- Changes in the Properties of Bio-Based Thermoplastic Polyurethanes with Different Chain Extenders
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea- 사슬연장제의 종류에 따른 바이오 열가소성 폴리우레탄의 물성변화 연구
*한국기술교육대학교 에너지, 신소재, 화학공학부, **친환경고성능화학소재연구소
Bio-based polyester polyol was synthesized by esterification using adipic acid and isosorbide. After the esterification, the bio-based polyurethanes (PUs) were synthesized by the reaction between the polyester polyol, isocyanate (4,4'-diphenylmethane diisocyanate) (MDI) and three chain extenders (1,3-propanediol (1,3-PD), 1,4-butanediol, or isosorbide) with a mixing ratio of 1:2.1:1. A thermoplastic polyurethane (TPU) without extender was used as a control. The structure analysis of bio-based TPUs were performed using FTIR, TGA, and DSC. Viscoelastic properties was investigated using a strain sweep test mode with a rubber process analyzer (RPA). The mechanical properties of samples (tensile strength and hardness value) were characterized with UTM and shore A hardness tester at room temperature. Water- and heat-resistance properties of the bio-TPUs were investigated using an oven and RPA with temperature sweep mode. The bio-based TPU which contained isosorbide as the chain extender showed the best mechanical and viscoelastic properties, and this polymer also showed great water and heat resistance during the test. The bio-based TPUs that contained liner chain extender 1,3-PD also showed excellent viscoelastic properties and heat resistance.
Adipic acid와 isosorbide를 사용하여 바이오 폴리에스터 폴리올을 합성하였다. 합성된 바이오 폴리에스터 폴리올을 4,4'-methylene bis(phenyl isocyanate)(MDI), isosorbide, 1,3-propandiol, 1,4-butanediol 3가지 사슬증량제와 1:2.1:1의 비율로 혼합하여 바이오 폴리우레탄을 합성하였다. 바이오 폴리우레탄의 구조를 분석하기 위해서 FTIR, TGA 및 DSC를 사용하였다. 고분자 가공분석기(RPA)의 변형 스윕(strain sweep) 및 온도 스윕(temperature sweep)기능을 사용하여 바이오 폴리우레탄의 점탄성 및 내열성을 조사하였다. UTM, shore A 기기를 사용하여 바이오 폴리우레탄의 인장강도, 경도를 측정하였다. Oven을 사용하여 바이오 폴리우레탄의 내수성을 조사하였다. 바이오 폴리에스터 폴리올, MDI와 isosorbide에 의한 바이오 폴리우레탄이 가장 좋은 기계적 물성과 점탄성을 보여주었다. 1,3-PD에 의한 바이오 폴리우레탄이 더 좋은 내열성을 보여 주었다.
Bio-based polyester polyol had been synthesized by the esterification method by adipic acid and isosorbide. the bio-based polyurethanes were synthesized by polyester polyol above, isocyanate (4,4’-diphenylmethane diisocyanate) and 3 kinds of chain extenders (1,3-propanediol, 1,4-butanediol and isosorbide), the bio-thermoplastic polyurethane which used isosorbide as chain extender showed the best mechanical and viscoelastic properties.
Keywords: bio-polyurethane, chain extender, viscoelastic, water resistance, heat resistance
Thermoplastic polyurethane (TPU) is the general term used for a polymer derived from the condensation of polyisocyanates and polyols having intramolecular urethane bonds (carbamate ester bond, -NHCOO-) (Figure 1).1 TPU has been used since the 1940s and is widely used as a base material in various industries. Considering sustainability and the high cost of petrochemicals, interest in bio-based materials has increased.2 Polyols from biomass have already found applications in the polyurethane (PU) elastomer industry.3 The main issue with bio-based polyester/polyether polyols is related to their heterogeneity in functionality within the same polyol groups as well as their relatively lower MW than necessary for flexible elastomers.4
As polyols, polyether and polyester polyols are generally used. TPU synthesized from polyester polyol is called as polyester TPU; and TPU synthesized from polyether polyol is termed as polyether TPU.5 Although most TPU used at present is polyether TPU, polyester TPU has recently attracted much interest because of its biodegradability and its advantages in waste treatment.6
TPUs are commonly prepared by two steps prepolymer method. The prepolymers have linear chains terminated by isocyanate groups resulting in low viscosity and thus enabling the melt processing of the prepolymer.7 First, prepolymers are prepared by reacting polyols with an excess of diisocyanate.8 Then, the prepolymers were reacted with a chain extender to obtain the final TPU, in which polyol is mainly considered as the soft segment (SS) and the diisocyanate as the chain extender for hard segment (HS).9 Both, molar mass of the polyol and concentration of HS in the matrix affect the thermal and mechanical properties of the final TPU.10 In general, TPUs with HS concentration lower than 50% are considered as soft rubbers and those with higher HS concentration (>50%), as rigid rubbers, both being of significant industrial importance.11
Biodegradable elastomers are versatile in many applications in soft tissue engineering. Herein, we report the synthesis and characterization of a series of biodegradable linear PU elastomers. In this article, bio-based polyester polyol was synthesized by esterification of adipic acid and isosorbide. MDI was used as polyisocyanate; 1,3-PD, 1,4-butanediol, and isosorbide were used as chain extenders. The bio-based PUs were synthesized with a ratio of 1:2.1:1 (polyol: polyisocyanate: chain extender), and a TPU sample without chain extender was used as a control. The chemical structure of products were confirmed with FTIR. The thermal decomposition behaviors of products were characterized by TGA and DSC. The mechanical properties (tensile strength, hardness value) and viscoelastic properties (storage modulus and loss modulus) were also investigated. Water- and heat-resistance properties of bio-based TPUs were investigated.

|
Figure 1 Synthesis of polyurethane. |
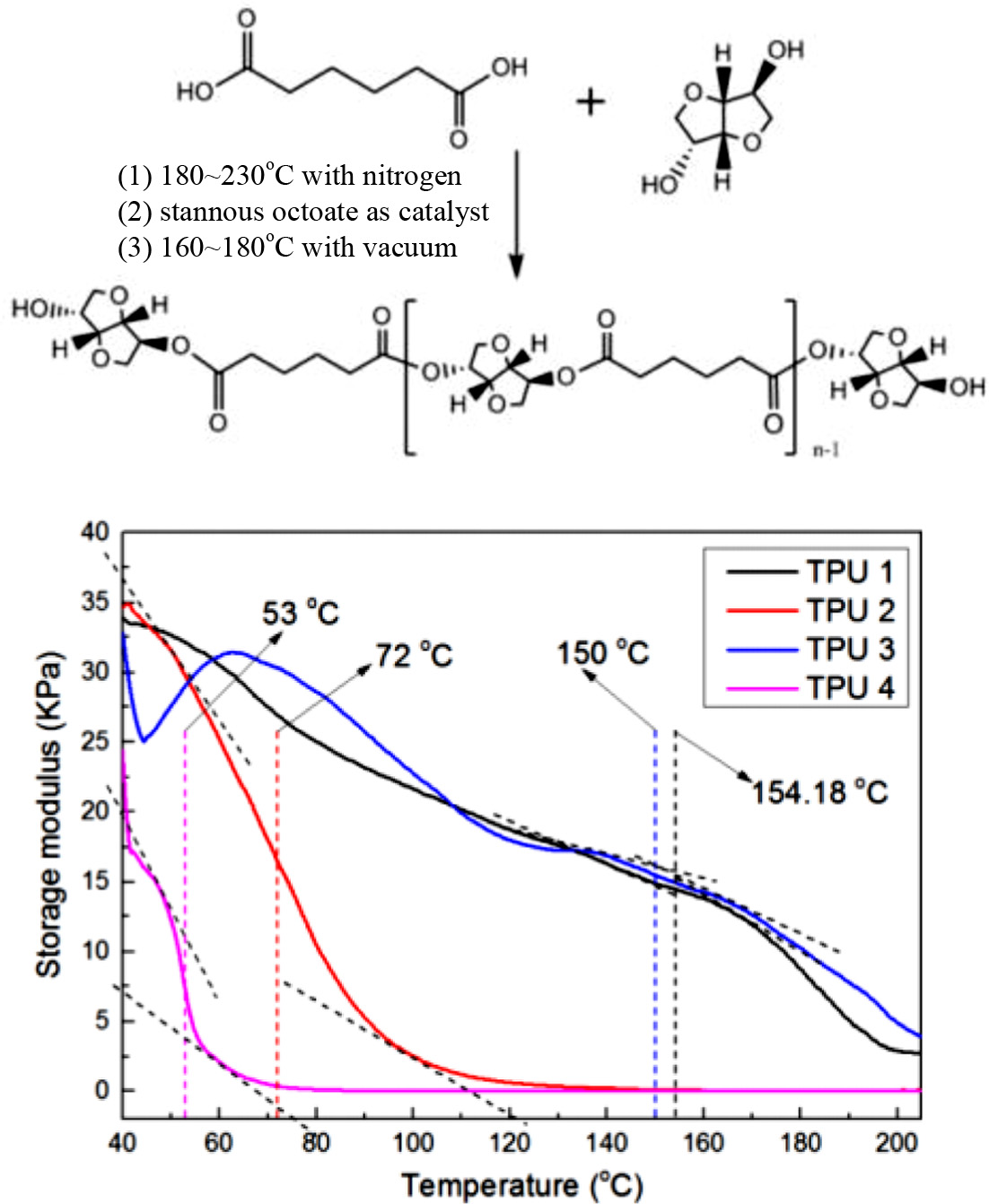
Materials and Reagents. The materials and reagents used in this experiment were shown in Table 1. All the materials were used as industrial pure class, and the nitrogen used as a protecting gas in polyester synthesis process was purchased from Daesung Co. The mechanisms for polymerization of polyester polyols were shown in Scheme 1. A typical polymerization reaction was carried out as follows: The fatty acid and polyol were mixed into the reaction system. The mixture was stirred by a magnetic stirrer at 400 rpm. And for the first 4 h, nitrogen was purged. Tin(II) 2-ethylhexanoate (stannous octoate) as a catalyst was added and for the final 4 h, nitrogen purging was ceased and vacuum system (2 torr) was set. After that, the system was cooled to below 60 oC, and the polyester polyol was obtained.
The reactant ratios for PU polymerization are shown in Table 2. Polyester polyol, diisocyanates, and chain extender were mixed, stirred with 200 rpm at room temperature, and the products were obtained.
Characterization. Thermal gravimetric analysis (TGA) of TPU samples were performed with TA Instruments TGA Q500 V20 from 30 to 800 oC at a heating rate of 10 oC min-1 under nitrogen atmosphere. Differential scanning calorimetry (DSC) was performed using TA Instruments DSC Q20 V24 from -50 to 150oC with a heating rate of 10oC min-1 under nitrogen atmosphere with two scans. Fourier transform infrared (FTIR) analysis was performed using a thin film of the polymer (thickness of about 200-300 μm) with Perkin Elmer Spectrum 100®. Samples were scanned in the wave range between 650 and 4000 cm-1 with a resolution of 4 cm-1. Viscoelastic properties were determined with a rubber process analyzer (RPA-V1, U-Can Dynatex Inc.). The strain sweep test from 0.01 to 20 degrees was operated at 60oC and 1 Hz on RPA according to ASTM D 6204-97. The hardness of samples was obtained by a shore durometer type A following ASTM D22-40. The tensile strength test was measured three times on a Tinius Olsen H5KT-0401 testing machine at a speed of 500 mm/min according to ASTM D412. The samples were made of a dumb-bell shape with the dimensions of 25×6×1 mm after the specimens formed on the heating press machine. Heat resistance was test by RPA-V1 with temperature sweep mode. Water resistance was tested according to ASTM D 570-98, hydrolysis resistance was tested by an aging chamber,12 put the samples into tubes filled with water, and set these tubes into rubber aging chamber at 100 oC for two weeks, after that, tested samples with tensile tester.
In order to determine the degree of esterification reaction, the acid number test was carried out according to ASTM D7253 (standard test method for PU raw materials). The acid number of polyol could be calculated from the equation below13:
Acid number = [A × N × 56.1]/W (1)
Where A = volume of 0.1 N KOH solution for the titration of the sample in mL, N = the normality of the KOH solution, and W = the weight of the sample used in g.
Determination of the hydroxyl number of polyol can be calculated from the theoretical molecular weight of polyol. The hydroxyl value was obtained according to ASTM E220 (standard test methods for hydroxyl groups using acetic anhydride acetylation). The hydroxyl number of the hydroxyl-containing compound was calculated from the equation below:14
Hydroxyl number = 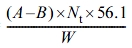 (2)
(2)
Where A = 1 N NaOH solution, mL, required for titration of the sample, B = NaOH solution, mL, required for titration of the blank, Nt = meq/mL of the solution at the temperature during analysis, W = sample used, g.
And the theoretical molecular weight of sample was calculated by the following equation15:
Mn = 56.11 × 2000 ÷ Hydroxyl number (3)
From the results of acid number and hydroxyl number of polyester polyol, M(PE) was determined to be 968.2 g/mol.
Figure 2 showed the IR spectra of the bio-based TPU samples, the typical bands of PU in the spectra were observed, in which the band at 1735 cm-1 is for C=O, the bands at 1540 cm-1 and 3200 cm-1 are for N-H.16 A small band at 2245 cm-1 in the specrum of TPU 1 suggests that almost –NCO groups were reacted. The band at about 3280 cm-1 is for the stretching vibration from the –NH groups. In general, the band of –NH should appear at about 3300 cm-1, but due to the hydrogen bonding effect, the band was shifted; and the peak showed in about 3500 cm-1 is for the intramolecular hydrogen bonds due to the isosorbide structure. Thus, the synthesis of bio-based TPU samples was successfully accomplished.
Figure 3 and Figure 4 showed the TGA thermograms. Because of the difference in the chain extender, the TGA curves showed considerable difference. TPU 4 showed linear decrease during the thermal decomposition step in Figure 3. But other bio-based TPU which mixed with the chain extender showed two or more plateau-like trends during the thermal decomposition step. From the curves of Figure 4, the same thermal decomposition temperatures about 310 oC and 400 oC were observed. The probable reason for that might be due to the same bio-based PE structure. And TPU 3 showed the lowest derivative weight peak in this temperature, which meant TPU 3 had the best thermal resistance before 400 oC. TPU 1 and TPU 3 showed 3 decomposition peaks about 470 oC, which meant these two bio-TPUs had better anti-heat resistance property than other two TPU samples.
DSC thermograms are shown in Figure 5. From the curves of TPU 1, TPU 2 and TPU 3, it was found the Tg of bio-based TPU samples increased. The probable reason for that is due to the increasing molecular weight of the chain extender and the steric hindrance effect. For the case of TPU 4, due to mixing without any other chain extender, the strength of this TPU molecules was lower than those of other TPUs. Thus, TPU 4 showed the lowest Tg.
Figure 6 and Figure 7 show the viscoelastic results of bio-based TPU samples by a RPA-V1 with a dynamic strain sweep test mode. Figure 6 was for storage modulus, and Figure 7 was for loss modulus, the bio-based TPU without chain extender showed the lowest modulus, meaning that the chain extender could improve the viscoelastic properties for bio-TPUs. In the figure of storage modulus, the curve of TPU 3 was higher than those of other TPUs, meaning it had the largest storage modulus during all the test process. But, in the curve of loss modulus, it could be found TPU 2 always showed the highest loss modulus value in the high strain degree region, which meant the structure destruction of TPU 2 was great in the strain sweep test, but with TPU 3, with the strain degree increased, the loss modulus value decreased sharply, meaning it might have a great stable structure. The probable reason for that may be due to the longer molecular chains of chain extender had more chances to produce the chain entanglement phenomenon, and resulting in uneven distribution of molecular structure and cracks,17 and led to the decline of viscoelastic properties.
Figure 8 showed the hardness value results of all the bio-TPU samples, TPU 3 also showed the highest hardness value in this test, and TPU 4 showed the lowest value, which meant chain extender could improve the hardness value of TPU material. And due to the similar isosorbide molecular structure in bio-based polyester polyol and chain extender, thus, TPU 3 showed the most compact structure in this research.
Figure 9 showed the moisture infiltration results, it could be seen that the curve of TPU 3 was always on the bottom, which meant TPU 3 had the best water resistance in this research, the probable reasons were showed in the following:
1. The polyester synthesized in this research is isosorbide dicarboxylate, which is also a typical plasticizer in the polymer field.
2. In PU field, the plasticizers always could improve the tensile stress, tear stress, abrasion resistance and water resistance during the synthesis process. But traditional plasticizer, due to the poor compatibility, the plasticizing effect is not ideal. Thus, to increase the compatibility of plasticizer, researchers use the polyesters as plasticizers which have similar molecular structure with PU molecular. In this research, bio-based polyester was also used as self-plasticizer.18
3. Isosorbide could increase the Tg of polyester, the reasons are: (1) the isosorbide groups could increase the aromatic heterocyclic structure, which might decrease the number of rotating single bonds, then the chain flexibility also decreases; and (2) The hydrogen atoms from isosorbide molecules could increase the hydrogen bonding effect of polyester molecules, it also could reduce the chain flexibility.19
4. The Mw (showed in Table 5) of TPU 3 was larger than any other TPU samples in this research, which meant in same mix ratio, isosorbide used as chain extender could provide the largest molecular weight for TPU synthesis, especially in hard segment parts of TPU molecules, and also meant the most compact matrix structure in TPU 3. The more compact matrix structure, the more intramolecular hydrogen bonds, and the better water resistance.
5. From the reasons above, when isosorbide was used as chain extender, it also could show some plasticizing effect, and it increased the stability of TPU molecular.
Table 3 and Table 4 showed the tensile results of all the bio-TPU samples at the room temperature and after hydrolysis resistance test. From these two tables, it could be seen that TPU 3 showed the best tensile strength and retention tensile ratio in this part, which meant this material had the best mechanical properties and hydrolysis resistance. And there was the highest ratio of isosorbide structures in TPU 3, which could improve the plasticizing effect of polyester. Thus, it showed the best hydrolysis resistance due to the self-plasticizing effect.20
Table 5 showed the GPC results of all the samples, from the results of TPU 4, it could be seen the TPU without chain extender showed the smallest PDI and molecular weight in this research, which meant chain extender could increase the molecular weight of TPU, and make the matrix structure complex. From the results of TPU 1 and TPU 2, it could be seen with the increase of chain extender’s carbon number, the molecular weight and PDI of TPU also increased. And for TPU 3, due to the specially structure, the chain extender (isosorbide) showed a better hydrogen bonding effect, which could provide more adsorption force and make more chaos in the matrix, so TPU 3 showed the largest molecular weight and PDI value in this research.
Figure 10 showed the results of heat resistance test by a RPA-V1 with a dynamic temperature sweep test mode. The temperature sweep range was from 40 to 200 oC. And from the figure, it could be found that the trends of all the curves were downward. It was due to with the temperature increased, the bio-TPU would undergo with phase transition from solid to liquid, thus, the storage modulus decreased rapidly, and after that state, TPUs lost the mechanical properties. Therefore, according to this phenomenon, the phase transition temperature could be found into the curves during the temperature sweep test, which also presented the heat resistance of TPU materials. In this figure, TPU 1 showed the highest phase transition temperature, which was about 154.18 oC, and TPU 3 was the second, which was 150 oC, TPU 4 also showed the lowest value, which was about 53 oC, it was due to no chain extender. But as TPU 2, it showed a lower value as about 72 oC in this research, the reason for that may be also due to the chain entanglement phenomenon, which reduce the stability of TPU structure and heat resistance.

|
Figure 2 FTIR spectra of bio-based TPU samples. |

|
Figure 3 TGA thermograms of bio-based TPU samples. |

|
Figure 4 Derivative of weight according to temperature changes of bio-based TPU samples. |

|
Figure 5 DSC thermograms of bio-based TPU samples. |

|
Figure 6 Storage modulus of bio-based TPU samples. |

|
Figure 7 Loss modulus of bio-based TPU samples. |

|
Figure 8 Hardness value of bio-based TPU samples. |

|
Figure 9 Moisture infiltration of bio-based TPU samples. |

|
Figure 10 Heat reistance results of all the bio-basedTPU samples. |
Bio-based polyester polyol was synthesized by adipic acid and isosorbide with an esterification method, 4,4'-diphenylmethane diisocyanate (MDI) was used as polyisocyanate, 1,3-propanediol, 1,4-butanediol and isosorbide were used as chain extender, the bio-based PUs were synthesized with a ratio of 1:2.1:1 (polyol: polyisocyanate: chain extender), and only one TPU without extender was set as control group. The chemical structure of bio-PU products was confirmed by FTIR. The thermal decomposition behaviors of products were characterized by TGA and DSC. The mechanical properties (tensile strength, hardness value) and viscoelastic properties (storage modulus and loss modulus) also had been tested. The water resistance and hydrolysis resistance was tested. After that, the heat resistance of bio-TPU samples had been characterized by RPA-V1 with dynamic temperature sweep. From the results of all the test, the TPU 3 which used isosorbide as chain extender showed the best mechanical and viscoelastic properties in this research, and this bio-TPU material presented great water & heat resistance during the test. The bio-based TPU synthesized by liner chain extender 1,3-PD also showed great viscoelastic properties and heat resistance. The reason could be explained at the level of molecular structure: similar isosorbide structure with bio-polyester, and it improved the self-plasticizing effect, which appeared as better water and hydrolysis resistance of bio-based TPU. As for linear chain extender, the longer molecular chain of chain extender, the more chances to form chain entanglements, and the lower mechanical or viscoelastic properties of TPU materials.
- 1. A. Kausar, S. Zulfiqar, Z. Ahmad, and M. I. Sarwar, Polym. Degrad. Stabil., 95, 2281 (2010).
-

- 2. A. K. Mohanty, M. Misra, and L. T. Drzal, J. Polym. Environ., 10, 19 (2002).
-

- 3. A. Sienkiewicz and P. Czub, EXPRESS Polym. Lett., 11, 4 (2017).
-

- 4. Z. S. Petrović, I. Cvetković, D. Hong, X. Wan, W. Zhang, T. W. Abraham, and J. Malsam, Eur. J. Lipid Sci. Tech., 112, 97 (2010).
-

- 5. C. Hepburn, Polyurethane elastomers, Springer Science & Business Media, New York, 2012.
-

- 6. A. Soroudi and I. Jakubowicz, Eur. Polym. J., 49, 2839 (2013).
-

- 7. F. Hermanutz, P. Hirt, O. Oess, and W. Oppermann, U.S. Patent 6485665 (2002).
- 8. T. Harjunalanen and M. Lahtinen, Eur. Polym. J., 39, 817 (2003).
-

- 9. A. K. Mishra, D. K. Chattopadhyay, B. Sreedhar, and K. V. S. N. Raju, Prog. Org. Coat., 55, 231 (2006).
-

- 10. M. Charlon, B. Heinrich, Y. Matter, E. Couzigné, B. Donnio, and L. Avérous, Eur. Polym. J., 61, 197 (2014).
-

- 11. L. Imbernon and S. Norvez, Eur. Polym. J., 82, 347 (2016).
-

- 12. Q. Q. Sun, Z. K. Duan, H. Peng, and L. N. Li, Mater. Rev., 24, 469 (2010).
- 13. Z. S. Petrovic, W. Zhang, and I. Javin, Macromolecules, 6, 713 (2005).
-

- 14. S. Y. Choi and U. R. Cho, Elastomers Compos., 40, 249 (2005).
- 15. S. O. Hwang, B. H. Lee, and U. R. Cho, Elastomers Compos., 47, 238 (2012).
-

- 16. K. H. Jin, M. S. Kim, and U. R. Cho, Elastomers Compos., 48, 190 (2013).
-

- 17. K. H. Jin and U. R. Cho, Elastomers Compos., 49, 31 (2014).
-

- 18. M. Yu, Q. Xu, and G. Y. Wang, Polyurethane Industry, 21(2), 1 (2006).
- 19. Y. L. Jin, G. H. Wu, T. Zhao, and X. L. Tan, Technology & Economics in Petrochemicals, 30, 21 (2014).
- 20. M. J. Aman, H. Karauzum, M. G. Bowden, and T. L. Nguyen, J. Bio-mol. Struct. Dyn., 28, 1 (2010).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2019; 43(4): 621-628
Published online Jul 25, 2019
- 10.7317/pk.2019.43.4.621
- Received on Mar 29, 2019
- Revised on May 9, 2019
- Accepted on Jun 19, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ryong Cho
-
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea - E-mail: urcho@koreatech.ac.kr
- ORCID:
0000-0003-4866-8109














 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.