- Study on Properties of Epoxidized Natural Rubber/Solution Styrene Butadiene Rubber Blend with Silica and Carbon Black in Different Filling Ratio
Xiang Xu Li*, Su Young Jeong*, Eun Ji Choi*, and Ur Ryong Cho*,**,†

*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea- 실리카 및 카본블랙이 보강된 ENR/SSBR 블렌드의 물성 연구
*한국기술교육대학교 에너지, 신소재, 화학공학부, **친환경고성능화학소재연구소
Epoxidized natural rubber (ENR) and solution styrene butadiene rubber were blended with a ratio of 150:50, which filled with silica and carbon black as reinforcement fillers. The curing properties of the rubber composites were tested by rubber processing analyzer and the morphological structure was characterized by SEM. The thermal decomposition behavior, tensile strength, hardness value, friction coefficient and resilience property were investigated to verify the property improvement of the SSBR/ENR composites. From the results of all the tests, it can be found that the heat resistance, hardness value, tensile strength and filler dispersion state of composites increased with increasing content of silica. The possible reason may be due to the fact that the silica could provide the better combination with ENR part due to the formation of hydrogen bond formation from O-Si-O. And with the increasing content of carbon black, the friction coefficient and resilience property were improved due to the lubrication and coupling effect of carbon black and blends.
에폭시화 천연고무(ENR)와 솔루션-스디렌-부다디엔 고무(SSBR)를 150:50로 혼합한 고무 블렌드를 실리카 및 카본블랙을 충전제로 보강하였다. 제조된 고무 복합체의 물성은 고무 가공 분석기로 측정되었고, 형태학적 구조는 SEM으로 확인되었다. 열무게 분석, 인장강도, 경도, 마찰계수 및 발반탄성은 ENR/SSBR 블렌드의 물성을 확인하기 위하여 조사되었다. 모든 실험 결과에서, 복합체의 내열성, 경도, 인장강도 및 충전제의 분산성은 실리카 함량이 증가함에 따라 향상되었음을 알 수 있었다. 그 원인은 실리카와 ENR부분 사이에 수소 결합이라는 더 나은 결합의 형성이었다. 또한 블렌드의 마찰계수과 발반탄성은 카본블랙 함량이 증가함에 따라 향상되었다. 그 원인은 카본블랙과 블렌드의 결합효과(coupling effect) 및 윤활성이 개선됨에 기인함을 알았다.
Keywords: epoxidized natural rubber, silica, carbon black, mechanical properties, viscoelastic properties
Epoxidized natural rubber (ENR) has been introduced as a modified form of natural rubber (NR). As the natural rubber is epoxidized, its chemical and physical properties change according to the extent to which the mole% of modification is introduced. For instance, the glass transition temperature, Tg, is raised, room temperature resilience is reduced, the rubber becomes increasingly more oil resistance and impervious to gases, polymer viscosity is increased and the polymer becomes more polar as the degree of epoxidation is increased.1
ENR obtained by epoxidation of 1,4-polyisoprene shows a higher glass transition temperature than the pristine 1,4-polyisoprene, with an increase in the polarity of the system while retaining the inherent high strength of NR. Some of these properties are more akin to those of synthetic rubbers than NR.2
The achievement of highly dispersed polarity filler involves the compatibility between the raw rubber materials and fillers. The formulas of polarity fillers/polar polymeric systems usually contain a polymeric compatibilizer.3-6 ENR obtained by epoxidation of 1,4-polyisoprene, depicts higher glass transition temperature and increased polarity. Accordingly, as a fine dispersion of polarity fillers is expected, ENR was chosen as a raw material with compatibilizer function in this study.7
And SSBR which has non-polarity8 could make good combination effect of both ENR and carbon black, thus SSBR was used as compatibilizer or blending agent in this study. In order to confirm the reinforcement of fillers, the blend ratio of ENR: SSBR was fixed into 150 phr: 50 phr.9
In this research, the O-Si-O groups in silica and the coupling effect of carbon black provide the great combination effect with the ENR/SSBR blends, due to the hydrogen bonding effect and other interaction force, the new physical crosslinking had been formed, which could provide the mechanical properties reinforcement for ENR. After filling with fillers in difference ratio, the vulcanizates were vulcanized during the curing process. Finally, the surface state, TGA, tensile strength, hardness, friction coefficient, resilience property had been characterized.
Materials. Epoxidized natural rubber 50 (EKOPRENA-50, epoxidation 50%), solution styrene butadiene rubber SOL- 5130H (styrene content 16 wt%, vinyl content 30 wt%, Kuhom Chem.), sulfur (S, powder, Daejung, 99%), stearic acid (SA, Samchun chemical, 95% EP), N-cyclohexyl-2-benzothiazole sulfonamide (CBS, Tokyo Chemical Industry Company, Japan, 95%), 2,2'-dibenzothiazolyl disulfide (DD, Tokyo Chemical Industry Company, Japan, 95%), zinc oxide (ZnO, Samchun chemical, 99%), silica (Evonik, Ultrasil 7000GR), and carbon black (C550, Shandong, China).
Compounding. The formulation of this research was shown in Table 1. The compounding process was conducted on a two roll mill. Note the sulfur and vulcanization promoters were added at the last step for avoiding the pre-vulcanization. After that, samples were vulcanized under 10 MPa for t90 at 160 ℃ in a heating press machine. The thickness of the samples was set as 1 mm.
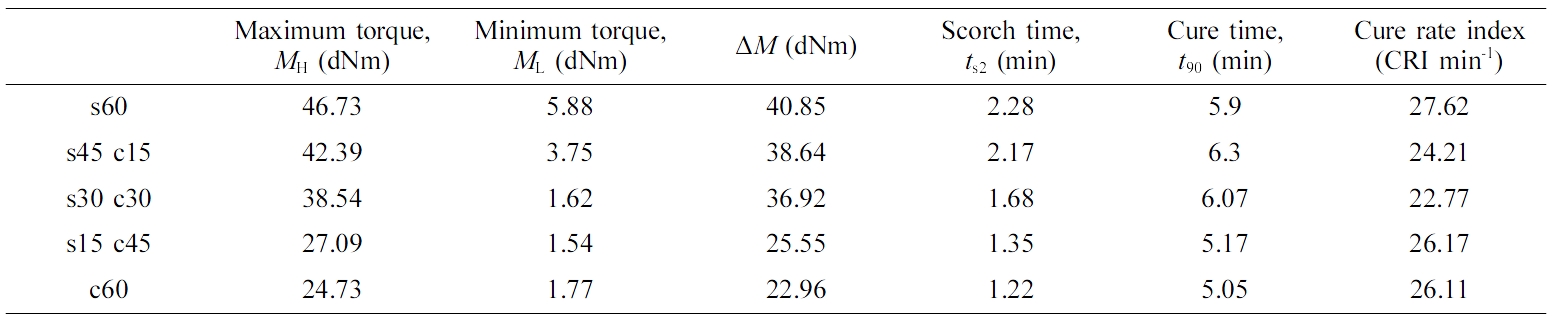
Characterization. The morphology of the samples after the tensile test was carried out on a FE-SEM with EDS (JSM- 7500F, JEOL Ltd. Japan). Thermal properties of composites were conducted on a thermogravimetric analyzer (TGA, Perkin Elmer 4000) by heating from 30 to 800 ℃ at a rate of 20 ℃/ min under the nitrogen flowing of 20 cm3/min. The cure/vulcanization characteristic of as-prepared samples were measured by a rubber process analysis (RPA) (RPA-V1, U-CAN DYNATEX INC.). The minimum torque (ML), maximum torque (MH), scorch time (ts2), and optimum cure time (t90) were determined by the above RPA. The cure rate index (CRI) was used to evaluate the cure rate of the rubber, and it was calculated by the following eq. (1):10

The hardness of samples was obtained by a shore durometer type A according to the ASTM D 22-40. The tensile strength test was measured three times on a Tinius Olsen H5KT-0401 testing machine at a speed of 500 mm/min according to ASTM D412. The samples were made of a dumb-bell shape with the dimensions of 25 mm×6 mm×1 mm after the vulcanization on the heating press machine. The resilience test was performed by Ball Rebound machine which according to the ASTM D3574-17 and ISO 8307. Friction factor test was performed at room temperature by friction testing machine TO-100-IC according to ASTM 1894.
|
Table 1 Formulation for Test Sample Blends |
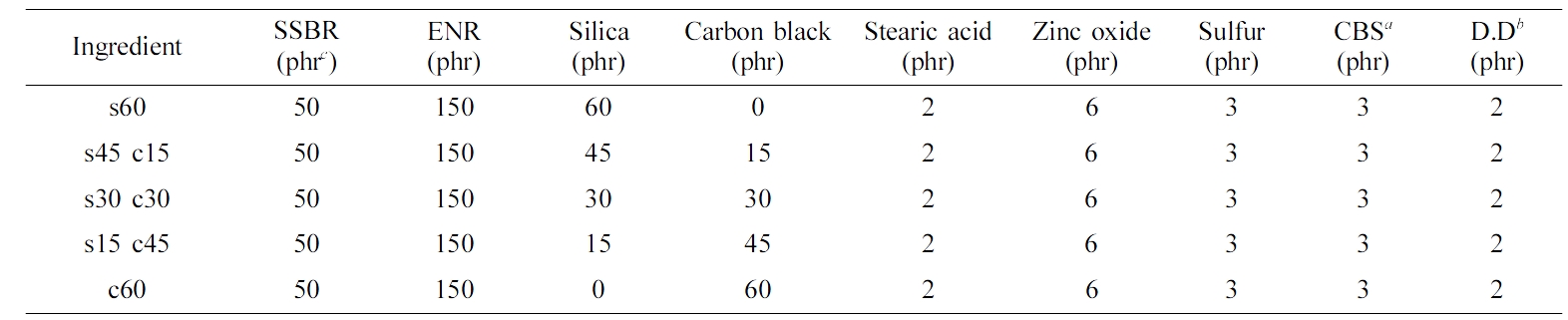
aN-Cyclohexyl-2-benzothiazole-sulfonamide. b2,2-Dibenzothiazolyl disulfide. cphr, part per hundreds of rubbers. |
Curing test results of composites were presented in Table 2. The torque values of other samples had been increased compared to the c60 blend which only filled with carbon black, due to the strength of fillers, which strongly restricted the deformation and consequently increased the mechanical properties of ENR/SSBR matrix. With the ratio of silica increasing, the MH and ΔM also increased, and silica 60 phr filler provide the largest MH, ΔM and CRI value, which meant silica not only could make better combination effect with ENR/SSBR blend, and improve the mechanical properties of blend,11 but also short the vulcanization time of composite. The possible reason is due to the formation of hydrogen bonds between the ENR part and silica. And both these two materials have oxygen atom, which would show the polarity, due to the like dissolves like principle, so silica may present a stable state in blend matrix, and the hydrogen bonds also short the distance of rubber chains, which could reduce the vulcanization time.12 But as carbon black, with the ratio of filling increasing, the ΔM reduced. The reason for this phenomenon is due to the ENR/ SSBR mixture blend system which is so complicated, carbon black cannot provide good combination effect with this whole system, then cause the worse combination of phases. Thus, some mechanical properties decreased, such as tensile strength, hardness, and resilience.
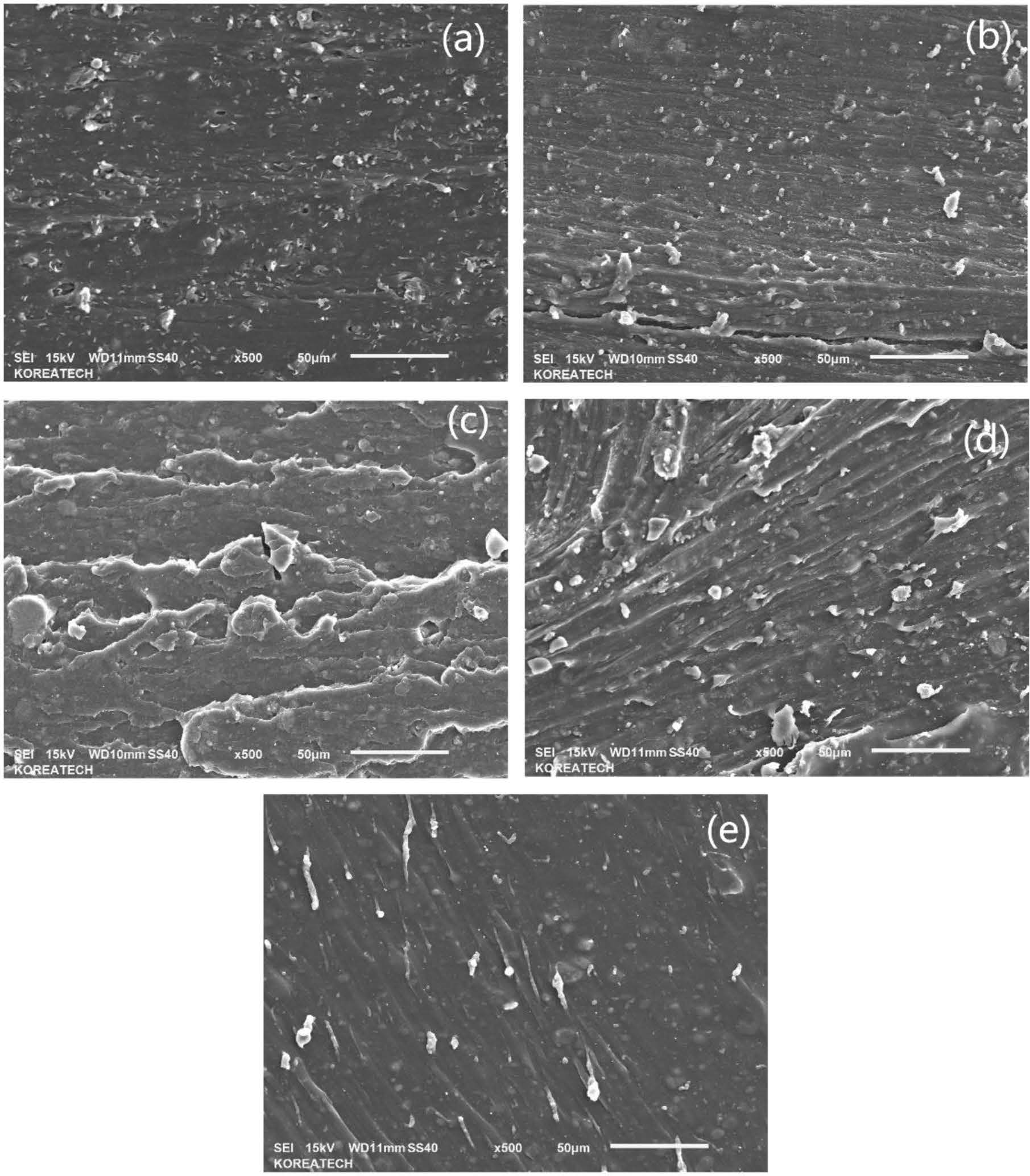
The morphology of samples was observed in Figure 1. From these pictures, it can be found from Figure 1(a) to (e) that with the content ratio of silica, composites showed more and more layer structure with less filler particle, which meant silica could make good combination with blend matrix. Compared to Figure 1(a), the Figure 1(b)-(d) presented more gully-like and more compact structure, which meant this filler provide better combination effect to ENR/SSBR blend. As for Figure 1(e), the layer structure turned into smooth surface, which meant s60 composite had the best compounding effect in this research. The probably reason is due to the hydrogen bonds between silica and ENR part of blend,13 with the silica ratio increasing, the hydrogen bond effect also had increased, which could improve the stability of matrix.
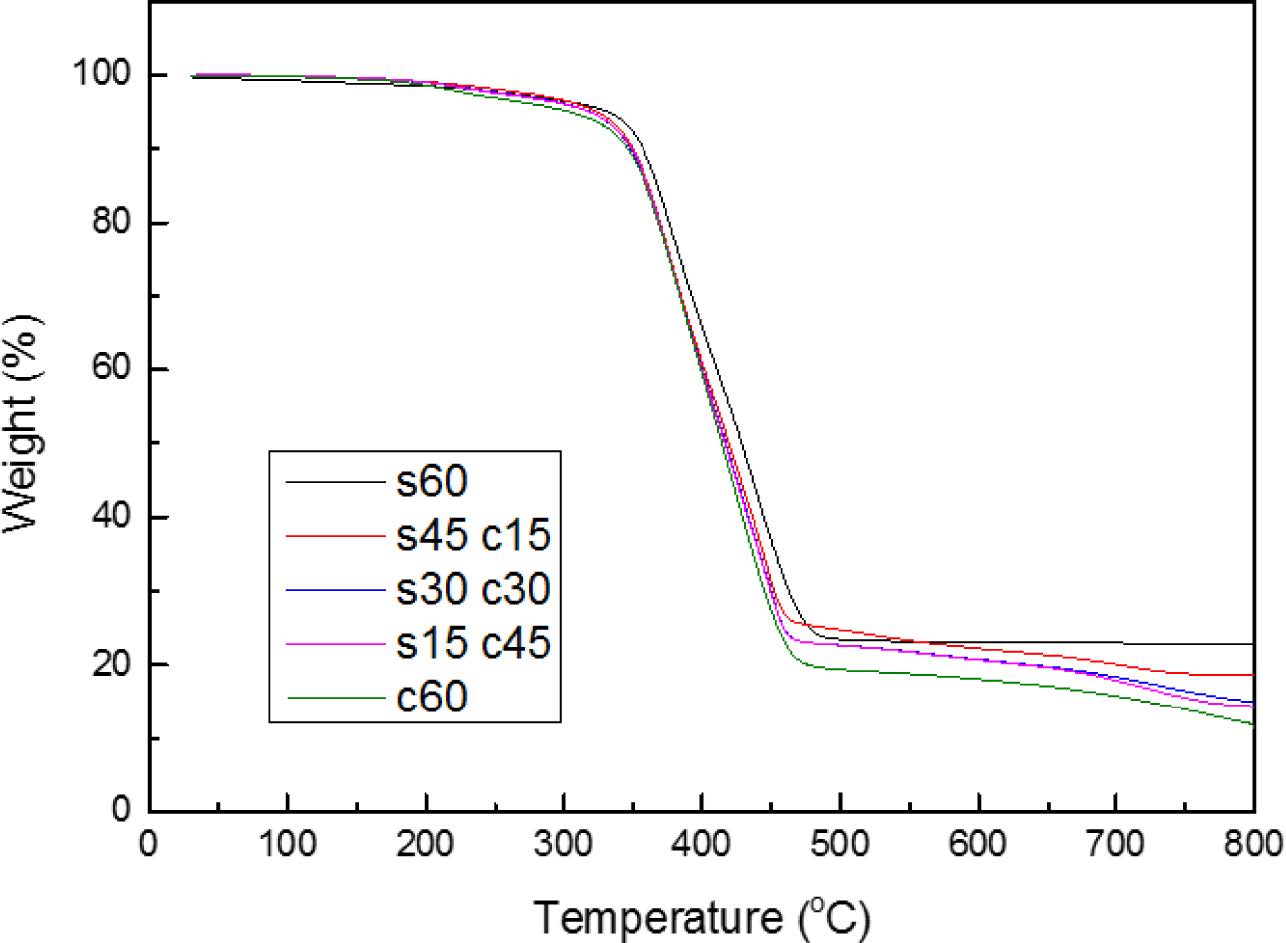
The TGA plot of the filling materials in nitrogen is shown in Figure 2. Before the temperature range of 300 ℃, the TGA plots of all the composites were same, but after 450 ℃, the difference of weight loss had become obvious, with the ratio of silica decreased, the weight loss of composites had been increased, the reason is also about hydrogen bonds, the more silica ratio, the more hydrogen bonds, and the better stability,14 and also in high temperature, such as 800 ℃, silica cannot be decomposed with high temperature, but as carbon black, it could decompose into gas state after 600 ℃, leading to further thermal decomposition.
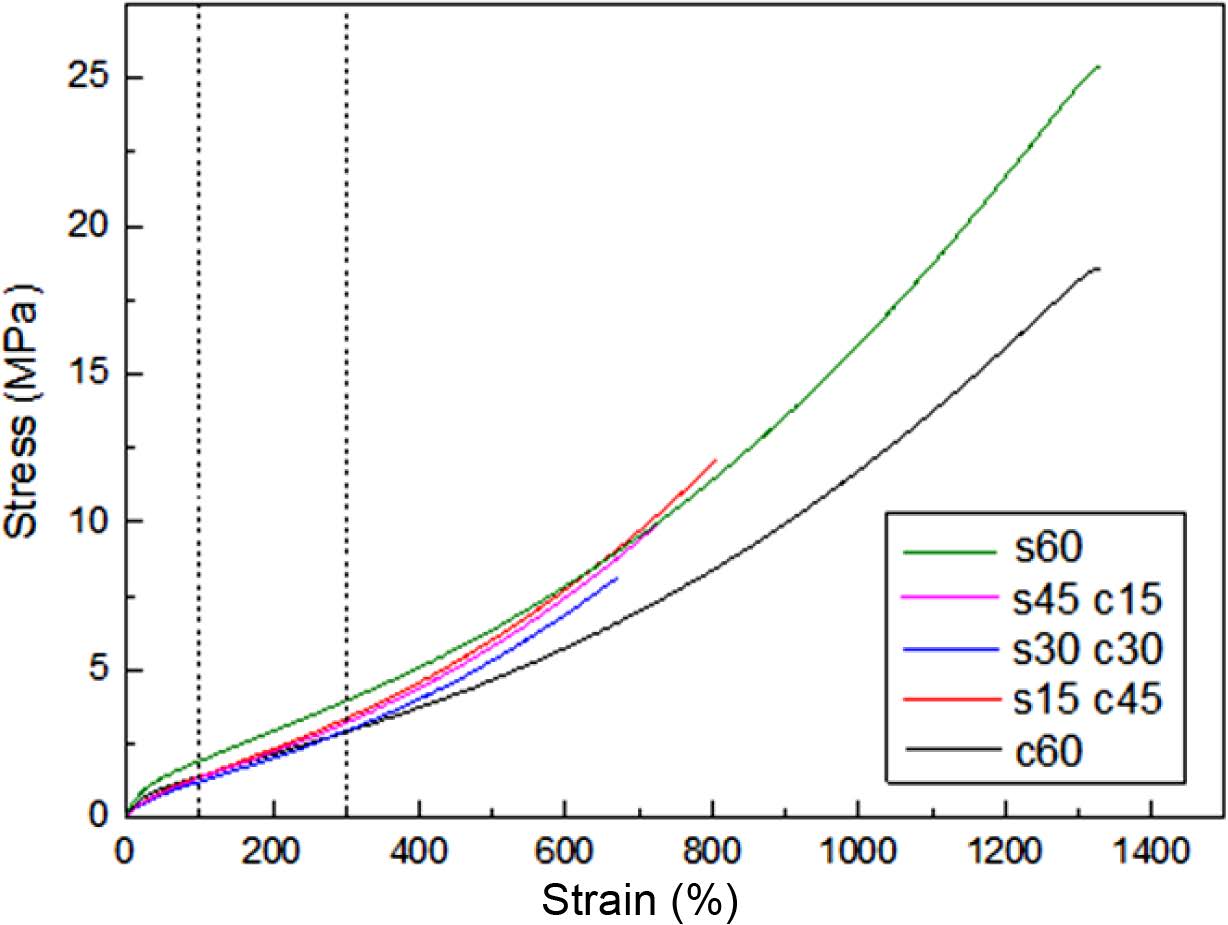
The results of tensile strength were shown in Figure 3. It could be found the stress strength value of s60 composite was the largest, and with the ratio of silica decreasing, the value of stress strength also decreased, but as s30 c30 composite, it showed lower curve than s15 c45, the probably reason is at the same ratio of silica and carbon black, the blend matrix system is very confused, and the filler in this ratio increased the chaos of the system greatly,15 and cannot make the combination and compatibility effect, but when the unilateral content of silica or carbon black exceeds equilibrium, it could provide mechanical reinforcing performance of the filler which content is more, and spontaneous reduce the chaos of system due to the compatibility principle, it also could be proved in Figure 1(b) and (d), which showed larger particle blocks of fillers. As for single filler, it could form a perfect whole composite matrix without cracks and gaps, but as hybrid filler, due to the uneven polarity, particle size, and binding force with rubber, so the rubbers filled with hybrid fillers had some cracks and gaps in the matrix, so there would be more uneven stresses during the tensile process. The tension could cause faster destruction of these composites filled with hybrid fillers than the composites filled with pure fillers.
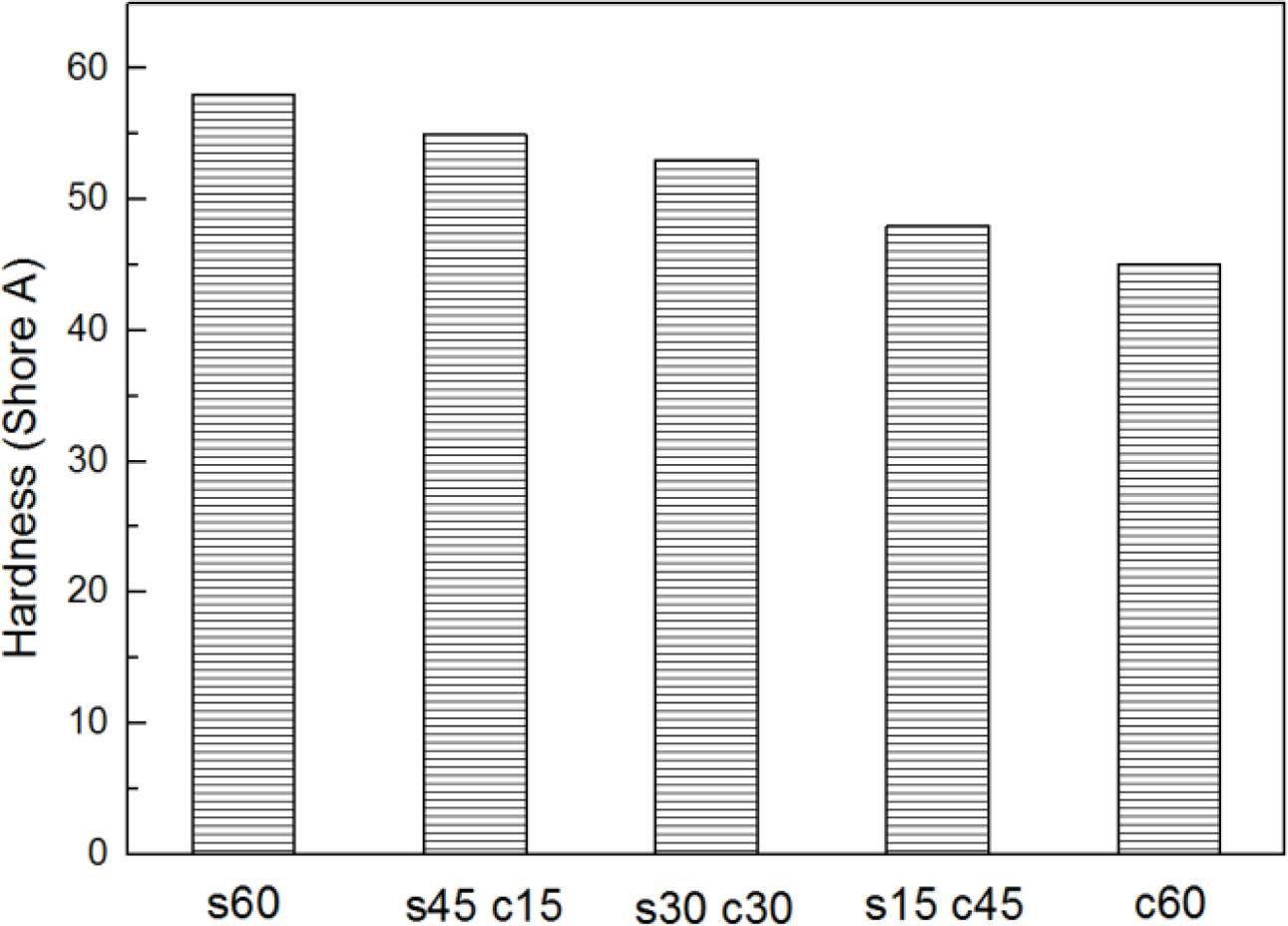
Figure 4 showed the hardness results of all the samples. From this figure, it can be found with the content of silica decreased, the hardness value of composites also decreased. Which meant the more silica content, the more compact matrix had formed during the filling process. the s60 composite showed the largest hardness value. The reason is due to the hardness of silica is higher than carbon black,16 and also could form the more compact structure with more hydrogen bonds from silica and ENR part.
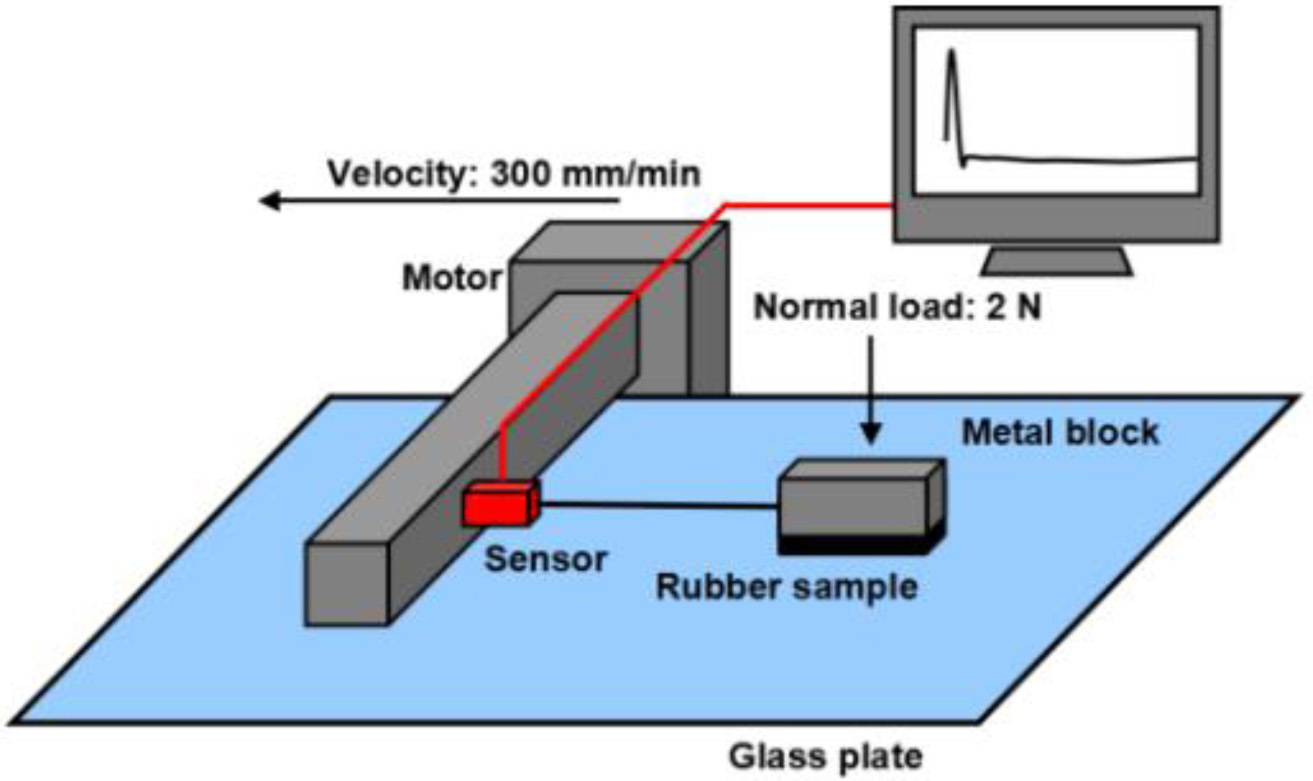
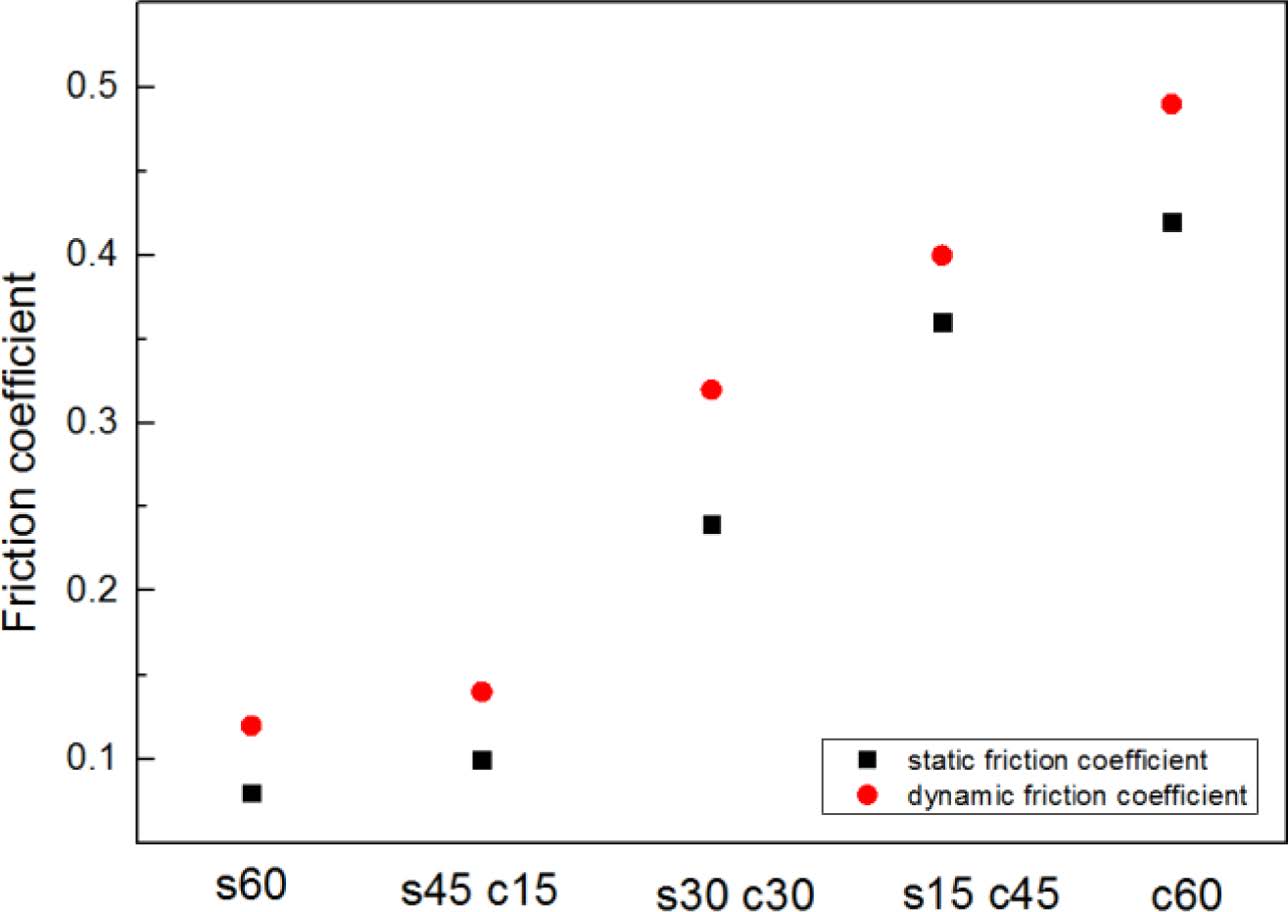
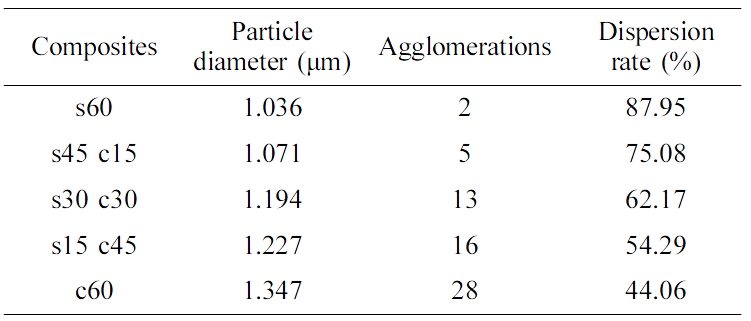
Figure 5 showed the mechanism of friction coefficient test, and the results of all the samples were shown in Figure 6, from this figure, it could be found the higher silica filling ratio, the smaller friction coefficient value, the s60 composite showed the smallest friction coefficient value (both static friction coefficient and dynamic friction coefficient). The probably reason of these results is about the particle dispersion,17 the dispersion results were shown in Table 3. From this table, it can be found s60 had the best dispersion effect, which meant this composite was closer to the whole, and presented a smoother surface with a lower friction coefficient.18
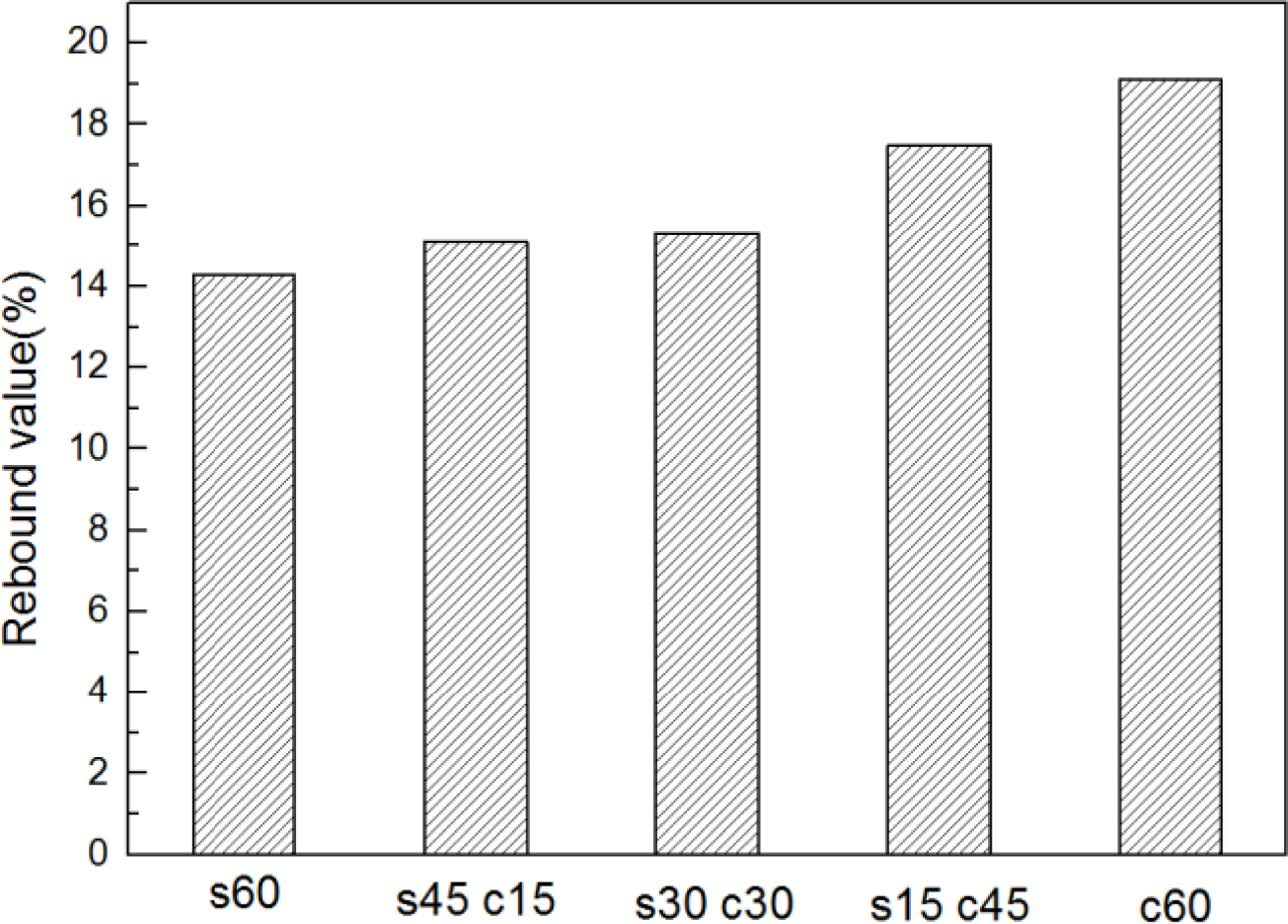
Figure 7 showed the results of resilience, which characterized by ball rebound tester, the resilience result (percentage rebound value R) can be calculated form the eq. (2) below:

Where h is the ball rebound height, expressed in millimeters; hmax is the height of the drop (500 mm).
From this figure, it can be found with the silica content increasing, the R values decreased, the main reason was about hardness, the larger hardness value, the composite showed more performance like resin, and smaller R value.
|
Figure 1 SEM pictures of blends: (a) c60; (b) s15 c45; (c) s30 c30; (d) s45 c15; (e) s60. |
|
Figure 2 TGA results of blends. |
|
Figure 3 Stress-strain curves of blends. |
|
Figure 4 Stress-strain curves of blends. |
|
Figure 5 Mechanism of friction coefficient test. |
|
Figure 6 Friction coefficient results of blends. |
|
Figure 7 The resilience results of blends. |
Epoxidized natural rubber (ENR)/solution styrene butadiene rubber (SSBR) blend with a fixed ratio of 150:50 was filled with silica and carbon black as fillers with different ratios. From the results of SEM, it could be found with the blend of ENR/SSBR showed the polarity, and make good combination effect with silica. From the results of TGA, it can be found that with the ratio of silica increasing, the heat resistance of ENR/ SSBR has been improved, from the hardness test and tensile strength, the more silica filling ratio, the higher mechanical properties. But also, with the filling ratio of carbon black increasing, the friction coefficient and resilience value19 also increased, these two properties are very important in tire industry.
- 1. H. Ismail and H. H. Chia, Polym. Test., 17, 199 (1998).
-

- 2. M. Arroyo, M. M. A. Lopez-Manchado, J. L. Valentin, and J. Carretero, Compos. Sci. Technol., 67, 1330 (2007).
-

- 3. R. Rajasekar, K. Pal, G. Heinrich, A. Das, and C. K. Das, Mater. Des., 30, 3839 (2009).
-

- 4. K. Pal, R. Rajasekar, D. J. Kang, Z. X. Zhang, J. K. Kim, and C. K. Das, Mater. Des., 30, 4035 (2009).
-

- 5. K. Sengloyluan, K. Sahakaro, W. K. Dierkes, and J. W. Noordermeer, Eur. Polym. J., 51, 69 (2014).
-

- 6. T. L. A. C. Rocha, R. H. Schuster, M. M. Jacobi, and D. Samios, Kautsch. Gummi Kunstst., 57, 656 (2004)
- 7. C. Kantala, E. Wimolmala, C. Sirisinha, and N. Sombatsompop, Polym. Adv. Technol., 20, 448 (2009).
-

- 8. N. M. Wasiuddin and M. M. Zaman, Int. J. Pavement. Eng., 3, 1 (2010).
- 9. S. H. Jang, X. X. Li, Q. Li, D. H. Lee, and U. R. Cho, Polym. Korea, 42, 106 (2018).
-

- 10. X. X. Li and U. R. Cho, Elastomers Compos., 53, 6 (2018).
-

- 11. X. X. Li, H. S. Jeong, and U. R. Cho, Elastomers Compos., 53, 48 (2018).
-

- 12. X. X. Li and U. R. Cho, Polym. Korea, 42, 492 (2018).
-

- 13. X. X. Li and U. R. Cho, Polym. Korea, 42, 841 (2018).
-

- 14. S. R. Raghavan, H. J. Walls, and S. A. Khan, Langmuir, 16, 7920 (2000).
-

- 15. P. Potschke and D. R. Paul, J. Macromol. Sci. C, 43, 87 (2003).
-

- 16. N. Rattanasom, T. Saowapark, and C. Deeprasetkul, Polym. Test., 26, 369 (2007).
-

- 17. M. R. Pourhossaini and M. Pazzaghi-Kashani, Polymer, 55, 2279 (2014).
-

- 18. X. X. Li and U. R. Cho, Elastomers Compos., 51, 43 (2016).
-

- 19. T. B. Edil and P. J. Bosscher, Geotech. Test. J., 17, 453 (1994).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(2): 321-326
Published online Mar 25, 2019
- 10.7317/pk.2019.43.2.321
- Received on Dec 26, 2018
- Revised on Jan 7, 2019
- Accepted on Jan 30, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ur Ryong Cho*,**
-
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea - E-mail: urcho@koreatech.ac.kr
- ORCID:
0000-0003-4866-8109









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.