- [Communication]
- Delayed Volatilization of Lavender Essential Oil Using Mesoporous Silica Nanoparticles
Min Ah Kim*, Young Eun Yeom**, Dong-Woon Kim***, Jin Kim****,†
 , and Chang-Moon Lee*,**,****,*****,†
, and Chang-Moon Lee*,**,****,*****,† 
*Department of Biomedical Engineering, Chonnam National University Graduate School, Yeosu 59626, Korea
**Interdisciplinary Program of Perfume and Cosmetics, Chonnam National University Graduate School, Gwangju 61186, Korea
***Department of Nursing Science, Gwangyang Health Sciences University, Gwangyang 57764, Korea
****Research Center of Healthcare Biomedical Engineering, Chonnam National University, Yeosu 59626, Korea
*****Department of Biomedical Engineering, Chonnam National University, Yeosu 59626, Korea- 메조다공성 실리카 나노입자로부터 라벤더 에센셜 오일의 지연된 휘발성
*전남대학교 바이오메디컬공학협동과정, **전남대학교 향장품학협동과정, ***광양보건대학교 간호학과, ****전남대학교 헬스케어의공학연구소, *****전남대학교 의공학과
Essential oils (EOs) can be volatilized and oxidized very quickly. The characteristics are impediments to apply EOs to various fields, despite their many beneficial effects. Here, we report on mesoporous silica nanoparticles (MSNs) for continuous diffusion of lavender EO (LEO). LEO could be loaded efficiently into the MSNs by hydrophobic interaction. The amount of residual LEO was measured over two weeks without closing by gas chromatography-mass spectrometry (GC-MS). Loading efficiency of LEO into the MSNs was 16.7% of total feed. Free LEO was volatilized by approximately 87%, whereas 48% of the LEO in the MSNs remained still after 16 days. In this study, the LEO loaded successfully into the core of the MSNs was continuously diffused out to air for a longer time. Our results indicate that the MSN is a useful formulation for encapsulation and continuous diffusion of EOs.
에센션 오일은 다양한 생리활성을 가지고 있지만 쉽게 휘발되고 산화되는 단점을 가지고 있다. 본 연구에서는 메조다공성 실리카 나노입자를 이용한 에센셜 오일의 휘발 지연과 지속적인 발향을 평가하였다. 제조한 나노입자의 메조다공성 구조는 라벤더 에센셜 오일을 효과적으로 담지할 수 있었다. 메조다공성 실리카 나노입자로부터 휘발되는 오일의 양은 GC-MS를 이용하여 2주 동안 평가하였다. 라벤더 에센셜 오일은 16일 동안 전체의 87%가 휘발된 반면 메조다공성 실리카나노입자 내 에센셜 오일은 느린 속도로 휘발되었고 48% 남아 있었다. 이러한 결과는 실리카 나노입자의 메조다공성 코어 구조가 에센셜 오일을 담지하고 휘발을 지연시키며 발향을 지속적으로 할 수 있게 한다는 것을 보여준다. 그러므로 메조다공성 실리카 나노입자는 에센셜 오일을 캡슐화하고 다양한 분야에 응용되는데 유용하게 활용될 수 있다.
Keywords: continuous diffusion, essential oil, mesoporous silica nanoparticles, volatilization
This work was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF- 2016M2A2A7A 03953181 and 2018R1D1A1B07045264).
Essential oils (EOs) are aromatic oily liquids extracted from plants. EOs are widely used in a variety fields with various formulations, such as soaps, perfumes, detergents, personal care, and aromatherapy products.1,2 Among many EOs, Lavandula angustifolia (common name lavender) oil is one of the most popular EOs for healthcare and cosmetic applications.3 Lavender oil has been traditionally used to treat headache and shows good antibacterial activity against various types of bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli. In addition, lavender oil has benefit in pain relief. For example, it has been reported that lavender oil is effective for back pain when stimulating acupuncture points with acupressure.4 Recently, lavender oil is widely used in massage and aromatherapy.5 Until now the use of lavender oil has been increasing steadily. However, the use of this EO has been limited by its poor physical properties, such as high hydrophobicity and rapid volatility, making it difficult to be used in pharmaceutical applications.6 In order to overcome these disadvantages and widen its range of use, this EO needs to be incorporated using nanotechnology into microparticles.7,8 For instance, liposomes can incorporate and protect EOs; in recent years, they have been extensively studied for incorporation and delivery of bioactive substances.9
Mesoporous silica nanoparticles (MSNs) are one choice for nanotechnology-based drug delivery systems.10 MSNs have unique properties, such as a large surface area, pore volume, and biocompatibility.11 In particular, the large surface area and pore volume provide great potential for drug loading into the pore channels by hydrophobic interaction. Furthermore, the pore sizes of MSNs can be adjustable, from 2 to 50 nm, controlling drug loading and release kinetics. Because of these excellent properties, MSNs have been studied in biomedical fields, including drug delivery systems, bioimaging, and tissue regeneration.12-14
The aim of this study is to propose a way to delay the rapid volatility of EOs so that they can be diffused steadily into the atmosphere using MSNs. Lavender essential oil (LEO) was incorporated into MSNs by hydrophobic interaction into the pore channels, and the volatility of the oil was evaluated over two weeks.
Materials. Cetyl trimethylammonium bromide (CTAB), diethanolamine, 3,3',5,5'tetramethyl benzidine (TMB), tetraethyl orthosilicate (TEOS), and hydrochloric acid were purchased from Sigma Aldrich Co., Ltd. (St. Louis, MO). Lavender essential oil (LEO) was obtained from HauYon Co. Ltd. (Yanyang, Republic of Korea). The other reagents in the experiments were analytically pure, and commercially available.
Preparation and Characterization of MSNs. MSNs were prepared by a method reported previously.15 We dissolved 0.78 g CTAB in 21.6 mL of DW and 0.15 mL of 2 M NaOH with stirring; 1.3 mL TMB and 3.4 mL of ethanol were added into the solution at 80℃. When the solution was dispersed completely, 2.2 mL of TEOS was dropped slowly to the solution over 6 min with vigorous stirring. After incubation for 2 h, the solution was centrifuged at 10000 rpm for 30 min to collect the nanoparticles, which were then washed with methanol twice. To remove CTAB, 1.4 mL HCl was added to the nanoparticles solution in methanol. The solution was stirred for 8 h at 60℃, and MSNs were obtained by centrifugation and drying. The shape of the MSNs was observed by using a transmission electron microscope (TEM, H-7500, Hitachi, Krefeld, Germany) and a focused ion beam scanning electron microscope (FIB, Helios G3 CX, FEI, Hillsboro, USA). The surface area of the MSNs was evaluated by nitrogen adsorption-desorption isotherm measurements on an ASAP2020 system (Micromeritics Instrument Co., GA, USA). The surface areas were calculated with Brunauer Emmett Teller theory using isotherm adsorption data at P/P0 from 0.05 to 0.30. The pore volume and pore size distributions of the MSNs were calculated from an adsorption branch using the Barrett Joyner and Halenda (BJH) method.16
Loading LEO into MSNs. We dispersed 0.1 g MSNs in 5 mL DW and 0.2 mL LEO was added to the solution. After sonication for 15 min (on 3 sec and off 1 sec), the bottle of solution was sealed and stirred for 24 h in the dark. The LEO-loaded MSNs were collected by centrifugation and washed with DW three times. To measure the amount of LEO, the MSMs were dispersed in hexane. After centrifugation to remove the nanoparticles, the supernatant was evaluated using a gas chromatography-mass spectrometry (GC-MS) method. GC-MS analysis was performed using QP-2010 Ultra system (Shimadzu Co., Kyoto, Japan) equipped with an Rxi-5ms capillary column (30 m×0.25 mm I.D., 0.25 μm film thickness). Helium was used as the carrier gas and the column temperature was initially programmed at 70℃ for 2 min and increased to 200℃ at 3 ℃/min. The ionization energy was 70 eV with a scan time of 0.3 sec.
Diffusion Study. The diffusion rate of LEO from MSNs was evaluated at 25℃ in accordance with a previous report.17 Then 10 mg MSN containing LEO or 0.01 mL LEO was weighted into 1.5 mL ependorf tubes in triplicate, respectively. At predetermined times, the amount of the remaining LEO was determined by the GC-MS. The amount of changes of the LEOs was monitored over two weeks.
Statistical Analysis. Quantitative data were expressed as mean values (±standard deviation). Mean values were compared by use of the independent samples t test, and p values of less than 0.05 were considered to be statistically significant.
MSNs have been studied and often proposed as a drug delivery carrier for controlled release and as a diagnostic agent for imaging and detecting biosignal from disease regions caused by their unique properties.18 In particular, their hydrophobic core is valuable for loading hydrophobic agents. In this study, we prepared and used MSNs as a carrier for continuous diffusion of LEO, as shown in Figure 1. LEO was loaded into the core of MSNs, and the remaining LEO was evaluated over two weeks. The loading efficiency of LEO into the MSNs was 16.7% of the feeding oil.
The MSNs obtained by the previous synthesis method were spherical with porous structure and had uniform particle size (Figure 2).
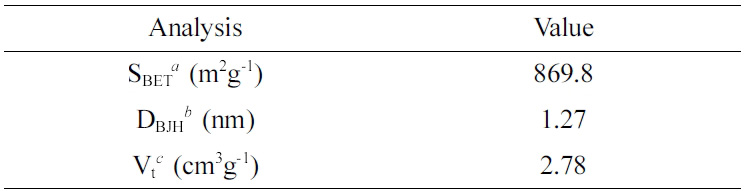
BET surface area, BJH pore size and total pore volume of the MSNs are presented in Table 1. The average pore diameter and total volume of the MSNs was 1.27 nm and 2.78 cm3/g, respectively. The surface area of the MSNs was 869.8 m2/g. The pore size and volume are appropriate for loading hydrophobic materials, such as EOs and anti-cancer drugs.19
The major volatile compounds of LEO are linalool and linalyl acetate.20 As shown in Figure 3, they were used as standard compounds for quantification and detected at 6.606 and 12.108 min by GC-MS analysis, respectively. We tested the diffusion of LEO from MSNs over two weeks.
Figure 4 shows profiles of the remaining LEO in the MSNs determined by GC-MS. The GC-MS results show that free LEO was volatilized into the air by around 87% of total LEO within 16 days (Figure 4). On the other hand, the LEO into the MSNs remained 48.4±1.9% of the total LEO during the same period. When LEO is loaded into the MSNs, more than twice as much was left in the core of the MSNs, that is, was not volatilized.
EOs have attracted attention in the cosmetic and pharmaceutical industries, because they have a wide spectrum of biological properties, such as antifungal, antioxidant, and antitumor effects.6,7 However, EOs are volatile and biologically unstable. In addition, EOs are sensitive to oxygen, light, and temperature, and poorly soluble in water.21,22
Therefore, encapsulation of EOs is necessary to improve their solubility in water, to control their volatilization, and to extend their use in various fields. For these reasons, various formulations, such as β-cyclodextrin, maltodextrin, and gelatin, have been used to incorporate EOs.23,24 In this study, MSNs were used as a formulation to encapsulate EOs. As results have shown, MSNs have a large surface and pore volume, resulting in efficient loading of the EO into the core. Volatilization of the EO in the core of the MSNs was delayed, and more than twice as much of the EO was retained in the MSNs han for the free EO. The use of MSNs for encapsulation of EOs can reduce the instability against oxidation and light, and can make EOs diffuse continuously. As a result, encapsulation of EOs in MSNs can widen their application range.
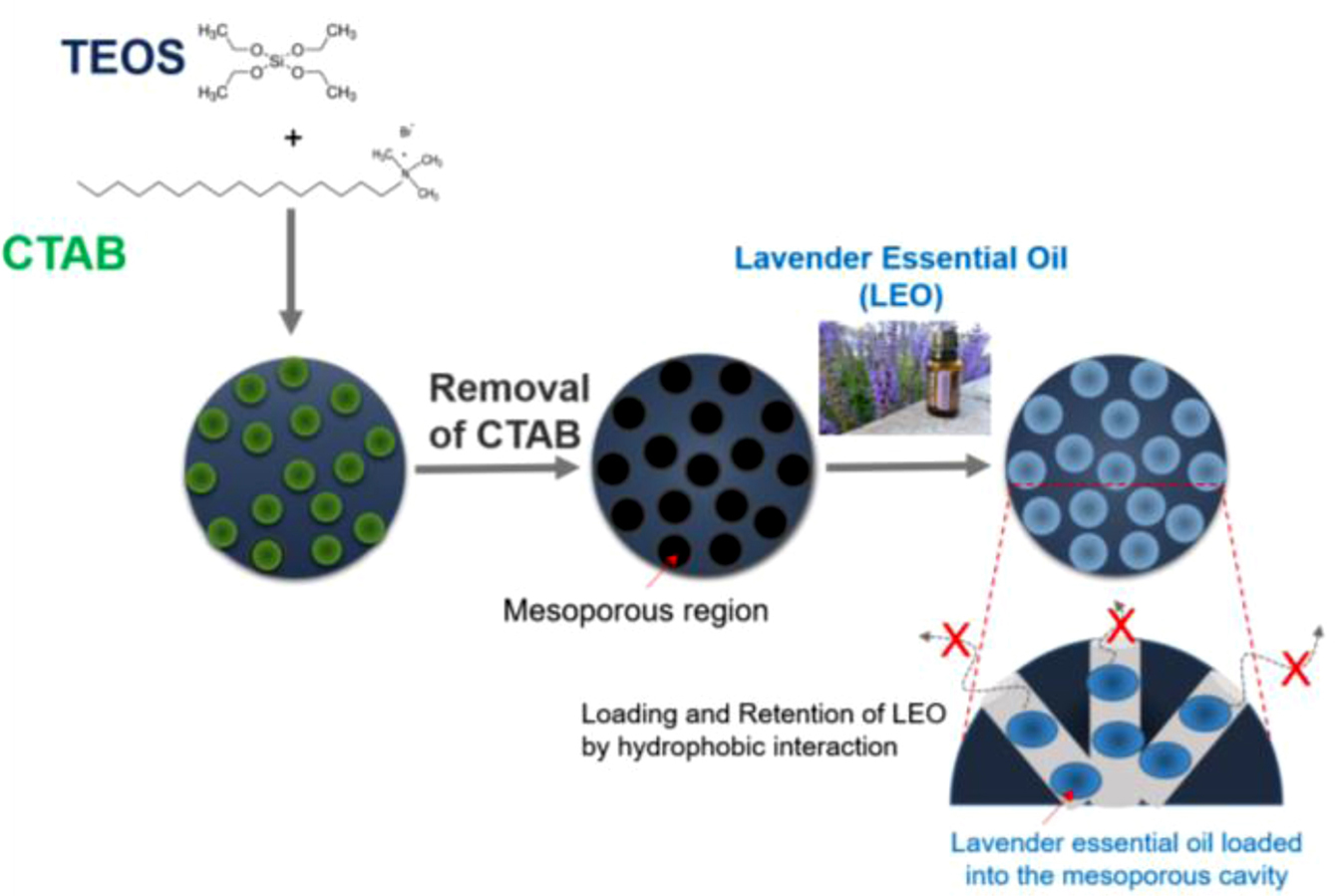
|
Figure 1 The schematic illustration for preparation of lavender essential oil-loaded MSNs and retention of the essential oil in the core of MSNs. |
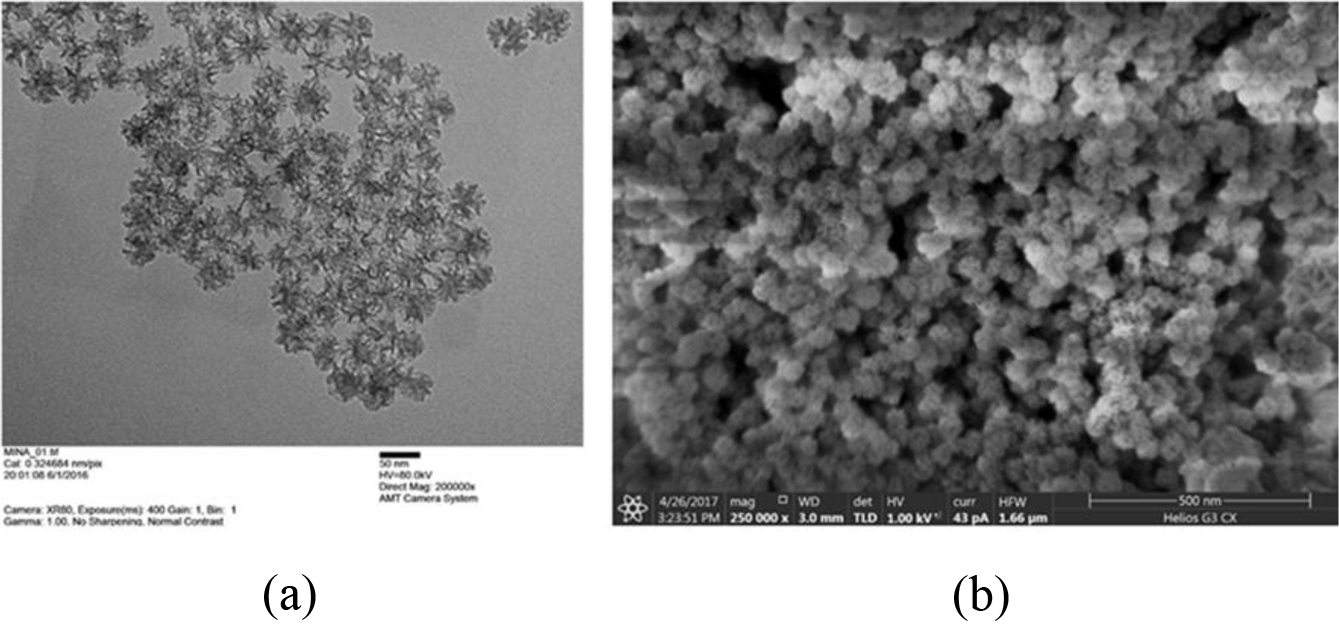
|
Figure 2 Electron microscopic images of lavender essential oilloaded MSNs: (a) TEM; (b) FIB images. Direct magnification: (a) 200000×; (b) 250000×. The scale bar: (a) 50 nm; (b) 500 nm. |
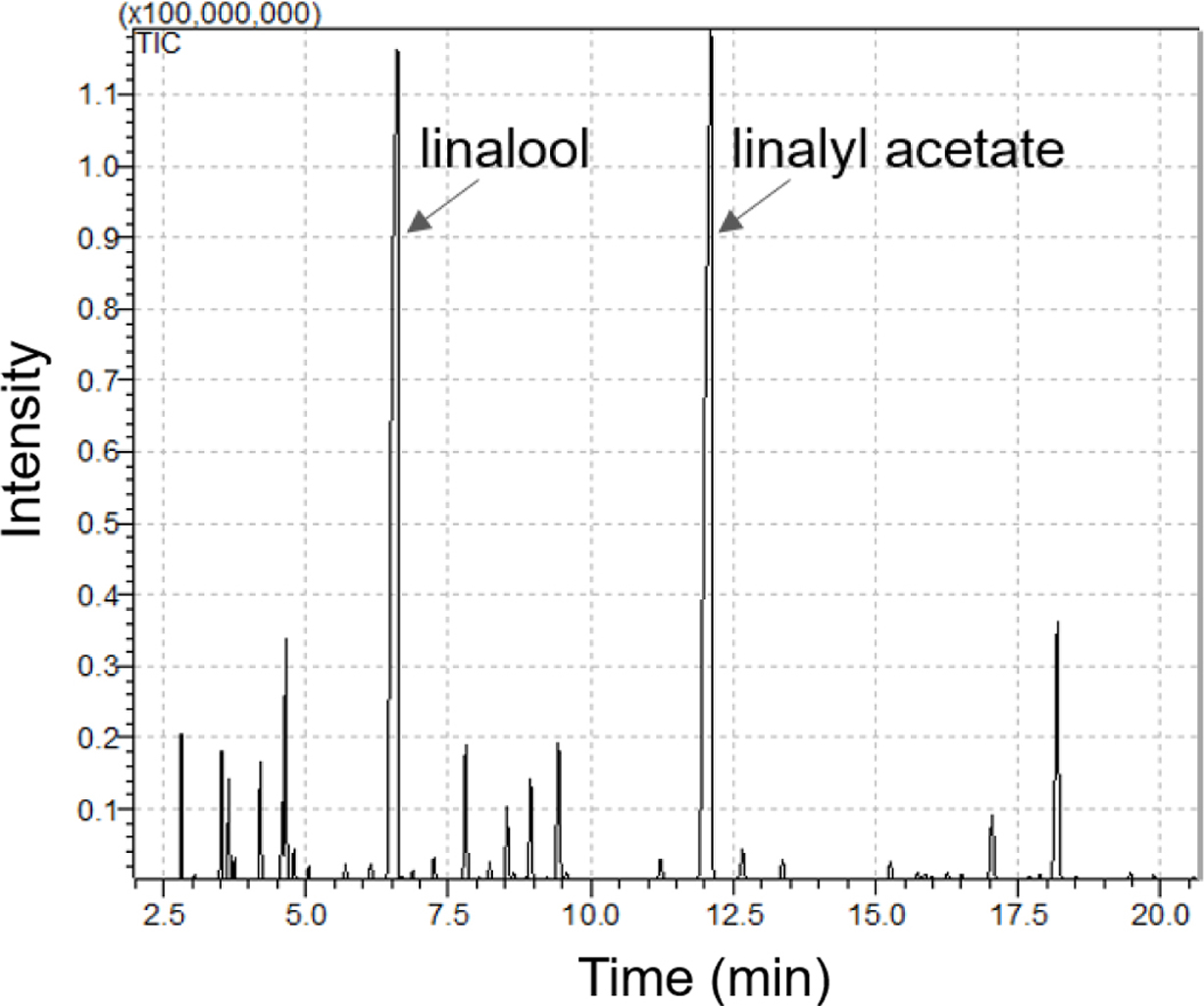
|
Figure 3 GC-MS chromatogram of lavender essential oil. |
|
Figure 4 Retention profiles of lavender essential oil in the core of the MSNs determined by the GC-MS. |
|
Table 1 BET and BJH Characteristics of the MSNs |
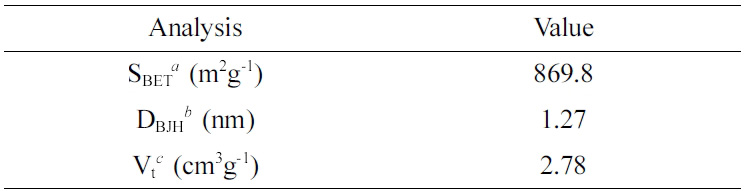
aSBET: specific surface area calculated from data in the range P/P0 < 0.3 using BET equation, bDBJH: pore diameter assigned from the maximum on the BJH pore-size distribution, cVt: total pore volume calculated at P/P0 = 0.99. |
The MSNs were used as a formulation to encapsulate LEO. LEO was loaded successfully into the core of the MSNs. The large pore size and volume of the MSNs are useful for loading LEO. More than twice as much of the loaded LEO than of the non-loaded LEO was retained in the MSNs for a longer time. Therefore, we anticipate that the MSN are a useful formulation for encapsulation of EOs and continuous diffusion.
- 1. S. Burt, Int. J. Food Microbiol., 94, 223 (2004).
-

- 2. T. Kulisic, A. Rodonic, V. Katalinic, and M. Milos, Food Chem., 82, 633 (2004).
-

- 3. M. Shrestha, T. M. Ho, and B. R. Bhandari, Food Chem., 221, 1474 (2017).
-

- 4. Y. B. Yip and S. H. M. Tse, Complement. Ther. Med., 12, 28 (2004).
-

- 5. L. Halcón and K. Milkus, Am. J. Infect. Control., 32, 402 (2004).
-

- 6. Y. H. Ma, Q. Wang, J. Gong, and X. Y. Wu, J. Pharm. Sci., 105, 1124 (2016).
-

- 7. A. R. Bilia, C. Guccione, B. Isacchi, C. Righeschi, F. Firenzuoli, and M. C. Bergonzi, Evid.-based Complement. Altern. Med., 2014, 1 (2014).
-

- 8. A. El Asbahani, K. Miladi, W. Badri, M. Sala, E. H. Ait Addi, H. Casabianca, A. El Mousadik, D. Hartmann, A. Jilale, F. N. R. Renaud, and A. Elaissari, Int. J. Pharm., 483, 220 (2015).
-

- 9. R. Gharib, L. Auezova, C. Charcosset, and H. Greige-Gerges, Food Chem., 218, 365 (2017).
-

- 10. F. Tang, L. Li, and D. Chen, Adv. Mater., 24, 1504 (2012).
-

- 11. J. L. Vivero-Escoto, I. L. Slowing, B. G. Trewyn, and V. S. Y. Lin, Small, 6, 1952 (2010).
-

- 12. I. L. Slowing, B. G. Trewyn, S. Giri, and V. S. Y. Lin, Adv. Funct. Mater., 17, 1225 (2007).
-

- 13. Z. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart, and J. I. Zink, Chem. Soc. Rev., 41, 2590 (2012).
-

- 14. Y. Chen, H. Chen, and J. Shi, Adv. Mater., 25, 3144 (2013).
-

- 15. A. B. D. Nandiyanto, S. G. Kim, F. Iskandar, and K. Okuyama, Microporous Mesoporous Mater., 120, 447 (2009).
-

- 16. H. Gu, Y. Guo, S. Y. Wong, Z. Zhang, X. Ni, Z. Zhang, W. Hou, C. He, V. P. W. Shim, and X. Li, Microporous Mesoporous Mater., 170, 226 (2013).
-

- 17. M. Sherry, C. Charcosset, H. Fessi, and H. Greige-Gerges, J. Liposome Res., 23, 268 (2013).
-

- 18. J. Lu, M. Liong, J. I. Zink, and F. Tamanoi, Small, 3, 1341 (2007).
-

- 19. M. Liong, J. Ku, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi, and J. Zink, ACS Nano, 2, 889 (2008).
-

- 20. L. Bing, F. Jihong, Z. Yan, and C. Ping, J. Oleo Sci., 67, 1327 (2018).
-

- 21. F. Donsi, M. Annunziata, M. Sessa, and G. Ferran, LWT-Food Sci. Technol., 44, 1908 (2011).
-

- 22. F. D. Goffman and C. Möllers, J. Agric. Food Chem., 48, 1605 (2000).
-

- 23. H. M. C. Marques, Flavour. Frag. J., 5, 313 (2010).
-

- 24. S. Krishnan, R. Bhosale, and R. S. Singhal, Carbohydr. Polym., 61, 95 (2005).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(3): 327-330
Published online May 25, 2019
- 10.7317/pk.2019.43.3.327
- Received on Jan 24, 2019
- Revised on Feb 13, 2019
- Accepted on Feb 25, 2019
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jin Kim****,†, and Chang-Moon Lee*,**,****,*****,†
-
*Department of Biomedical Engineering, Chonnam National University Graduate School, Yeosu 59626, Korea
**Interdisciplinary Program of Perfume and Cosmetics, Chonnam National University Graduate School, Gwangju 61186, Korea
***Department of Nursing Science, Gwangyang Health Sciences University, Gwangyang 57764, Korea
****Research Center of Healthcare Biomedical Engineering, Chonnam National University, Yeosu 59626, Korea
&a - E-mail: cream4251@daum.net, cmlee@jnu.ac.kr
- ORCID:
0000-0002-2558-8643, 0000-0002-9119-6154








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.