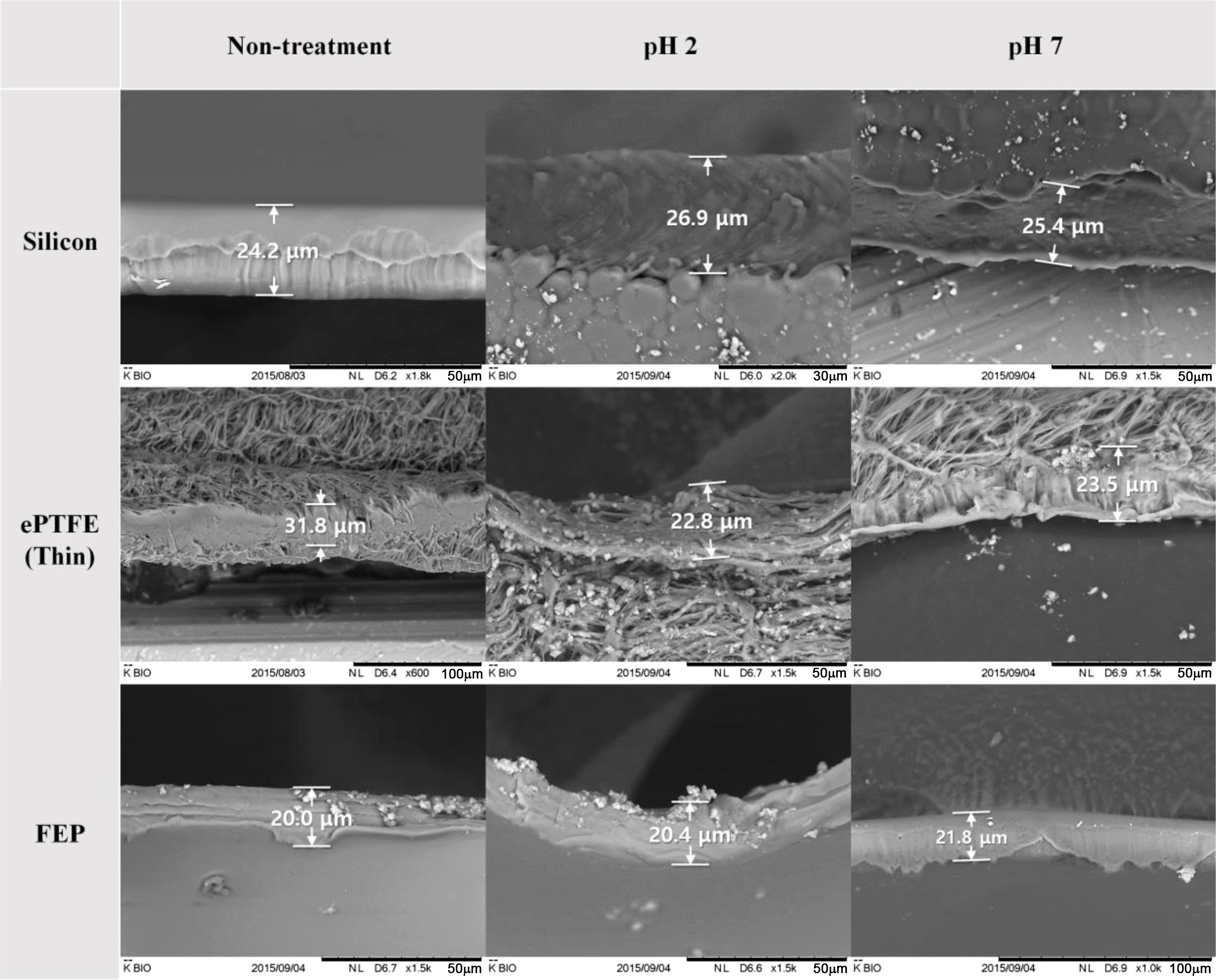
In addition, the change in the membrane thickness was investigated after the accelerated aging test with different buffer solutions (pH 2 and pH 7). The initial thicknesses of the silicone, e-PTFE and FEP were 25.4±1.2, 27.2±1.7, and 21.4± 0.9 μm, respectively. No noticeable change was observed after the accelerated aging test (Figure 3). Therefore, the accelerated aging test and the pH of the buffer solution were not found to affect the thickness of the membrane.
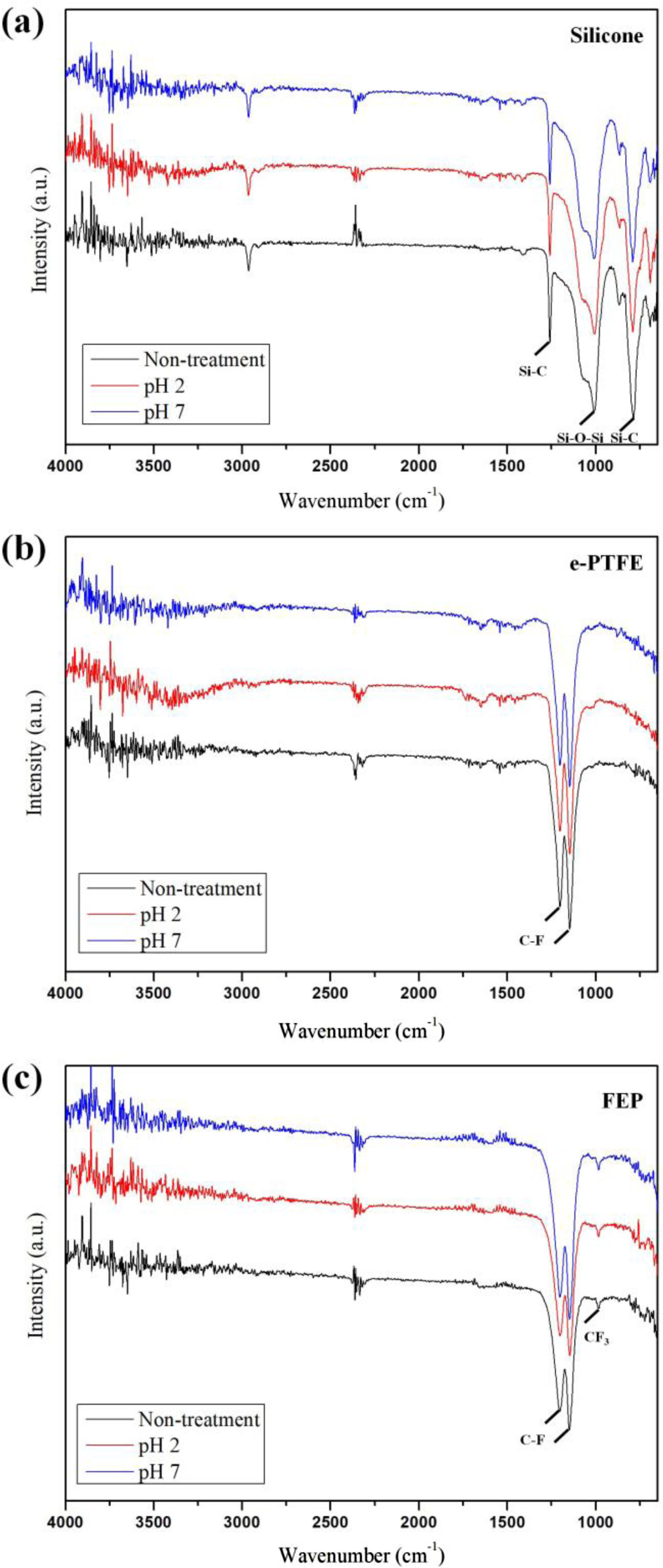
The chemical structure of each substance was analyzed via FTIR. In the silicone, characteristic peaks such as 1010, 1258 and 785 cm-1, corresponding to Si-O-Si and Si-C were respectively observed (Figure 4(a)).25 In Figure 4(b) and 4(c), the two strong peaks at 1200 and 1148 cm-1 were attributed to -CF2- asymmetric and symmetric stretching vibrations in CF2 groups. Only at FEP, an additional peak corresponding to the CF3 side group was observed at 984 cm-1.24,26,27 A structural analysis revealed that no chemical structural change occurred in all samples after the accelerated aging test at pH 2 and pH 7. This suggests that the accelerated aging test led to morphological deformation of some materials, but it did not cause chemical changes. Based on the above results, the e-PTFE and FEP with chemical stability and no microstructural deformation were suitable for use as dietary stent materials.
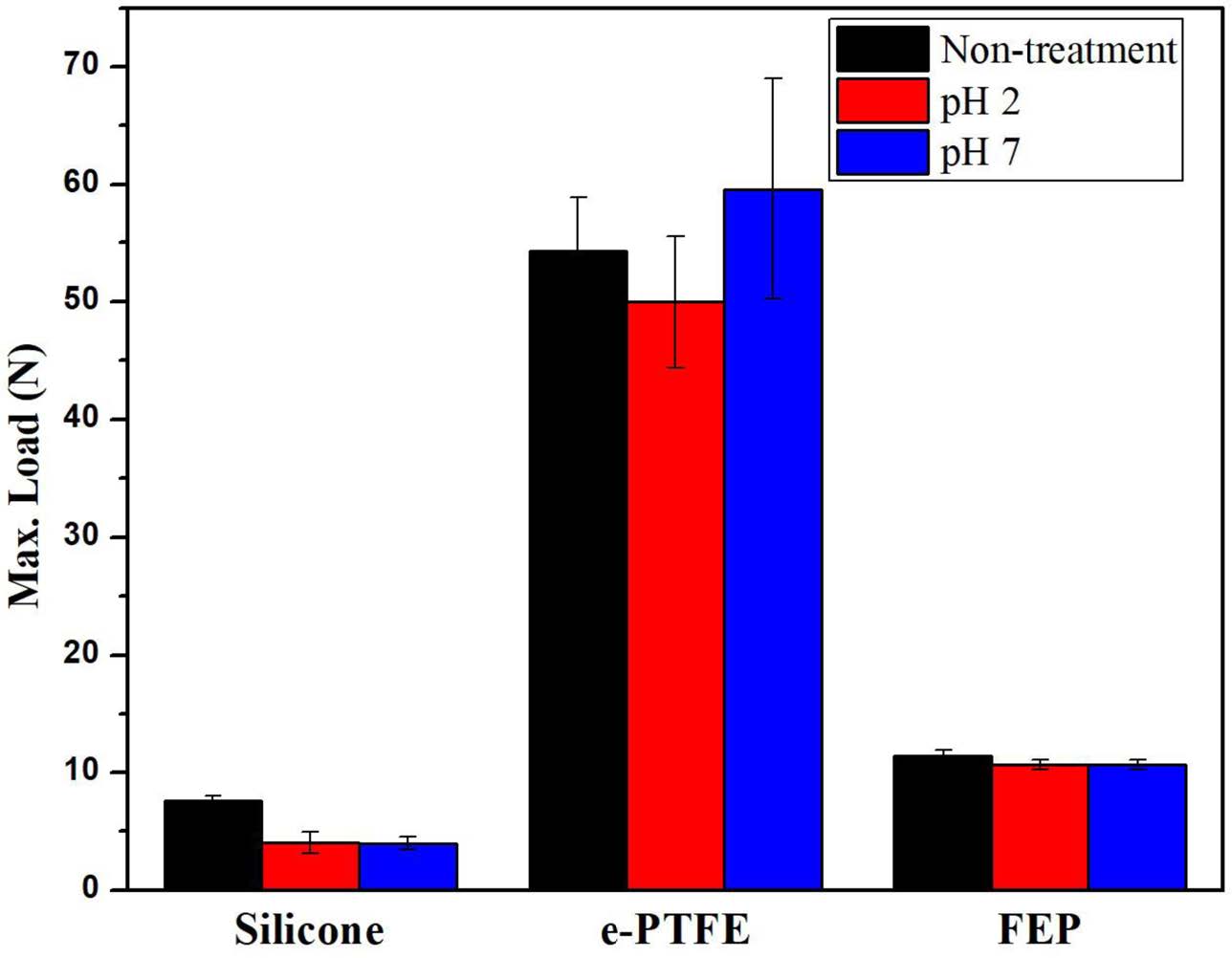
Figure 5 shows the mechanical strength before and after the accelerated aging test in different buffer solutions (pH 2 and pH 7). The silicone showed a significant decrease in the mechanical strength after the accelerated aging test, regardless of the pHs, comparing to original mechanical strength before test. On the other hand, e-PTFE and FEP showed little change in the mechanical strength before and after the accelerated aging test, regardless of the pHs. No change in the mechanical strength indicates no chemical deformation in the samples. The bypass liner of the stent should maintain a certain degree of strength, and therefore, the e-PTFE with durability and tensile strength is the most suitable as a dietary stent for use in the body.
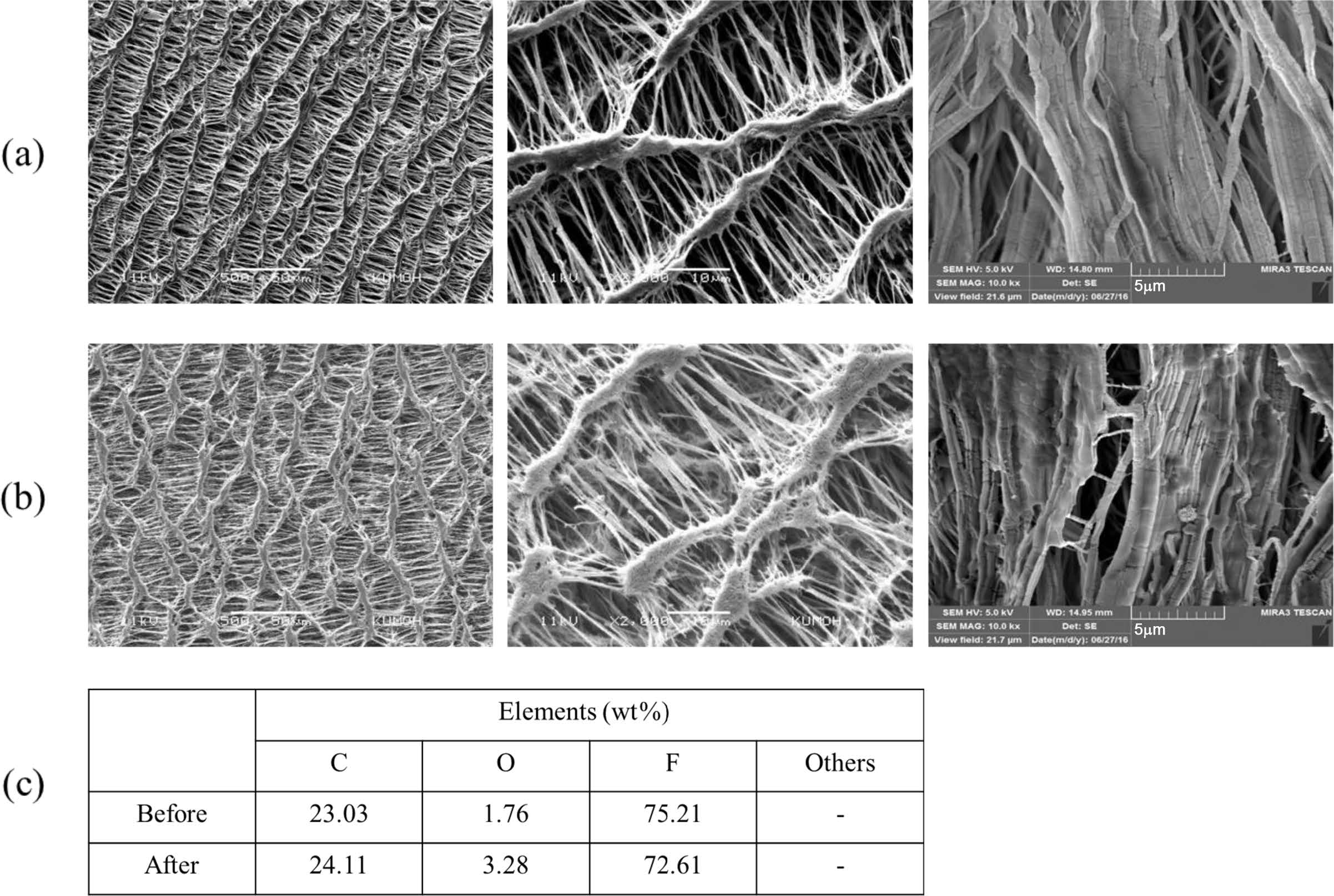
Porcine Bile Permeation Experiment. In vitro experiments were conducted using porcine bile to determine whether e-PTFE can be used as a bypass liner for the stent to treat obesity. The outer surface of the e-PTFE tube containing the porcine bile was subjected to an elemental analysis through EDS after a certain period of 72 h, and the results are shown in Figure 6. To be useful as a bypass liner for a stent to treat obesity, the passage of nutrients must be prevented. In this study, porcine bile was used instead of nutrients, and it should not permeate the samples. The results from Figure 6 showed that no bile elements were found on the e-PTFE surface that did not contact the bile before and after the bile permeation experiment. From this, the e-PTFE did not permeate the nutrients. Therefore, e-PTFE is suitable as a bypass liner for a dietary stent because it prevents the absorption of nutrients.
|
Figure 1 Photographic images of the samples: Silicone (left), e-PTFE (middle), FEP (right). |
|
Figure 2 SEM images of surface before and after the accelerated aging test at the pH of buffer solution. |
|
Figure 3 SEM images of cross-section before and after the accelerated aging test at the pH of buffer solution. |
|
Figure 4 FTIR before and after the accelerated aging test at the pH of buffer solution: (a) silicone; (b) e-PTFE; (c) FEP. |
|
Figure 5 Mechanical strength of silicone, e-PTFE, and FEP before and after the accelerated aging test. |
|
Figure 6 SEM morphologies of e-PTFE before (a); after (b) in vitro test using porcine bile; (c) amount of elements by EDS analysis. Magnification: surface (left) 500X, surface (middle) 2000X, cross-section (right) 10000X. |
The silicone, e-PTFE and FEP showed no significant differences in the chemical and physical properties after the accelerated aging test at different pH conditions. It is considered to be a suitable material because it is stable for use as a bypass liner of the stent to treat obesity. In the case of silicone, there is a slight deformation, and as mentioned above, as a medical device for interventional procedure, there is a risk of stenosis when loading for a long time. On the other hand, the e-PTFE and FEP, which are fluorine compounds, have excellent chemical resistance and are not affected by the pH condition. It is also expected to remedy stenosis caused by silicone due to its excellent hydrophobicity. There is much clinical verification with a ready-made bypass liner using FEP materials. However, since FEP uses film form, kinking occurs in the body, which may result in the loss of function as a bypass liner. In addition, the bile acid infiltration experiment confirmed that e-PTFE prevents the penetration of substances. Therefore, the accelerated aging test and in vitro test indicate that the e-PTFE is most suitable as a bypass liner inhibiting the absorption of the stent to treat obesity. There is a need to confirm the validity and stability of the material through further experiments.
- 1. World Health Organization (WHO), Obesity and overweight, 2015.
- 2. F. T. Yazdi, S. M. Clee, and D. Meyre, PeerJ, 2015; 3:e856. DOI:10.7717/peerJ.856.
-

- 3. S. Bleich, D. Cutler, C. Murray, and A. Adams, Annu. Rev. Public Health, 29, 273 (2009).
-

- 4. G. D. Foster, T. A. Wadden, A. P. Makris, D. Davidson, R. S. Sanderson, D. B. Allison, and A. Kessler, Obes. Res., 11, 1168 (2003).
-

- 5. B. M. Wolfe, E. Kvach, and R. H. Eckel, Circ. Res., 118, 1844 (2016).
-

- 6. D. W. Haslam and W. P. James, Lancet, 366, 1197 (2005).
-

- 7. S. Z. Yanovski and J. A. Yanovski, JAMA, 311, 74 (2014).
-

- 8. J. L. Colquitt, K. Pickett, E. Loveman, and G. K. Frampton, Cochrane DB Syst. Rev., 8, 1 (2014).
-

- 9. I. Imaz, C. Martínez-Cervell, E. E. García-Alvarez, J. M. Sendra-Gutiérrez, and J. González-Enríquez, Obes. Surg., 18, 841 (2008).
-

- 10. R. F. Kushner, Prog. Cardiovasc. Dis., 56, 465 (2014).
-

- 11. J. Wells, M. Miller, B. Perry, J. A. Ewing, A. L. Hale, and J. D. Scott, Am. Surg., 81, 812 (2015).
- 12. A. Srivastava and A. Niranjan, J. Minim. Access Surg., 6, 91 (2010).
-

- 13. https://www.nhlbi.nih.gov/health/health-topics/topics/stents.
- 14. K. T. Sato and C. Takehana, Semin. Intervent. Radiol., 24, 391 (2007).
-

- 15. P. Koehestanie, C. de Jonge, F. J. Berends, I. M. Janssen, N. D. Bouvy, and J. W. Greve, Ann. Surg., 260, 984 (2014).
-

- 16. N. Gupta, M. V. Kavya, Y. R. G. Singh, J. Jyothi, and H. C. Barshilia, J. Appl. Phys., 114, 1 (2013).
-

- 17. S. C. Park, N. Park, D. Kim, J. Nah, Y. T. Jeen, H. J. Cho, E. S. Kim, B. Keum, Y. S. Seo, H. S. Lee, H. J. Chun, S. H. Um, C. D. Kim, and H. S. Ryu, Polym. Korea, 38, 351 (2014).
-

- 18. S. Majumder and J. Birk, Surg. Endosc., 27, 2305 (2013).
-

- 19. P. Koehestanie, B. Betzel, K. Dogan, F. Berends, I. Janssen, E. Aarts, M. Groenen, and P. Wahab, Surg. Endosc., 28, 325 (2014).
-

- 20. M. Takeuchi, T. Kuratani, S. Miyagawa, Y. Shirakawa, K. Shimamura, K. Kin, T. Yoshida, Y. Arai, T. Hoashi, N. Teramoto, K. Hirakawa, N. Kawaguchi, and Y. Sawa, J. Thorac. Cardiovasc. Surg., 148, 4 (2014).
-

- 21. D. W. L. Hukins, A. Mahomed, and S. N. Kukureka, Med. Eng. Phys., 30, 10 (2008).
-

- 22. K. J. Hemmerlich, Med. Plast. Biomater., 5(4), 16 (1998).
- 23. ASTM F1980-16, Standard guide for accelerated aging of sterile barrier systems for medical devices.
- 24. M. Lv, F. Zheng, Q. Wang, T. Wang, and Y. Liang, Mater. Des., 85, 162 (2015).
-

- 25. L. M. Johnson, L. Gao, C. W. Shields IV, M. Smith, K. Efimenko, K. Cushing, J. Genzer, and G. P. López, J. Nanobiotechnol., 11, 1 (2013).
-

- 26. S. Yakunin, M. Fahrner, B. Reisinger, H. Itani, C. Romanin, and J. Heitz, J. Biomed. Mater. Res. Part B, 100, 170 (2012).
-

- 27. A. Kitamura, T. Kobayashi, T. Satoh, M. Koka, T. Kamiya, A. Suzuki, and T. Terai, Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms, 307, 614 (2013).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(2): 261-267
Published online Mar 25, 2019
- 10.7317/pk.2019.43.2.261
- Received on Nov 14, 2018
- Revised on Nov 26, 2018
- Accepted on Nov 29, 2018
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Min Young Jung**, and Won Ho Park*
-
*Department of Advanced Organic Materials and Textile System Engineering, Chungnam National University, Daejeon 34134, Korea
**R&D Institute of Intervention, M. I. Tech Co., Ltd., Pyeongtaek 17711, Korea - E-mail: myjung@mitech.co.kr, parkwh@cnu.ac.kr
- ORCID:
0000-0001-5616-3704, 0000-0003-1768-830X









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.