- Formulation of Double-layer Tadalafil and Amlodipine Complex Tablets to Treat Erectile Dysfunction and Hypertension
Eun Yeong Shin, Jong Ho Park, Myeong Eun Shin, Jeong Eun Song, and Gilson Khang†

Department of BIN Convergence Technology, Department of Polymer Nano Science &Technology and Polymer Materials Fusion Research Center, Chonbuk National University, 567 Baekje-daero, Deokjin-gu, Jeonju-si, Jeollabuk-do, 54896, Korea
- 발기부전과 고혈압을 치료하기 위한 이중층 타다라필 및 암로디핀 복합 정제 제조
전북대학교 BIN 융합 공학과, 고분자나노공학과, 고분자융합소재연구소
Many men over the age of 40 suffer from erectile dysfunction (ED) for a variety of reasons. In addition, more than 40% of ED patients have hypertensive disease at the same time. Tadalafil (TD) is a selective and reversible inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase type 5 (cGMP-specific PDE-5). It is an insoluble drug with biopharmaceutics classification system class-II and was prepared by wet granulation with a solubilizing agent. Amlodipine besylate (AM) is a calcium channel antagonist that is effective in preventing systolic hypertension and stroke, and is manufactured using physical mix method. We used TD and AM to fabricate a double-layer complex tablet that can prevent or treat two diseases at the same time. Scanning electron microscope (SEM), Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimeter (DSC), X-ray diffraction (XRD) were analyzed for confirming chemical and physical characterization. Also, in vitro dissolution test was conducted to evaluate the dissolution rate of tablet at pH=1.2. In conclusion, the rapid release of two drugs in the double layer tablet was confirmed and could be applied as a pharmaceutical therapeutic agent.
40세 이상의 많은 남성들은 여러 가지 이유로 발기부전(erectile dysfunction, ED)의 고통을 가지고 있다. 또한 ED 환자의 40% 이상이 고혈압 질환을 동시에 가진다. 타다라필(tadalafil, TD)은 선택적이고, 가역적인 cGMP- 특이적 PDE-5억제제이다. 이는 생물 약제 분류 시스템 클래스-II의 불용성 약물로, 가용화제로 습식 과립제로 제조되었다. 암로디핀 베실레이트(amlodipine besylate, AM)는 수축기 고혈압 및 뇌졸중 예방에 효과적인 칼슘 채널 길항제이며, 물리적 혼합 방법을 사용하여 제조하였다. TD와 AM을 2중층 복합정제로 제작하여 사용하였고, 이는 동시에 두 가지 질병을 예방하거나 치료가능하다. 주사 전자 현미경(SEM), 푸리에 변환 적외선 분광법(FTIR), 시차 주사열량계(DSC), X선 회절(XRD)은 화학적 및 물리적 특성평가를 위해 실시하였다. 또한 생체외 용출 테스트는 pH=1.2에서 정제의 용출률을 평가하기 위해 실시하였다. 결론적으로, 이중층 정제에서 두 가지 약물의 빠른 방출을 확인하였고, 따라서 이는 약학적 치료제로서 적용될 수 있다.
Keywords: tadalafil, amlodipine besylate, double-layer tablet, erectile dysfunction, hypertension
This research was supported by a grant of the Korea Health Technology R&D Project (HI15C2996) through the Korea Health Industry Development Institute (KHIDI), the International Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017K1A3A7A03089427).
In recent years, many men around the world are suffering from diseases that cannot erect for satisfactory sexual function.1,2 The development of various industries and technologies has extended the life span of human and the number of patients suffering erectile dysfunction (ED) will increase accordingly.3,4 ED cannot be sustained temporarily during sexual reactions at first, and the frequency of such an event gradually increases, resulting in sudden erectile dysfunction.5 ED is classified as an organic ED and psychogenic ED.6,7 More than 50% of patients with ED experience organic ED, and aging, cardiovascular disease, including diabetes and chronic hypertension, are the most common cause.8,9 In fact, about 41% of patients with ED have hypertenstion (HT) and about 55% of patients with HT have ED.10,11
HT is chronic disease in which blood pressure is higher than normal, and may be caused by genetic factors, stress, dietary, or environmental factors.12,13 HT is a disease that needs to be regulated, not a completely cured disease. However, taking a HT medication is likely to cause ED, so if the patient takes the medication at the same time, both diseases can be prevented and treated together. Also, providing a complex tablet that can be taken as a single formulation for a patient in need of administration of two active ingredients on a daily, drug compliance may increase.14,15
Tadalafil (TD) is a selective and reversible phosphodiesterase type 5 inhibitor, used as an oral therapeutic for ED, and known in Korea as a Cialis. The chemical structure of TD is described in Figure 1. The recommended dose is 10 mg, with a half-life of 17.5 h, and a Cmax (maximum serum concentration) of 2-4 h after dosing.16,17 It is much longer than the half-life of 3.7 h of sildenafil, which was previously used as an ED treatment.18 TD is a BCS (biopharmaceutics classification system) class-II with low solubility (2 μg/mL) and high biopermeability. 19 In order to increase solubility, we made wet granulation using polyethylene glycol (PEG) as a water soluble solubilizer.
Amlodipine besylate (AM) is a persistent functional calcium channel blocker, used in the treatment of HT, and known in Korea as Norvasc. The structure of AM is described in Figure 1.The dose of AM is 5 mg, and has a long half-life of 40 h. Cmax is reached after 6-8 h after dosing. AM is a BCS class-I and does not require any special binder because of its high solubility.20,21
The aim of this study is to fabricate a double-layer complex tablet for simultaneous treatment of HT and ED. We used PEG as a solubilizer to improve the solubility of TD, and to control the initial release rate of TD and AM, sodium starch glycolate (SSG) was prepared in various contents. FTIR was performed to identify the chemical molecular structure, and DSC and XRD were used to analyze the crystallinity and thermal properties of the drug. SEM was used to confirm the formation of TD granules and physical mixing of AM. In vitro dissolution analysis was also used to determine the drug release rate of the tablets produced.
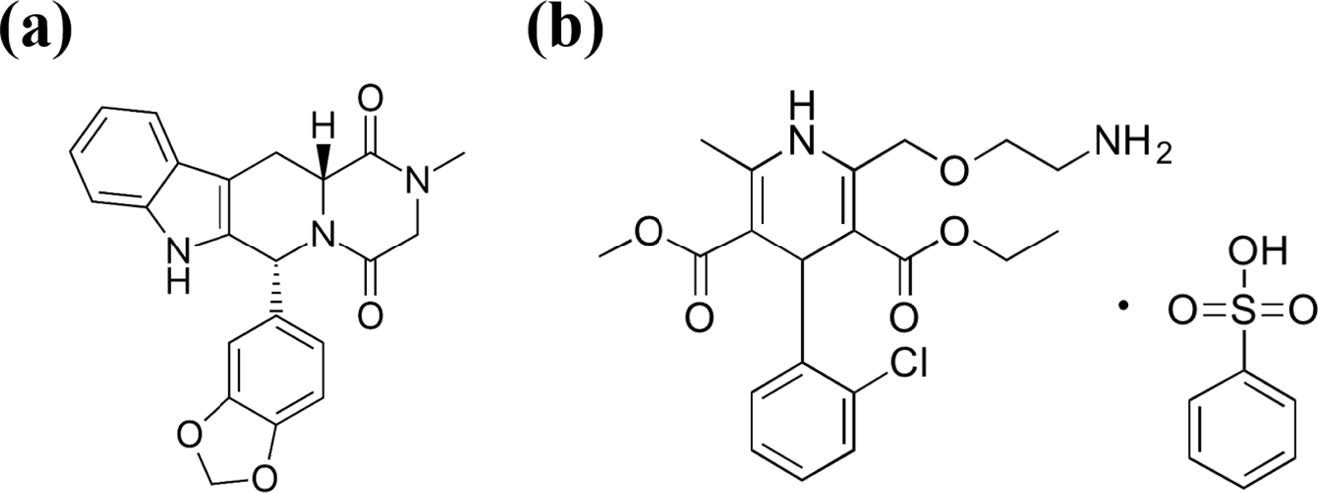
|
Figure 1 Chemical structure of tadalafil and amlodipine. |
Materials. TD and AM was obtained from. Povidone (BASF Chemical Co., Germany), PEG 6000 (Sigma-aldrich, USA), Mannitol (Junsei chemical Co., Japan), microcrystalline cellulose (MCC), sodium starch glycolate (SSG, JRS pharma), Magnesium stearate (Mg.st., Deahwa pharm, Korea) are purchased from each company. All experimental solvent and reagent is high performance liquid chromatography (HPLC) grade.
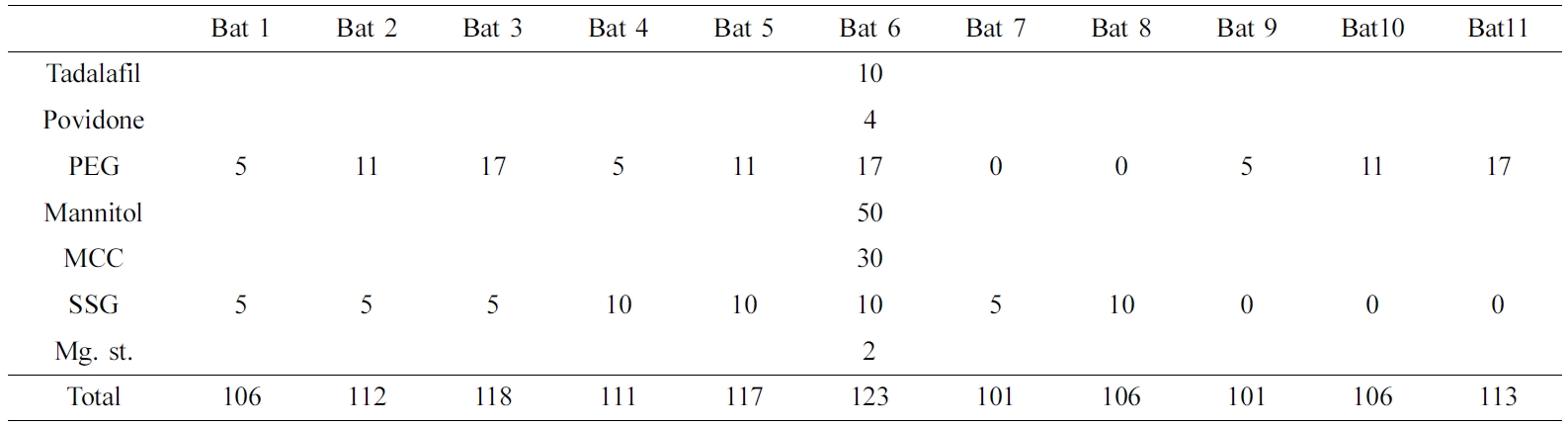
Preparation of Tadalafil Using Wet Granulation. TD was prepared by the wet granulation method by varying the content of polyethylene glycol (PEG), a solubilizer, and SSG, a disintegrant. Prepared wet granulation was dried in oven at 60 ℃ for 30 min to remove the remaining solvent. Then, the dried granules were sieved through a 30-mesh sieve and mixed with Mg. st., a lubricant (Table 1).
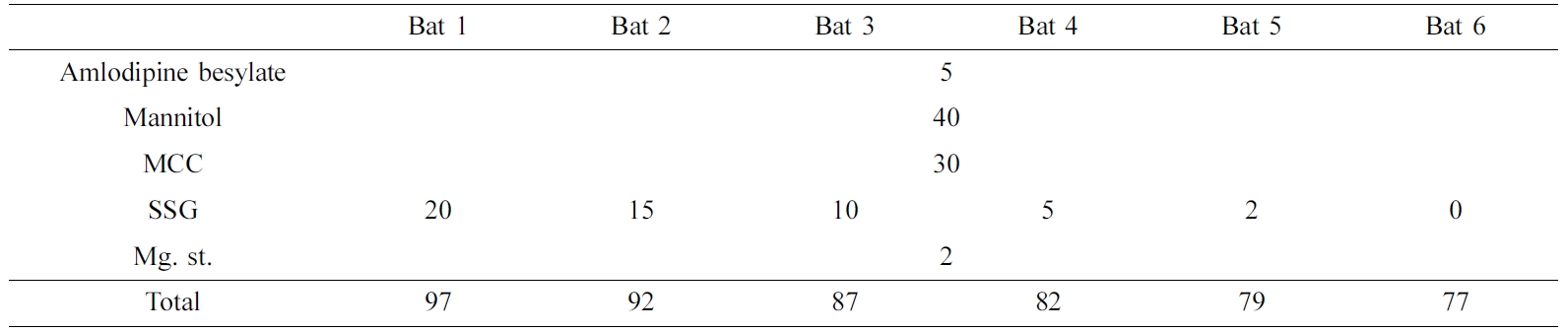
Preparation of Amlodipine Using Physical Mix Method. AM is BCS class-I and has a good solubility. To control the rate of disintegration, SSG was added by the content. It was used after physical mixing with all additives (Table 2).
Preparation of Tablet. First, 11 tablets of TD and 6 tablets of AM were prepared, as shown in Table 1 and 2, respectively. When preparing the double layer tablet later, 5 mg of SSG was used as the disintegration layer. The tablet was made by applying a pressure of 40 kg/cm2 for 15 sec using a 5 mm compression mold Press (MH-50Y CAP 50 ton, Japan). The tablet was stored in a desiccator until use.
Fourier-transform Infrared Spectroscopy (FTIR) Analysis. Infrared spectrum of powders were measured by FTIR spectrometer (Perkin Elmer, USA) in wavenumber range of 650-4000 cm-1 to analyze changes in the physicochemical characterization.
Morphological Characterization. The morphological characterization of TD wet granules AM physical mix powders and additives were observed through scanning electron microscope (SEM, SN-3000 Hitachi, Japan) at 20.0 kV. The samples were coated using platinum-palladium under argon gas.
X-ray Diffraction (XRD) Analysis. XRD (MAX 2500 X-ray diffractometer, Japan) was analyzed to confirm the crys tallinity of samples. The step size is 0.02°, and the measuring angle is from 10° to 50° at speed of 4°/min on 30 mA, 40 kV conditions.
Differential Scanning Calorimeter (DSC) Analysis. Thermal analysis was performed by DSC (DSC 4000, Perkin Elmer, USA). The samples of 5-10 mg were sealed in aluminum pens and heated at a rate of 30 ℃/min over a temperature range of 0-300 ℃.
Dissolution Assay. Dissolution assay was performed using USP dissolution apparatus II (paddle method) in dissolution bath with 900 mL of the second dissolution medium (the simulated gastric juice pH 1.2) specified by USP dissolution medium apparatus at 37±0.5 ℃ and the paddle speed of 100 rpm using dissolution tester (DST-610, Fine Sci, Korea). Dissolution assay of API, TD tablet, AM tablet, double-layer tablet and a commercially available product such as Cialis and Norvasc, were conducted by the second dissolution medium. The samples were taken each 1 mL at 5, 10, 15, 30, 45, 60, 90 and 120 min from the bath of dissolution tester. After that, the second dissolution medium of 1 mL was refilled in bath. The taken samples were filtered by 0.45 μm PTFE membrane filter and analyzed using RP-HPLC.
Reverse Phase-high Performance Liquid Chromatography (RP-HPLC) Analysis. RP-HPLC composed with NS-4000 HPLC and NS-6000 Autosampler (Futecs, Korea) was conducted to evaluate the dissolution behavior of tablets. The reverse-phase column of 20 μm C18 (250X4.6 mm, Bischoff chromatography) was used, and the flow rate of mobile phase was 1.0 mL/min (n=3). The TD’s mobile phase consist of methanol and distilled water (50:50) and AM’s mobile phase consist of methanol, acetonitrile and buffer (35:15:50). The wavelength of UV detection of TD is 225 nm, and that of AM is 237 nm. Measurements were made with injection volume 100 μL, and running time for 30 min.
FTIR Analysis. The physicochemical characterization of the samples were evaluated using FTIR spectroscopy (Figure 2). The spectra of TD displays characteristic peaks at 751 cm-1 due to the benzene and 1644 cm-1 due to C=C aromatic. In addition, there were various peaks at 1037 cm-1 (C-O-C stretching), 1432 cm-1 (C-N stretching), 1688 cm-1 (C=O), 2901 cm-1 (C-H stretching) and 3322 cm-1 (N-H stretching).22 AM spectrum shows peaks at 3300 cm-1 (primary amine), 3233 cm-1 (secondary amine), 2987 cm-1 (C-H stretching), 1688 cm-1 (C=O stretching). A characteristic peak at 1101 cm-1 was caused by S=O stretching in besylate.23 TD Bat 1-11 had both peaks due to the intrinsic peaks of TD, C=C aromatic and C=O. AM Bat 1-6 was also confirmed to have C=O stretching peak, which is the characteristic peak of AM. However, TD and AM batches showed a pattern similar to that of mannitol as an additive. The peak of mannitol appeared at 3400 cm-1 (OH stretching) and 1080 and 1020 cm-1 (C-O).24 Based on these results, it was confirmed that the APIs and additives of each sample were mixed.
SEM Analysis. SEM images were used to evaluate the crystalline of API, additives, batches and wet granulation (Figure 3). The shape of the TD API is very irregular and has a long rectangular shape (Figure 3(a)). The SEM images of AM API showed that the form of prismatic crystals of different sizes including very fine particles and large one (Figure 3(b)). PEG shows a small size of particles and a crystal form of the smooth surface where the small particles were cohered (Figure 3(c)). The morphology of the SSG is a pebble shape of various sizes (Figure 3(d)). The images of the TD batches were observed to confirm if they were wet granules (Figure 3(e-o)). All the granules were well formed and the surface was rugged and larger than those produced from each component. In addition, the morphology of pure TD was disappeared. AM batches were manufactured by a simple physical mixing method, so that the shapes of the components were not deformed and all of the various component types appeared (Figure 3(p-u)). The results of SEM indicate that TD and AM are crystalline, granules are well formed, and AM batches imply that all ingredients are uniformly mixed.
XRD and DSC Analysis. Figure 4 shows XRD results to find out the crystallinity of samples. The characteristic peaks of TD at 10-20°, 21. 54° and 24. 08°. AM peaks were observed at 13.48° and 23.44°, but the intensity was weak. The peak of mannitol was very strong at 18.74°, 21.08° and 23.34°, and PEG at 19.06° and 23.2°. Povidone and SSG, which are other additives, are in an amorphous state, so no peak was confirmed. The batches of TD and AM had similar patterns due to the strong peaks of mannitol and PEG crystals. One of the differences between patterns of TD batches and AM batches is that the inherent peak of TD appears around 21.5°.
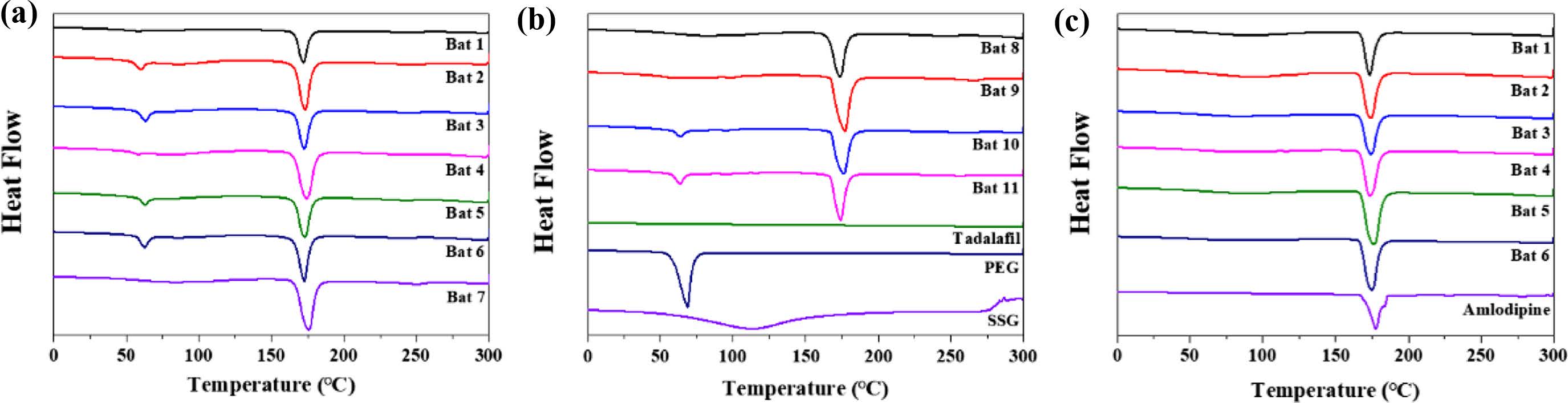
In Figure 5, DSC results of API, additives, samples were shown. SSG displays a broad endothermic peak because of dehydration at high temperature during the DSC measurements. The endothermic peak of PEG was observed at 68.93 ℃. TD showed a single peak at over 300 ℃ (It is not shown in the data) and AM at around 175 ℃. Although not shown in the data, mannitol has an endothermic peak at 168 ℃, resulting in strong peaks in all batches. The peak of AM and the peak of mannitol were overlapped so that no clear peak of AM was found in the result of AM batches. In the case of TD Bat 7 and 8 in which PEG was not added, the peak of PEG was not shown around 68.93 ℃. The results show that the intrinsic peak of the pure drug is maintained because the batches were prepared using a simple physical mixing method and wet granulation method without large temperature changes.
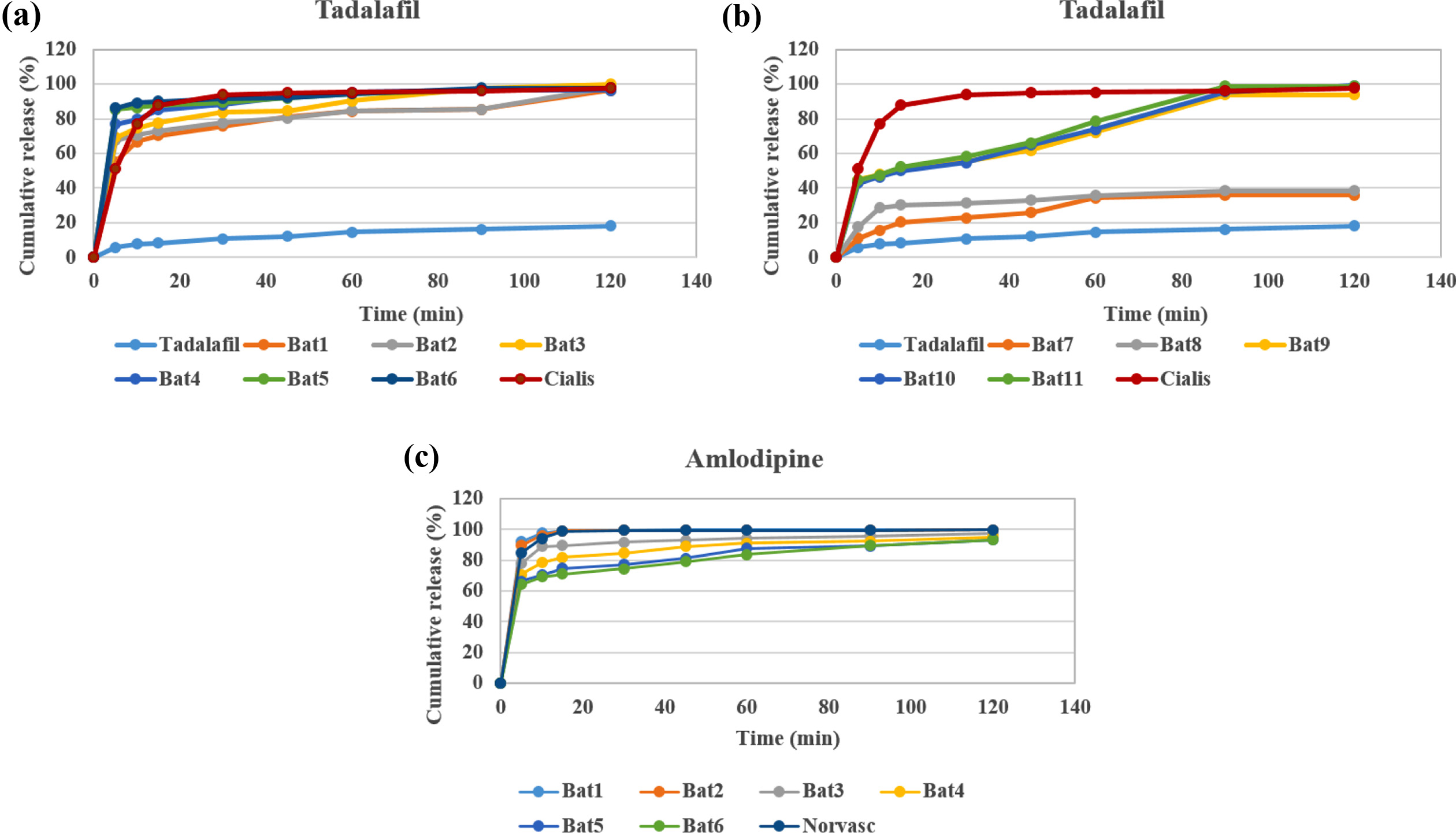
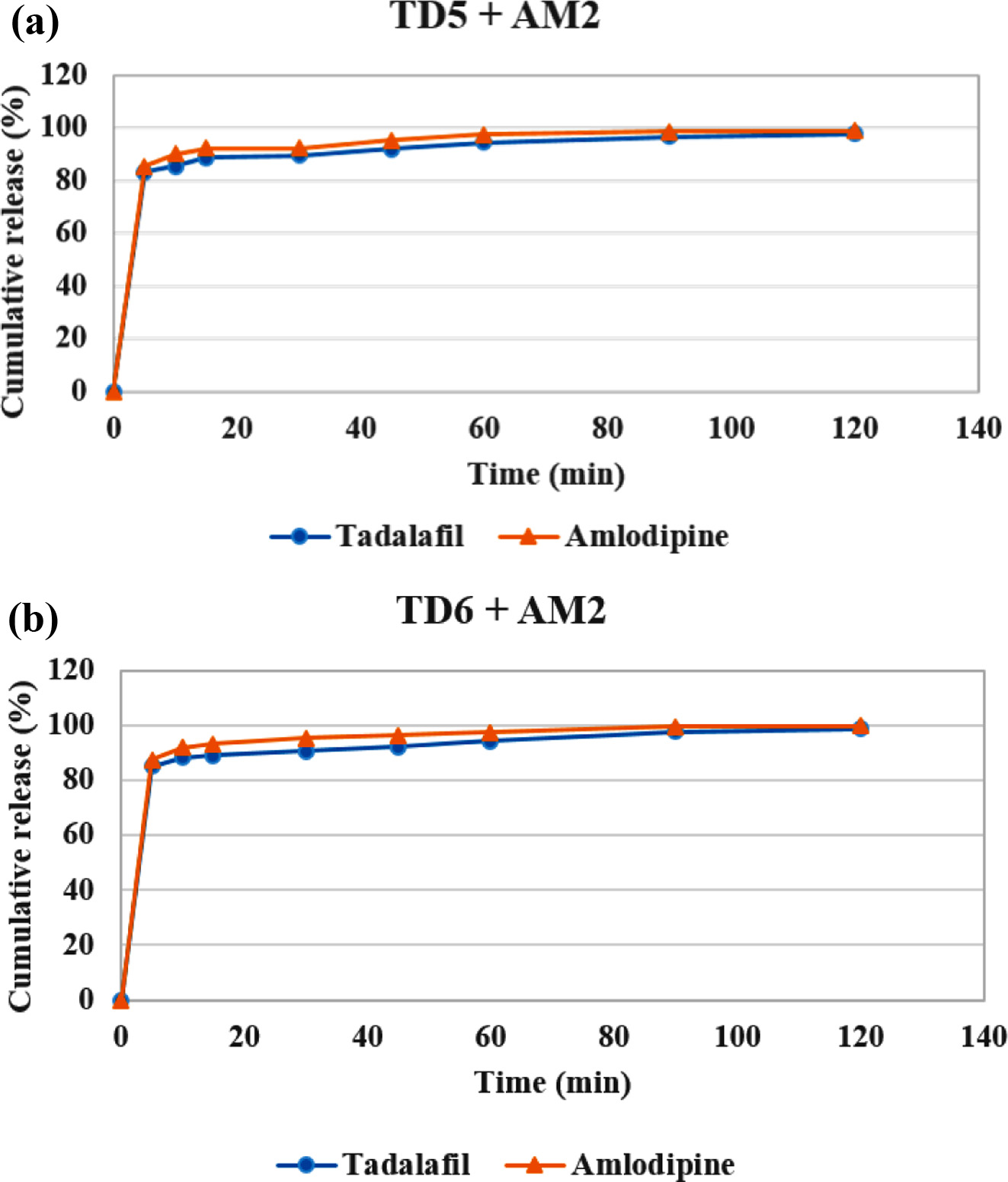
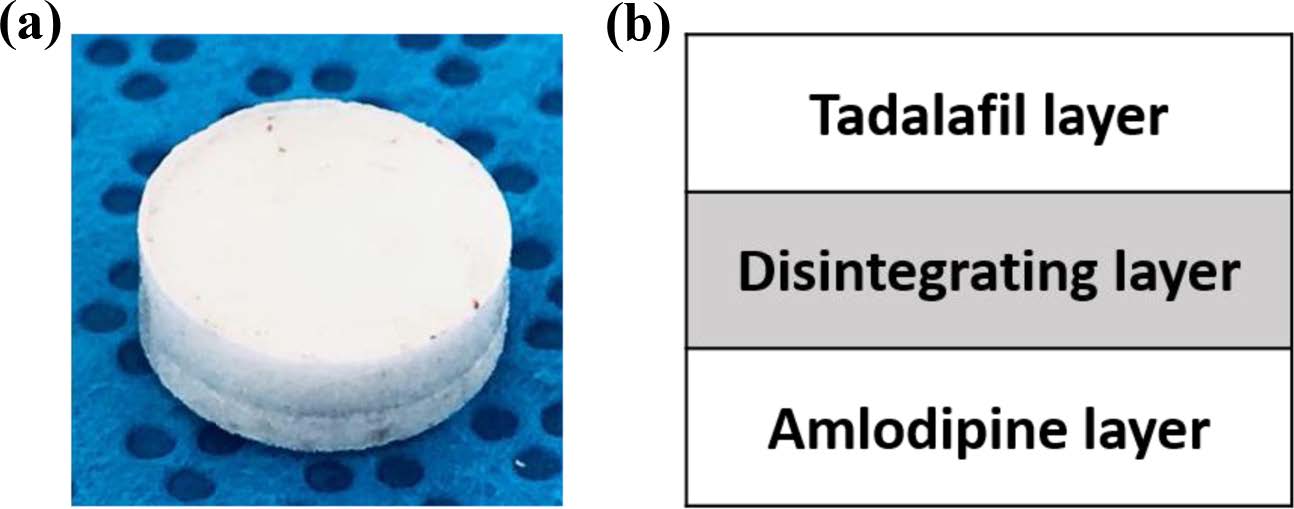
Drug Release Behavior. Dissolution test was conducted in the simulated gastric fluid (pH=1.2) to confirm the release behavior of TD and AM (Figure 6 and 8). TD and AM have a half-life of 17.5 and 40 h, respectively, so the active ingredient may remain in the body for a long time after taking it.16,20 Therefore, Cialis and Norvasc, sold in Korea, are made to release quickly after taking. First, the dissolution test of TD API, TD Bat 1-11 and Cialis were performed to determine the TD dissolution rate of the TD monolayer tablet (Figure 6). Because TD is a poorly soluble drug of BCS class-II, only about 20% of the drug was released in 120 min. TD Bat 7 and 8 without PEG, which is a water-soluble solubilizer, did not increase the solubility of TD and reached a final dissolution rate of 40%. In the case of TD Bat 9-11 without SSG, the drug could be released close to 100% after 90 min because the disintegration of the tablet was not accelerated. TD Bat 4-6, which has high SSG content, eluted more than 80% within 15 min and had higher initial dissolution rate than Cialis. Since AM has a high solubility, it was not tested separately. In the results of AM Batches, the initial dissolution rate decreased as SSG content decreased, and AM Bat 2 showed the most similar dissolution profile to Norvasc. Therefore, a double-layer tablet was prepared by combining TD Bat 5, 6 and AM Bat 2. The gross image and schematic diagram of the double-layer complex tablet is shown in Figure 7. In both tablets, TD and AM released more than 80% after 5 min and the dissolution rate reached almost 100% after 90 min (Figure 8). Therefore, it was confirmed that double-layer tablet compose of TD and AM could treat ED and HT at the same time.
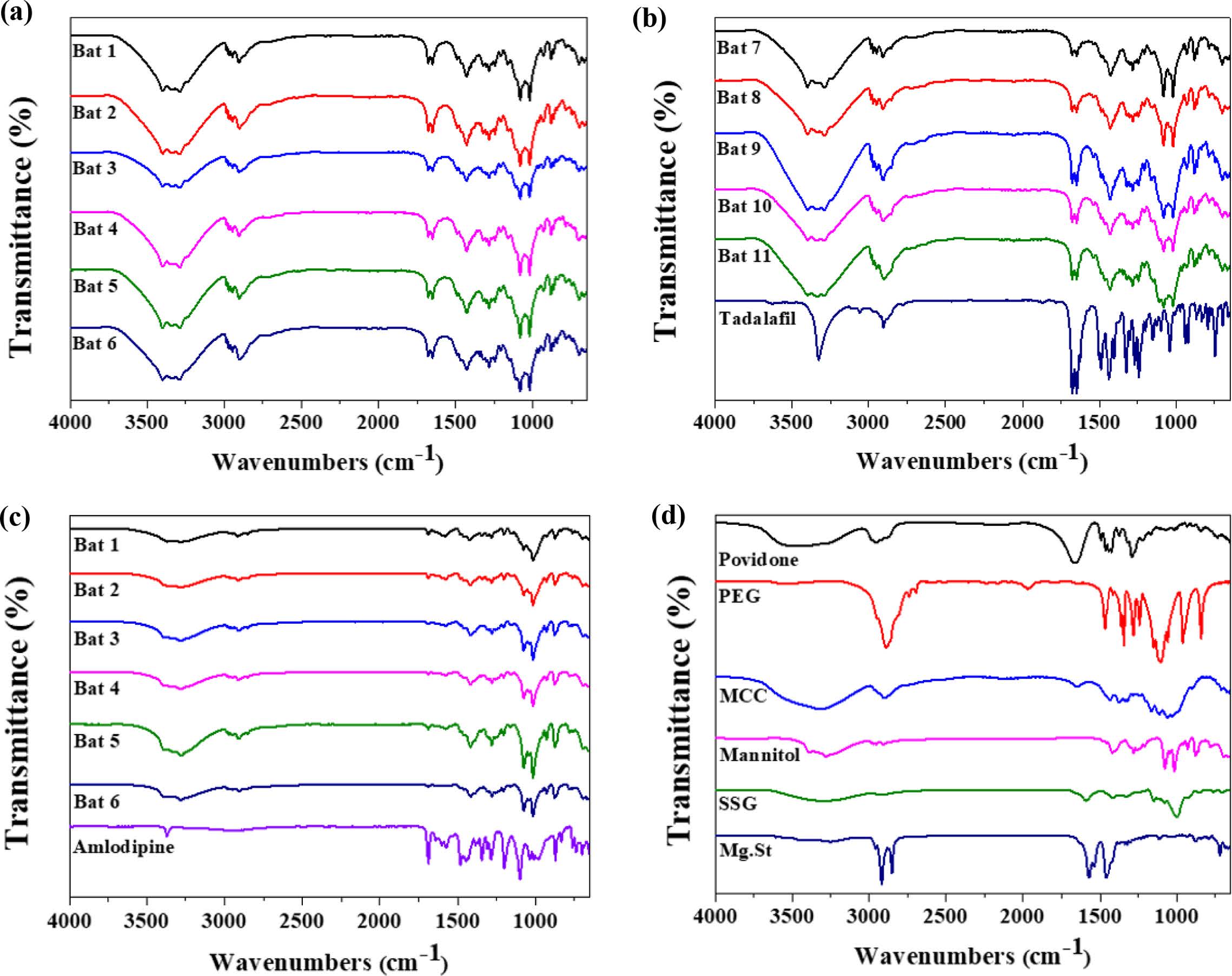
|
Figure 2 FTIR spectra of (a) TD Bat 1-6; (b) TD Bat 7-11 and Tadalafil; (c) AM Bat 1-6 and Amlodipine; (d) additives. |
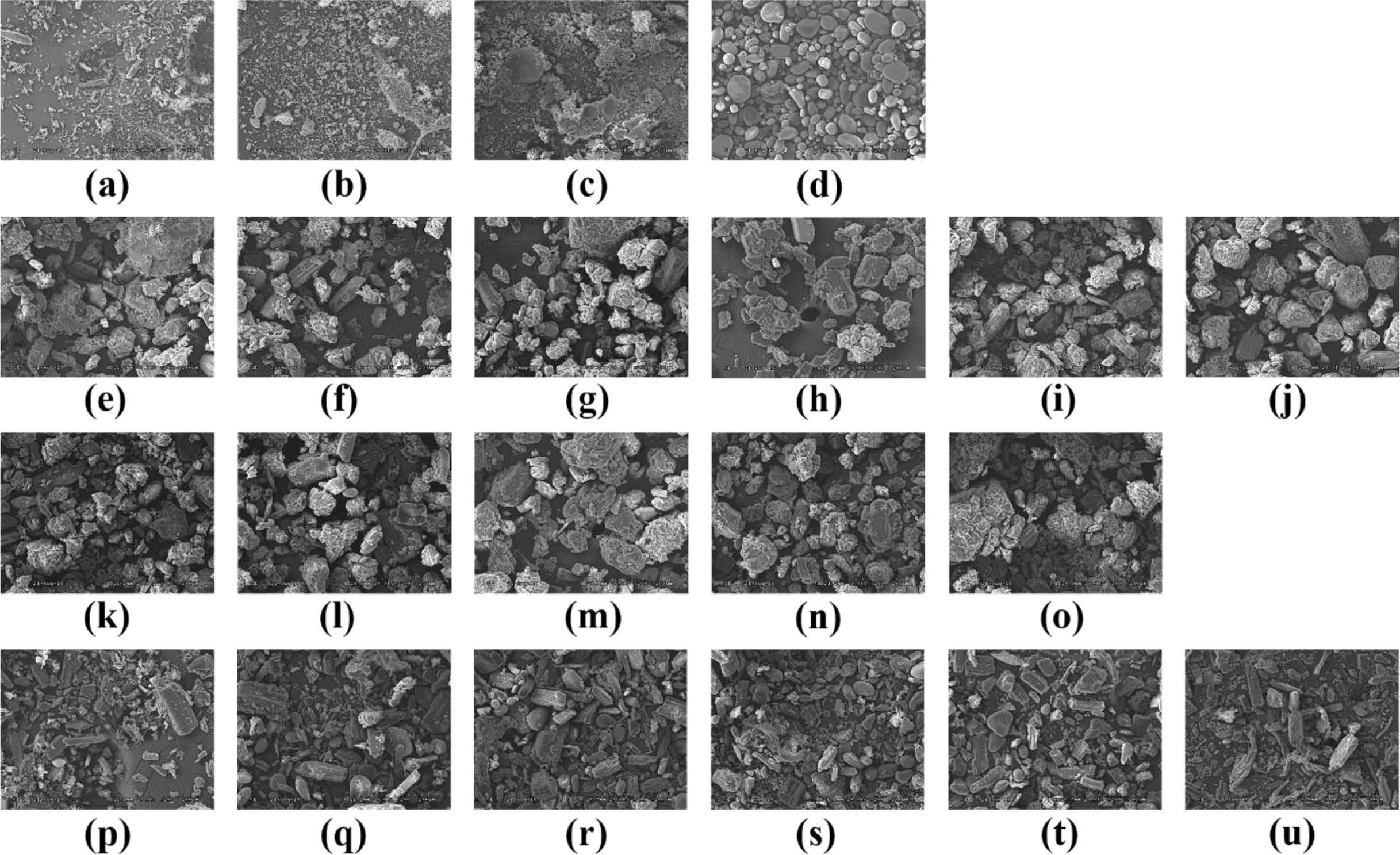
|
Figure 3 SEM images of (a) Tadalafil; (b) Amlodipine; (c) PEG; (d) SSG; (e)-(o) TD Bat 1-11; (p)-(u) AM Bat 1-6. |
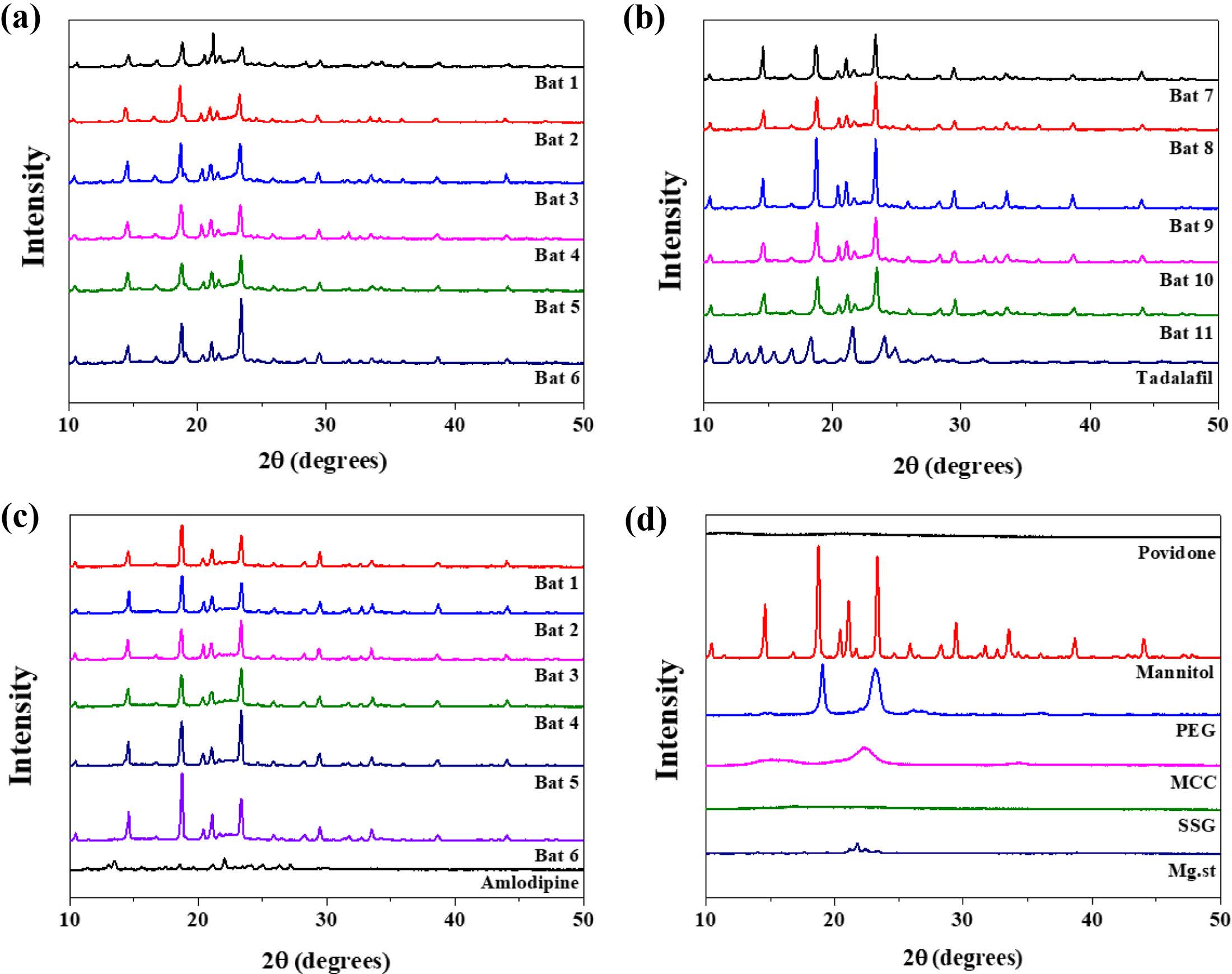
|
Figure 4 XRD spectra of (a) TD Bat 1-6; (b) TD Bat 7-11 and Tadalafil; (c) AM Bat 1-6 and Amlodipine; (d) additives. |
|
Figure 5 DSC spectra of (a) TD Bat 1-7; (b) TD Bat 8-11; Tadalafil, PEG and SSG; (c) AM Bat 1-6 and Amlodipine. |
|
Figure 6 Drug release behavior (a) TD Bat 1-6; (b) TD Bat 7-11; (c) AM Bat 1-6 (pH=1.2). |
|
Figure 8 Drug release behavior (a) double-layer tablet of TD Bat 5 and AM Bat 2; (b) double-layer tablet of TD Bat 6 and AM Bat 2 (pH=1.2). |
|
Figure 7 (a) Gross image; (b) schematic diagram of double-layer complex tablet. |
In this study, we fabricated a double-layer complex tablet consist of TD and AM to treat ED and HT at the same time. To improve the poor solubility of TD, wet granulation was performed by adding PEG, and SSG was added to control the disintegration rate of TD and AM. In the XRD and DSC data, the changes of peak of the API were not clearly identified due to the strong peaks of the additive, mannitol. However, in the release behavior of TD, the release rate of tablet containing 100 mg of SSG and 10-15% of PEG was similar to that of Cialis. In the case of AM, the release rate of the tablet containing the most SSG was similar to that of Norvasc. In addition, it was confirmed that more than 80% of TD and AM were released within 15 min in both double-layer complex tablets. Therefore, it is suggested that this new complex tablet with immediate release has the potential to simultaneously treat ED and HT.
- 1. A. Nicolosi, E. D. Jr. Moreira, M. Villa, and D. B. Glasser, J. Affect. Disord., 82, 235 (2004).
-

- 2. A. D. Smith, Urol. Clin. North. Am., 15, 41 (1988).
- 3. B. A. Inman, J. L. Sauver, D. J. Jacobson, M. E. McGree, A. Nehra, M. M. Lieber, V. L. Roger, and S. J. Jacobsen, Mayo. Clin. Proc., 84, 108 (2009).
- 4. K. Esposito, F. Giugliano, C. D. Palo, G. Giugliano, R. Marfella, F. Andrea, M. Armiento, and D. Giugliano, Jama-J Am. Med. Assoc., 291, 2978 (2004).
-

- 5. C. B. Johannes, A. B. Araujo, H. A. Feldman, C. A. Derby, K. P. Kleinman, and J. B. Mckinlay, J. Urol., 163, 460 (2000).
-

- 6. R. C. Rosen, Urol. Clin. North. Am., 28, 269 (2001).
-

- 7. J. P. Hatch, A. M. Delapena, and J. G. Fisher, J. Urol., 138, 781 (1987).
-

- 8. J. P. W. Heaton and M. A. Adams, Endocrine, 23, 119 (2004).
-

- 9. I. M. Thompson, C. M. Tangen, P. J. Goodman, J. L. Probstfield, C. M. Moinpour, and C. A. Coltman, Jama-J. Am. Med. Assoc., 294, 2996 (2005).
-

- 10. M. Burchardt, T. Burchardt, L. Baer, A. J. Kiss, R. V. Pawar, A. Shabsigh, A. de la Taille, O. R. Hayek, and R. Shabsigh, J. Urol., 164, 1188 (2000).
-

- 11. F. A. Giuliano, A. Leriche, E. O. Jaudinot, and A. S. de Gendre, Urology, 64, 1196 (2004).
-

- 12. R. A. Augustyniak, M. Tuncel, W. Zhang, R. D. Toto, and R. G. Victor, J. Hypertens., 20, 3 (2002).
- 13. M. Esler, N. Eikelis, M. Schlaich, G. Lambert, M. Alvarenga, T. Dawood, D. Kaye, D. Barton, C. Pier, L. Guo, C. Brenchley, G. Jennings, and E. Lambert, Clin. Exp. Pharmacol. Pharmacol., 35, 498 (2008).
-

- 14. A. Okunlola, Front. Pharmacol., 5, Article 294 (2015).
- 15. S. R. Vaithiyalingam and V. A. Sayeed, Int. J. Pharmaceut., 398, 9 (2010).
-

- 16. I. Eardley and J. Cartledge, Int. J. Clin. Pract., 56, 300 (2002).
- 17. I. S. de Tejada, G. Anglin, J. R. Knight, and J. T. Emmick, Diabetes Care, 25, 2159 (2002).
-

- 18. U. Gresser and C. H. Gleiter, Eur. J. Med. Res., 7, 435 (2002).
- 19. P. R. Vuddanda, M. Montenegro-Nicolini, J. O. Morales, and S. Velaga, Eur. J. Pharm. Sci., 109, 372 (2017).
-

- 20. P. A. Meredith and H. L. Elliott, Clin. Pharmacokinet., 22, 22 (1992).
-

- 21. J. L. Morgan, B. K. Kogutt, C. Meek, E. K. Stehel, D. D. Mclntire, J. S. Sheffield, and S. W. Roberts, Pregnancy Hypertens, 11, 77 (2018).
-

- 22. R. T. Rad, S. A. Mortazavi, A. Vatanara, and S. Dadashzadeh, Iran. J. Pharm. Res., 16, 1335 (2017).
-

- 23. N. Mubtasim, E. R. Kabir, A. K. Podder, and S. Bhadra, Saudi. Pharm. J., 24, 689 (2016).
-

- 24. K. P. R. Chowdary and A. S. Rao, Asian J. Chem., 20, 4581 (2008).
- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(2): 274-281
Published online Mar 25, 2019
- 10.7317/pk.2019.43.2.274
- Received on Nov 24, 2018
- Revised on Dec 28, 2018
- Accepted on Jan 4, 2019
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Gilson Khang
-
Department of BIN Convergence Technology, Department of Polymer Nano Science &Technology and Polymer Materials Fusion Research Center, Chonbuk National University, 567 Baekje-daero, Deokjin-gu, Jeonju-si, Jeollabuk-do, 54896, Korea
- E-mail: gskhang@chonbuk.ac.kr
- ORCID:
0000-0002-6452-5653









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.