- Synthesis of a Coconut Oil-based Bioplasticizer and Its Effects on the Rheological and Fusion Properties of Poly(vinyl chloride)
Department of Polymer Science and Engineering, Chungnam National University, 99, Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
- 코코넛 오일 기반 바이오 가소제 합성 및 폴리염화비닐의 유변학적 및 용융학적 특성에 대한 영향
충남대학교 고분자공학과
Phthalate esters have been extensively used as plasticizers to improve the flexibility and processability of poly(vinyl chloride) (PVC) resin. However, the use of phthalate plasticizers is prohibited, and they have been replaced by non-phthalates due to the controversy over their harmful effects on the human body and the environment. Therefore, in this paper, a coconut oil-based bio-plasticizer (COBP) was synthesized via bioconversion of coconut oil using an enzyme catalyst. The rheological and fusion effects of the COBP on the PVC resin were compared with the effects of commercially available phthalate plasticizers in terms of processability, and a predictive model for correlating the mechanical and thermal properties was suggested.
프탈레이트 에스터계 가소제는 폴리염화비닐(PVC) 소재의 유연성과 가공성을 향상시키기 위해 광범위하게 사용되어 왔다. 그러나 인체 및 환경에 대한 유해성 논란으로 인해 사용이 규제되고 있으며, 친환경적이며 PVC 소재와의 가공성이 우수한 비프탈레이트계 가소제로의 대체가 증가하는 추세이다. 본 연구에서는 친환경적 가소제 개발을 위해 코코넛 오일 기반의 바이오 가소제를 생물학적 전환 공정에 의해 합성하였다. 합성된 가소제가 PVC 소재의 가공성 측면에서 유변학적 및 용융학적 특성에 미치는 영향을 상용 프탈레이트 가소제와 비교하였고, 기계적 및 열적 특성과의 상관관계에 대한 예측 모델을 제안하였다.
A coconut oil-based bio-plasticizer (COBP) was developed through bioconversion of coconut oil using an enzyme catalyst. The COBP has superior rheological flow and excellent fusion behavior compared to commercially available phthalate plasticizers.
Keywords: coconut oil based bioplasticizer, poly(vinyl chloride), rheological effect, processability, mechanical properties
This work was supported by research fund of Chungnam National University.
A plasticizer is a material that is incorporated into rigid and hard plastics to increase their flexibility, or elongation, and their workability. Addition of the plasticizer may decrease the elastic modulus, the melt viscosity or the glass transition temperature of the second-order transition.1 Over 90% of the plasticizers by volume are used in the PVC industry. The reason for such high usage with poly(vinyl chloride) (PVC) is that the benefits afforded to the plasticized PVC are far greater than those afforded to other polymers. PVC would rarely be used alone without the wide range of available additives, e.g., plasticizers, stabilizers, fillers, lubricants, and pigments. PVC is easily mixed with various additives to obtain a broad variety of material properties. The PVC offers not only a wide range of physical, chemical, and mechanical properties but also durability and cost effectiveness. All these properties allow PVC to be used in many applications, such as packaging, healthcare devices, toys, wall coverings, floorings, building materials and electrical wire insulation.2,3
Phthalate esters have been used as plasticizers in plastic materials since the 1920s. However, the world phthalate plasticizer market has recently decreased from 87% in 2005 to 80% in 2014.4 This reduction is the result of human and environmental hazard controversies. The harmful effects of phthalate exposure on health have been investigated in studies involving rodents and humans for more than 35 years.5-8 Phthalate esters have been reported to cause serious harm to human health, including changes in hormone levels, respiratory difficulties, reproductive malformations, chronic toxicities, and hepatic tumorigenesis.9-16 For these reasons, the European Union has restricted the use of several phthalate esters (less than C8), especially for PVC toys for young children.9 Korea has further limited the use of C9 and C10 phthalate plasticizers in addition to adhering to the EU regulations, according to the domestic laws and regulations trend data of KOVEC (Korea Vinyl Environmental Council).
Recently, given the increasing concerns about the fatal influence of phthalate esters, extensive research has been carried out to develop safer plasticizers with lower toxicity and migration rates. These plasticizers could replace petrochemical-based phthalate plasticizers for medical and other commodity PVC products because of their economic feasibility, technical advantages and environmental stability. Recently, there has been increasing interest in natural-based plasticizers derived from renewable and eco-friendly biomass. However, these epoxidized triglycerides prepared from natural resource such as soybean oil, linseed oil, castor oil, sunflower oil, glucose ester, and fatty acid esters (FAEs) are used more as lubricants, processing aids or antifogging agents than as plasticizers.17-25 Therefore, it remains a challenge to manufacture effective plasticizers with excellent performance, environmental stability and commercial availability in practical industry.
The aim of this study is to develop an environmentally friendly bioplasticizer to replace the current phthalate esters and to evaluate its effect on the rheological and fusion properties of PVC resin. The bioplasticizer is synthesized from edible coconut oil through bioconversion with lipase enzyme. The coconut oil-based bioplasticizer (COBP) was compared with commercially available phthalate plasticizers in terms of the processability, and a predictive model for correlation with the mechanical and thermal properties was suggested.
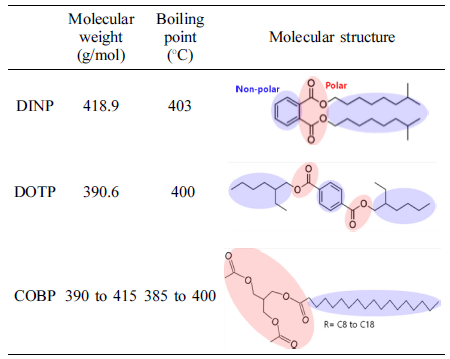
Materials. For the synthesis of COBP, the refined, bleached and deodorized (RBD) coconut oil, triacetin (food grade), and lipase catalyst were purchased from Dongsuh Co., Ltd. (Korea), Zhonglan Industry Co., Ltd. (China), and Novozymes Co., Ltd. (Denmark), respectively. The PVC resin with a K value of 75 was purchased from Hanwha Chemical Co., Ltd. (Korea). The commercial plasticizers, di-isononyl phthalate (DINP) and di-octyl terephthalate (DOTP), were supplied by LG Chemical Co., Ltd. (Korea). The heat stabilizer, LFX-910, was obtained from KD Chemical Co., Ltd. (Korea).
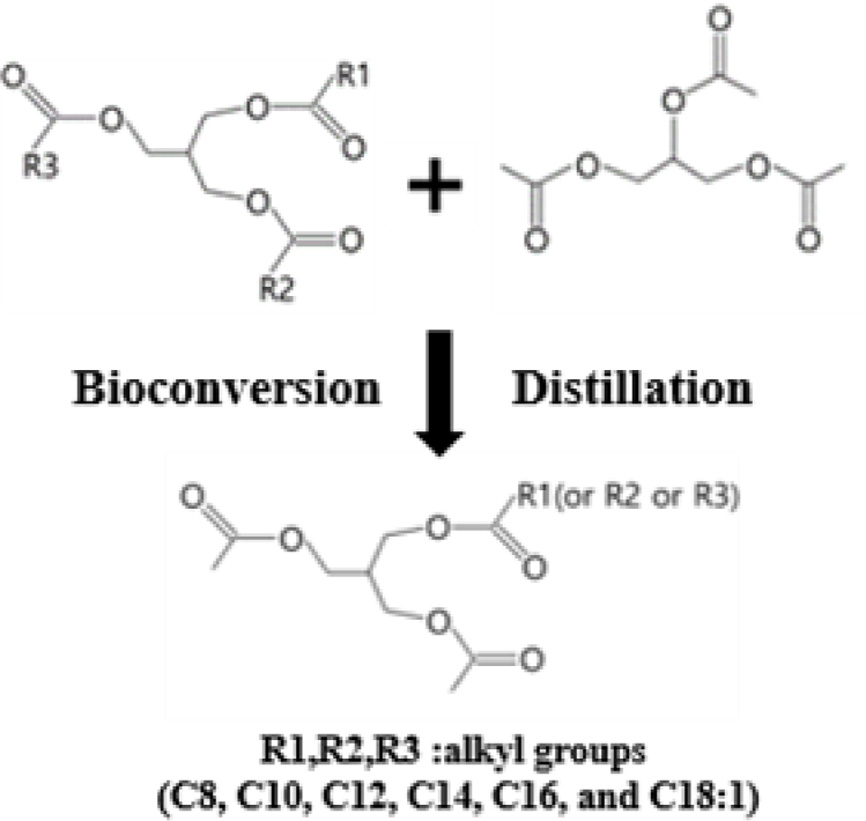
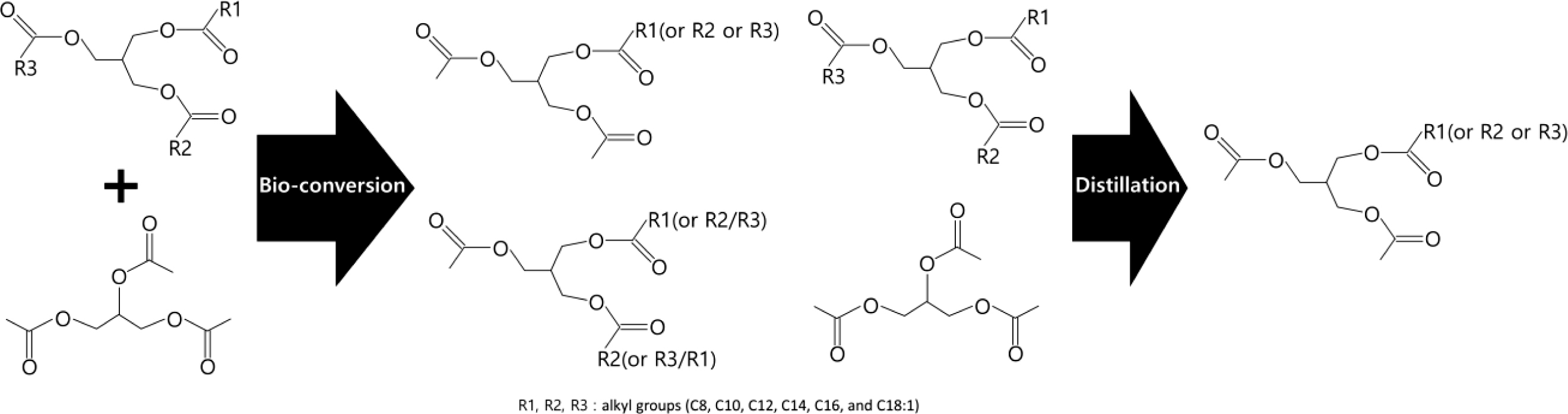
Synthesis of the Coconut Oil-based Bioplasticizer (COBP). The COBP was synthesized by the transesterification reaction of coconut oil and triacetin using lipase (Figure 1). The refined, bleached and deodorized (RBD) coconut oil (360 g) was mixed with triacetin (240 g) in a 1 L four-neck flask equipped with a mechanical stirrer, a thermostat, a vacuum pump, and a nitrogen inlet. The mixture of coconut oil and triacetin was preheated to approximately 100 ℃ with vigorous stirring under vacuum for 1 h to eliminate moisture. After purging with nitrogen, the mixture was kept at 40 to 45 ℃ for 30 min, and then the lipase (60 g) was added to initiate the reaction. The mixture was reacted under stirring at a low speed of 150 to 200 rpm for 1 h. After the reaction, the lipase was separated from the mixture by centrifugation, and the reaction product was transferred to a distillation apparatus. To obtain the desired composition of the monoglycerides, the unreacted triacetin and coconut oil, and other side products were sequentially separated by a series of distillation processes at 60, 120, and 150 ℃, respectively, under vacuum.
Preparation of the PVC Films. Three kinds of plastisols were prepared by mixing the dry PVC resin with three different plasticizers, COBP, DINP, and DOTP, in the standard proportion of 60 parts plasticizer and 3 parts heat stabilizer to 100 parts resin by weight. These mixtures were thoroughly blended using a laboratory-type mixer at speeds of 500, 700, and 1000 rpm sequentially for 20 min and then degassed for 30 min under vacuum (pressure ≤ 100 Pa). After one day of aging, the plastisols were spread on release papers. The coated release papers were heated in a vacuum oven at 210 ℃ for 2 min to gel and fuse. The PVC films were obtained by removing the release papers at room temperature.
Characterization. The COBP was identified using gas chromatography (GC) with a GC–FID HP7890A apparatus (Agilent Technologies, USA), Fourier transform infrared spectroscopy (FTIR) with a VERTEX 80v FTIR spectrometer (Bruker, Germany), and 1H NMR spectroscopy with an AVANCE III 600 spectrometer (Bruker BioSpin, Germany) at 600 MHz.
The rheological properties of the plastisols were examined using a HAAKE MARS-I Rheometer (Thermo Electron GmbH, Germany) with a cone and plate geometry (0.2 mm plate diameter) according to temperature factors. The effects of the plasticizers on the processability of the plastisols were estimated based on the rheological analysis. The viscosities of the plastisols were measured using a Brookfield (BF) viscometer at room temperature for 2 months.
The fusion properties of the plastisols were investigated using the HAAKE MARS-I Rheometer with a parallel plate geometry (0.2 mm plate diameter) under temperature-programmed conditions at temperatures from 20 to 200 ℃ for 20 min. The effects on the processability of the plastisols were predicted from the fusion behavior analysis. The analysis to correlate the processability with the mechanical and thermal properties was carried out. The Young’s modulus, tensile strength and elongation at break were determined at room temperature with a universal testing machine LS 1 (LLOYD, UK) at a constant rate of 500 mm/min by using a 20 N load cell according to ASTM D-638. The analysis of the glass transition temperature (Tg) was carried out on a differential scanning calorimeter (DSC) (SDT Q600, TA Instrument, USA) with a heating rate of 5 ℃/min from -20 to 120 ℃ under inert atmosphere. The analysis of the thermal stability was performed on a thermogravimetric analyzer (TGA) (Thermo plus EVO II, Rigaku Co., Japan) at a 10 ℃/min heating rate from room temperature to 600 ℃ under nitrogen flow.
|
Figure 1 Bioconversion process for the synthesis of COBP. |
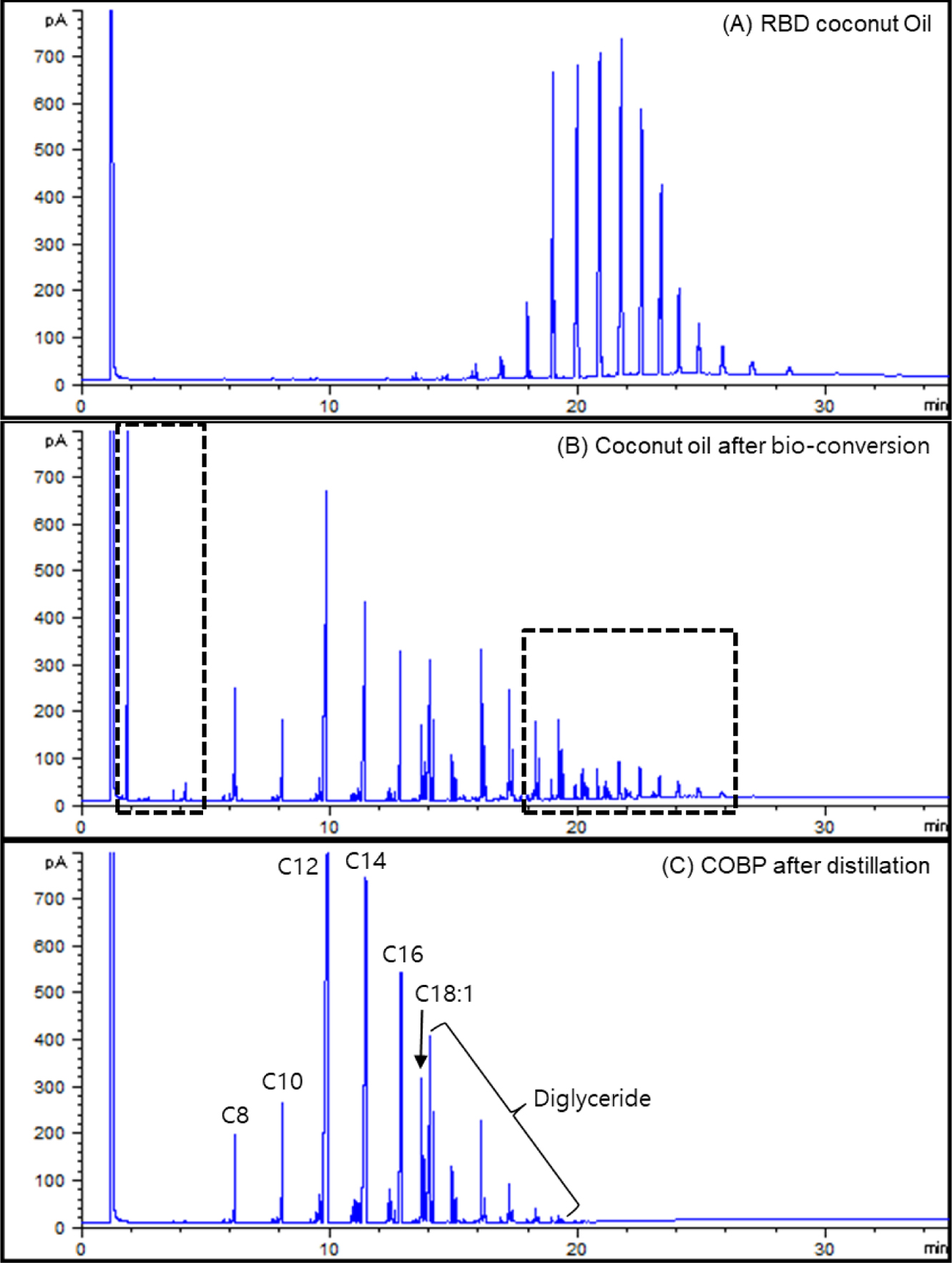
Synthesis and Characterization of the COBP. Gas Chromatography (GC-FID): Classical gas chromatography (GC) has been frequently used for the characterization of various edible oils.26-28 Figure 2 shows the GC-FID chromatograms of (A) the RBD-coconut oil, (B) the coconut oil after bioconversion, and (C) the COBP after distillation. Figure 2(A) shows the approximate concentrations of the fatty acids in the coconut oil as follows: 7% caprylic saturated C8, 8% decanoic saturated C10, 48% lauric saturated C12, 16% myristic saturated C14, 9% palmitic saturated C16, 6% oleic monounsaturated C18:1, and 6% polyunsaturated fatty acids and the other fatty acids.
The RBD coconut oil was converted to low molecular weight (fatty acids) mono-, di-, and triglycerides diversely through the bioconversion, as shown in Figure 2(B). Considering the compatibility and processability of PVC resin, the low molecular weight (less than C8) and high molecular weight (triglycerides) fatty acids marked by dotted lines in Figure 2(B) should be removed by the distillation process. Low molecular weight is considered the major factor that affects the problems of migration, thermal stability and mechanical properties in plasticized PVC. Additionally, high molecular weight compounds especially interfere with the fusion of PVC resin due to their bulky molecular structure and high molecular weight and further affect the mechanical and thermal properties of PVC film because of the tardy and incomplete fusion. As shown in Figure 2(C), the desired composition of the COBP, which consists of 85 to 90% monoglycerides and 15 to 10% diglycerides, was successfully obtained.
Fourier Transform Infrared (FTIR): The functional groups of the RBD coconut oil and COBP were analyzed by FTIR spectroscopy. The frequencies and intensities clearly confirm the presence of the relevant functional groups in the coconut oil and COBP. The various absorption bands of the spectra were assigned based on data given in the literature.29-31
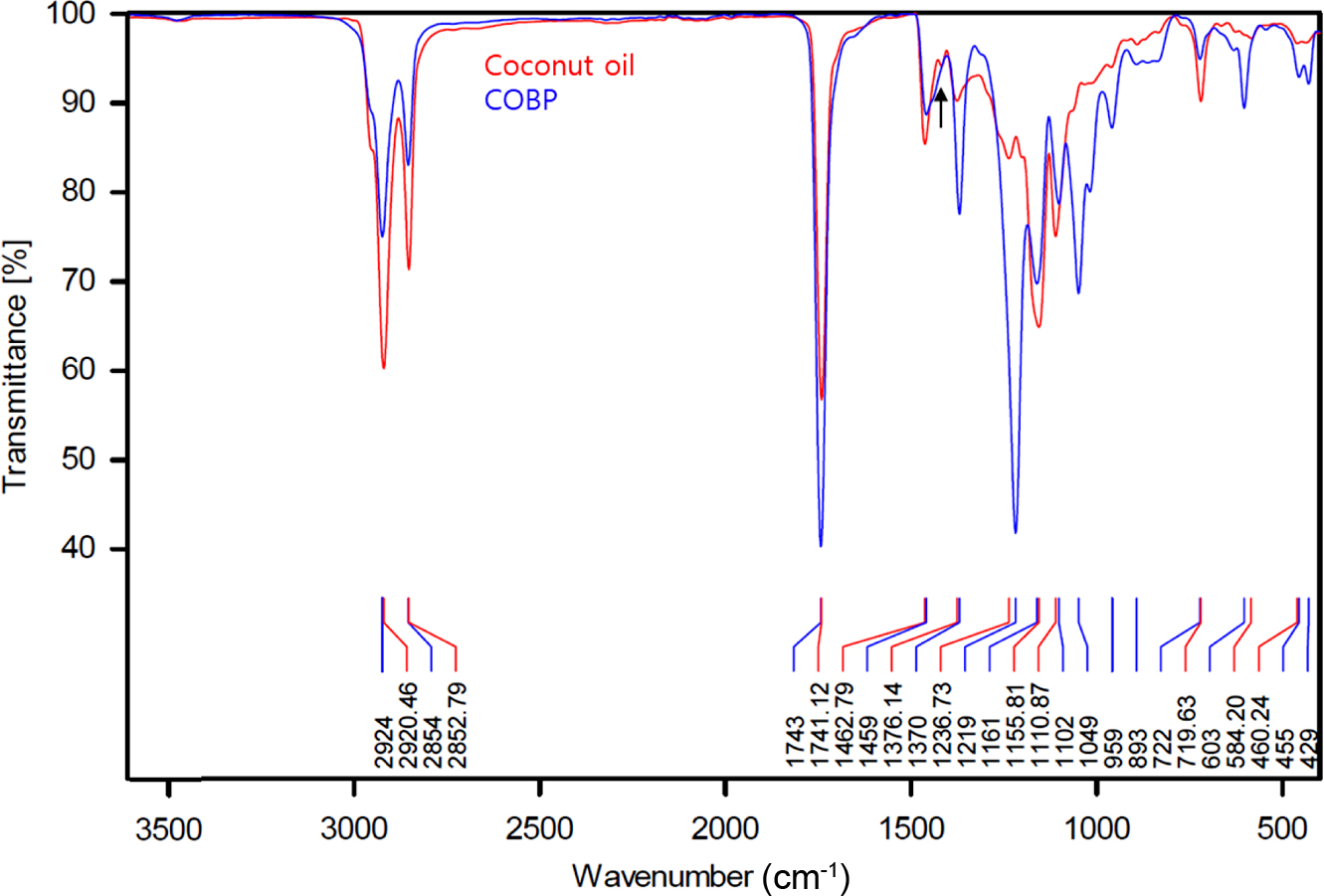
Figure 3 shows the FTIR spectra of the coconut oil and COBP. There are several indications that the triglycerides in the coconut oil were converted into mono- and di-glycerides in the COBP, namely, there were decreases in the peaks (~2925 and ~2854 cm-1) of the asymmetric and symmetric stretching vibrations of the C-H of the aliphatic CH2 groups; an increase in the peak (1741 and 1743 cm-1) of the stretching vibration of the ester carbonyl functional groups; an increase in the peak (1370 cm-1) of the bending symmetric vibration of the C-H bonds of the monoglyceride CH2 groups and a slight decrease in the peak of the bending vibration of the CH3 aliphatic groups in the peaks (1462 and 1459 cm-1); increases in the peaks (~1237 and ~1050 cm-1) of the stretching vibrations of the C-O ester groups; and finally, a decrease in the peak of the -(CH2)4- rocking vibration in the peaks (722 and 719 cm-1). These results suggest that the coconut oil, with high molecular weight constituents, successfully transformed into the COBP, with low molecular weight constituents, via bioconversion processes. Furthermore, there were no observed peaks corresponding to the carbonyl band (1704 cm-1) and the broad OH band (~3300 cm-1) of free fatty acids, which can cause various problems such as migration and decrease in thermal stability and processability.
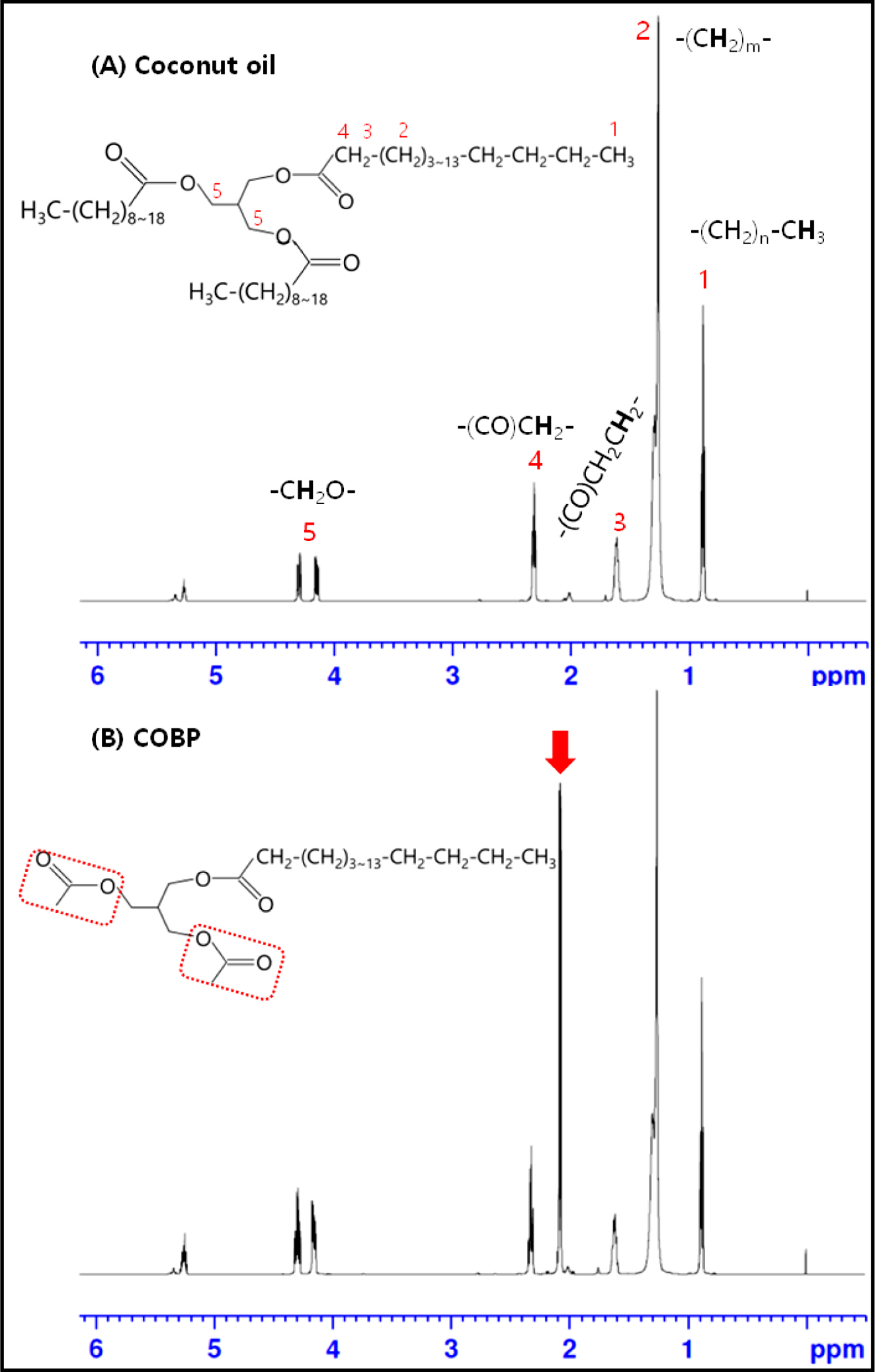
1H NMR Measurements: The chemical compositions of the RBD coconut oil and the COBP were characterized by 1H NMR spectroscopy. The presence of triglycerides in the coconut oil and monoglycerides in the COBP are traced in Figure 4. The characteristic peaks of the coconut oil are observed clearly in Figure 4(A). The two sharp peaks at 4.1 and 4.3 ppm were attributed to the methylene protons on the glycerol backbone. The peaks at ~2.3, ~1.6, ~1.3, and ~0.9 ppms were assigned to the proton of the methylene alpha to the carbonyl, the proton of the methylene group adjacent to the carbonyl, the proton of the methylene group adjacent to the methyl terminal, and the terminal methyl protons, respectively. The presence of unsaturated groups in the coconut oil was confirmed by the peaks at ~2.0 ppm (-CH2CH=CHCH2-) and 5.2 to 5.5 ppm (-CH=CH-). The characteristic peak of the COBP was observed at ~2.1 ppm, marked by the red arrow in Figure 4(B). Additionally, the absence of peaks at 3.5 and 3.8 ppm confirmed that there was no free glycerol. These results indicated that the COBP was successfully synthesized from the coconut oil by a series of bioconversions and distillation processes.
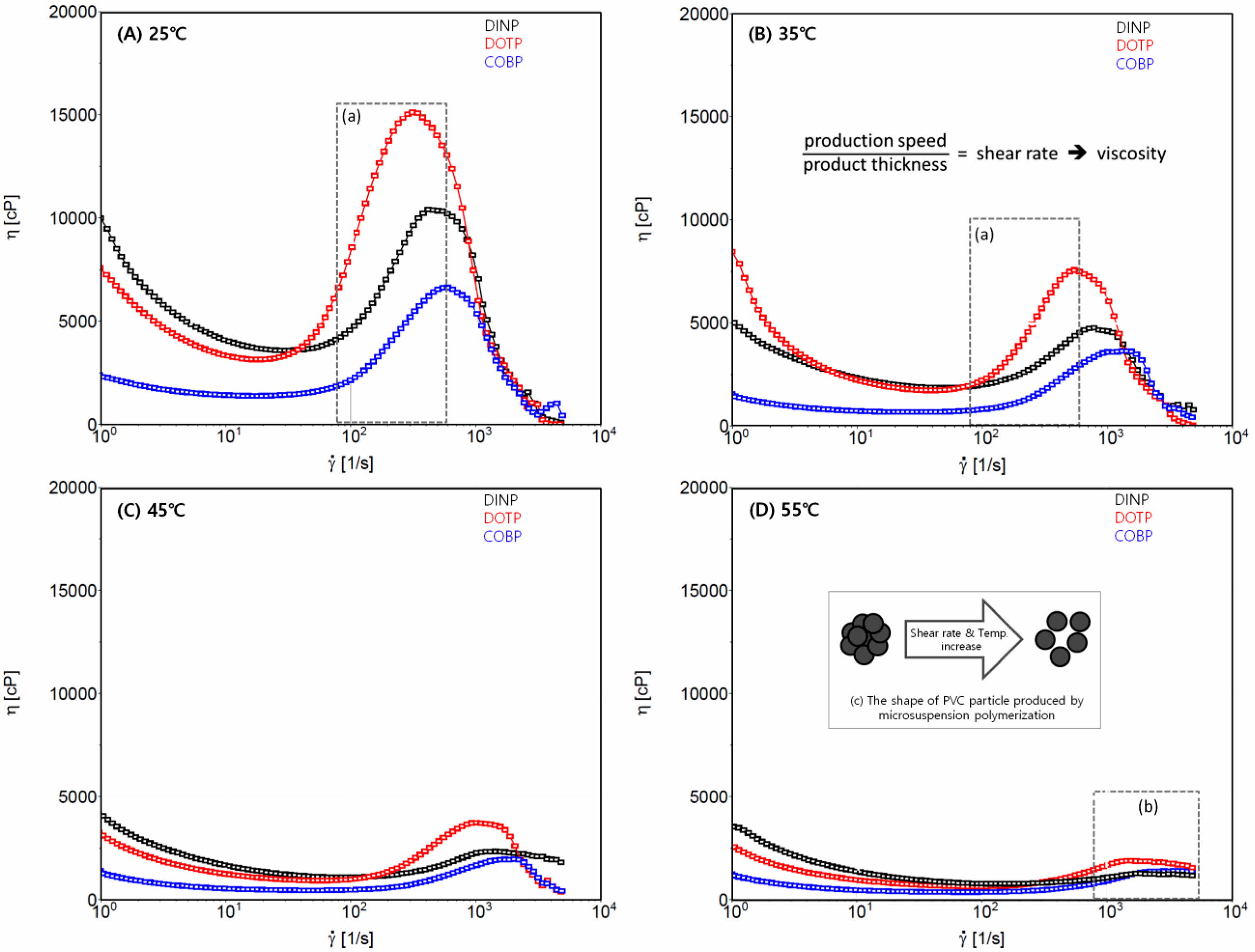
Rheological Behavior of the Plastisols. The rheological analysis of the plastisols provides much useful information for the estimation of the processability of plasticized PVC. The processability is one of the core criteria for determining whether a substance can be used as a plasticizer.32 Wilkes et al. described processability as the ease with which various processes combine the liquid plasticizer with the PVC resin to compound and form the end product. Figure 5 shows the rheological behavior of the plastisols at different processing temperatures (A) 25 ℃, (B) 35 ℃, (C) 45 ℃, and (D) 55 ℃. The dilatant and pseudoplastic characteristics were observed for three kinds of plastisols. The dilatant and pseudoplastic behaviors of all the plastisols significantly diminished as the processing temperature increased. Eventually, all the plastisols showed a behavior corresponding to a Newtonian flow at 55 ℃. These phenomena are mainly due to the morphology of the agglomerates of the PVC resin and the type of emulsifier used for the polymerization. The PVC resin used in this study was manufactured by microsuspension polymerization. The PVC agglomerates produced by the polymerization and subsequent drying and milling processes formed spherical shapes with diameters of 15 to 0.2 µm that consisted of primary particles, as shown in Figure 5(D)-(c). More importantly, the agglomerates contained holes of various sizes, which is a critical factor in the performance of the PVC resin and the rheology behaviors of the plastisol.35,36 Therefore, PVC particles with large pores gave rise to the rapid and easy absorption of the plasticizer and swelling of the agglomerates, resulting in the plastisol behaving like a dilatant. The subsequent plasticization steps brought about the disassociation of the PVC particles, which were loosened and transformed into primary particles and exhibited pseudoplastic and Newtonian flow. These phenomena were accelerated as the shear rate and processing temperature increased. Additionally, Newtonian behavior was demonstrated by the plasticizer acting as a buffer or lubricant between the primary PVC particles and the emulsifier used in the polymerization.
According to the rheological analysis of the plastisols, the COBP showed less dilatant and pseudoplastic flow than did the DOTP and DINP at low processing temperatures and more Newtonian-like flow at high temperatures, indicating that the COBP was more advantageous than were the DINP and DOTP in terms of processability. The Newtonian behavior related to the processability generally helps to achieve a usable plasticized PVC product. In addition, it is necessary to determine whether changes are needed in the processing conditions of the PVC plastisol for use in industrial applications.33,34 PVC products using plastisol are manufactured by coating and dipping processes. The former is commonly carried out with a production speed of 10 to 30 m/min and a product thickness of 0.2 mm at 25 to 30 ℃. The resultant shear rate is in the range from 83 to 416/s, as indicated in Figures 5(A) and 5(B). The latter is generally performed with a production speed of 1 to 5 m/min and a product thickness of 0.2 mm at 50 to 55 ℃. The resultant shear rate is in the range of 833 to 2500/s, as indicated in Figure 5(D). These indicate that the slight change in production speed can affect the shear rate, which causes a change in the viscosity and, subsequently, a variation in the product thickness. Eventually, undesirable consequences on the mechanical and thermal properties of the PVC film can result because of the deviation of the product thickness and the heat history (i.e., processing temperature). Thus, less dilatant and pseudoplastic flow and more Newtonian-like flow of the COBP are preferred to the contrary flow of the DOTP and DINP to prevent adverse processing properties.
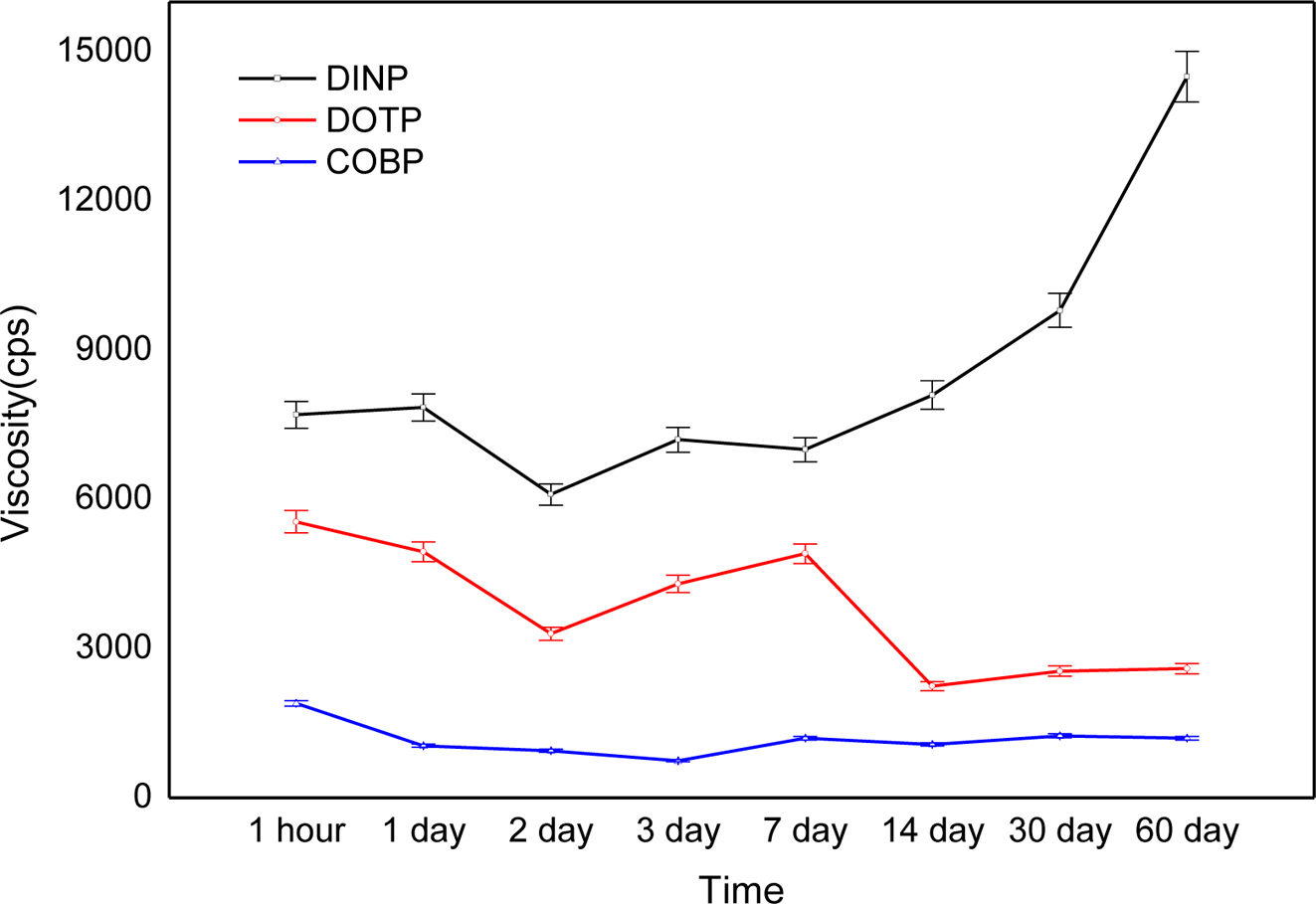
Figure 6 shows viscosity changes of the plastisols over a long period of time. The COBP had excellent short-term and long-term viscosity stability compared to that of the DINP and DOTP. In general, the viscosity changes noticeably, as the plastisol behaves like a dilatant or pseudoplastic flow. The plastisols using the DINP and DOTP showed similar viscosity changes until 1 week and displayed contrary results after 1 week. These changes were mainly due to the correlation between the polar-nonpolar balance, the interactions with negatively charged PVC particles, the molecular structure of the plasticizers and the morphology of the PVC particles. As shown in Table 1, the COBP had a typical surfactant structure, which can lead to “PVC particles in bio-based plasticizer emulsion” and therefore cause a small and uniform viscosity change. On the other hand, the DOTP and DINP had molecular structures that were different from the typical surfactant structure. Furthermore, the DOTP had a low molecular weight compared to that of the DINP and the COBP but had a relatively bulky molecular structure and benzene ring structure, known as the hard segment. Thus, the viscosity of the DINP increased with time, which can be described as a fast absorption rate into the PVC particles. The viscosity of the DOTP decreased with time, which brought about a phase separation due to the poor absorption rate. Similar results have been reported showing that the change of viscosity with time is related to the particle morphology of the PVC resin, the molecular size and shape, and the solvent power of plasticizer.37
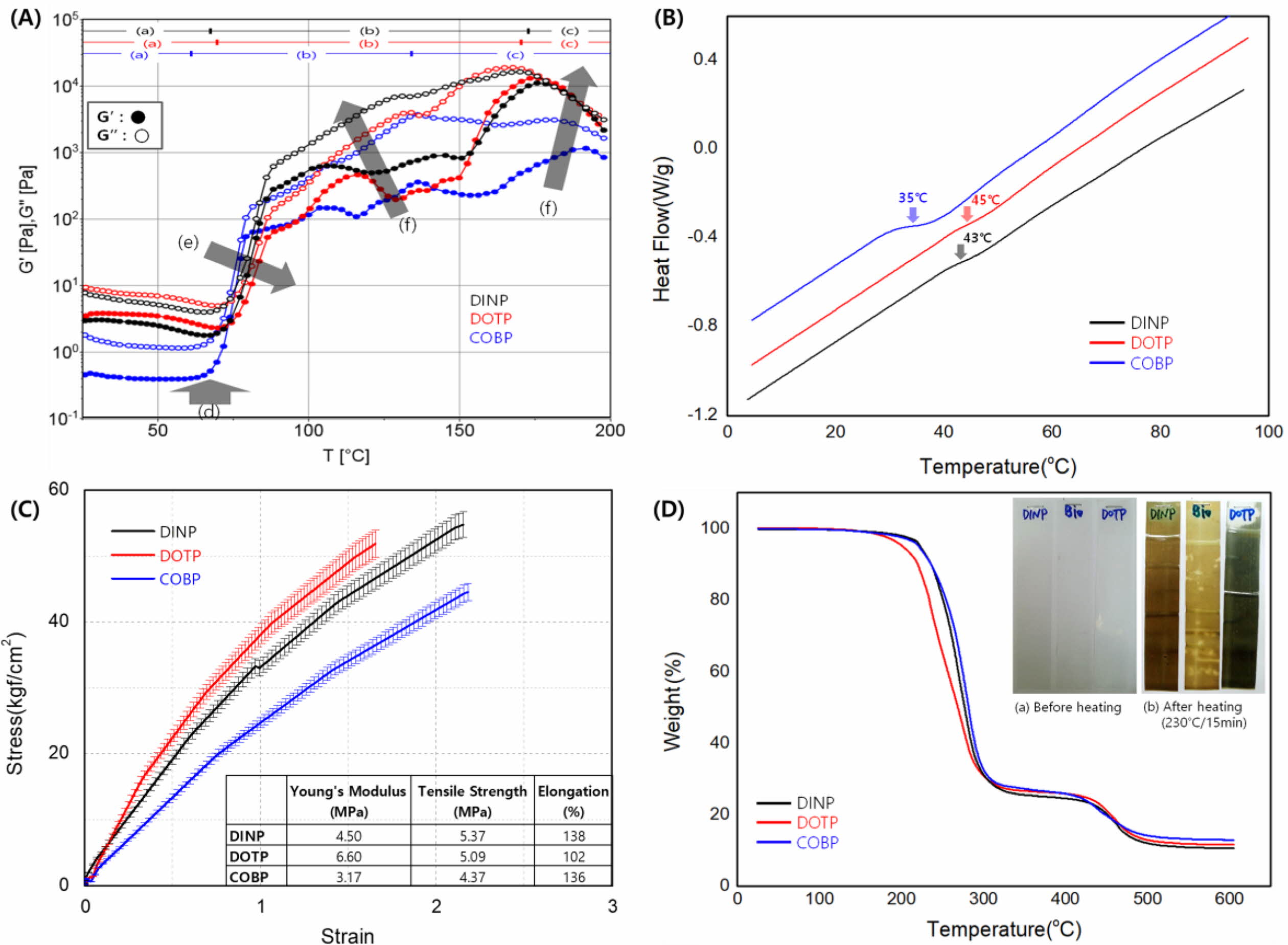
Fusion Behavior of the Plastisols. The processability requirements in PVC processing depend on its viscoelastic behavior, which is related to the gelation and fusion of the plastisols. The characterization method for the progress of continuous gelation and fusion over the temperature range of interest has been developed by using viscoelastic measurements.33,38-40 Figure 7 shows the viscoelastic behavior of the plastisols and correlated processing properties, providing practical information on the estimation of the mechanical and thermal properties of the plasticized PVC. In Figure 7(A), the range of (a) indicates the plastisol as an initial mixture of the PVC resin, plasticizer and thermal stabilizer. As the processing temperature increased, the liquid additives penetrated the PVC resins, which is defined as a gelation step (b) including the concept of swelling. Further temperature increases led to the dissolution of PVC resins, a fusion step (c).
As shown in Figure 7(A), there was a slight decrease in G' and G'' in the temperature range (a) due to the decreasing viscosity of the plasticizer as the plastisols were heated. The minimum value of G' corresponds to Tg, as indicated by the black arrow at (d), and the beginning of gelation. The values of Tg for the COBP, DINP and DOTP were approximately 52.5, 63.1 and 67.8 ℃, respectively. The values of Tg for the plastisols were confirmed by DSC analysis, as shown in Figure 7(B), and followed a similar trend. The COBP had the lowest Tg because of its soft and flexible molecular structure consisting of hydrocarbons, while the DINP and DOTP had relatively higher Tg values due to the hard benzene ring structure.
As the temperature increased further, the G' and G'' began to dramatically increase because the PVC particles swelled from the absorption of the plasticizer and induced gelation. The maximum of G' corresponded to the end of gelation and the beginning of fusion. The gelation characteristics and the plasticizer absorption rate are measures of the ability of a plasticizer to fuse with the polymer to give a product of maximum elongation and softness (i.e., maximum plasticization). The COBP had the fastest gelation rate, and the rates for the DOTP and DINP followed. Thus, the elongation of the COBP is expected to be the highest, followed by that of the DINP and DOTP. These results are consistent with those shown in Figure 7(C). This behavior is attributed to the soft and flexible segment of the hydrocarbon structure of the COBP and the long methylene chain that is likely to be transformed into an entanglement. The plasticizer absorption rate into the PVC particles decreased in the direction of the black arrow (e). The COBP had the fastest plasticizer absorption rate, and the rates for the DINP and DOTP followed. The plasticizer absorption rate is closely related to the thermal stability of the plasticized PVC. In general, the faster the plasticizer absorption rate is, the better the thermal stability is. Furthermore, the slope of G' and G'' shown in Figure 7(C)-(f) is an important indication of the thermal stability of PVC systems. The steeper the slope was, the more the thermal stability decreased. This trend occurred because the thermal stabilizer contributing to the thermal stability of the PVC system was quickly absorbed by the plasticizer. The thermal stability of the plastisols was confirmed by the weight loss indicated by the TGA analysis and isothermal heating test, as shown in Figure 7(D). In general, the sharp weight loss in the temperature range of 200 to 300 ℃ indicated the dehydrochlorination of the PVC resin. The color of the PVC sheet sequentially changed to yellow, red, brown and black as the thermal degradation proceeded during the isothermal heating. The decrease in thermal stability resulted in dehydrochlorination and discoloring simultaneously.
In the temperature range of fusion (c) in Figure 7(A), additional swelling and dissolution occurred through the interaction between the polymer and plasticizer molecules, and the G' and G'' began to decrease gradually. The fusion characteristics are a measure of the ability of a plasticizer to fuse with the polymer and cause further structural rearrangement (i.e., entanglement) that may improve its mechanical properties. The ease of the fusion of plastisols has been clearly connected to the polarity and solvating power of the plasticizer used. To achieve a useful plasticized PVC product, the polymer and plasticizer must be fully solvated or fused. In general, the mechanical properties of the PVC films increase in the direction of the black arrow (f) shown in Figure 7(A). The mechanical properties of the DOTP and DINP are expected to be higher than those of the COBP. The mechanical properties of the PVC films are shown in Figure 7(C) and followed a trend similar to those shown in Figure 7(A)-(f). The benzene ring, a hard and rigid segment of the DOTP and DINP, is attributed to the high Young’s modulus and tensile strength. However, the mechanical properties excluding elongation are generally not key requirements in soft PVC products. The Young's modulus is required to be as low as possible, and the tensile strength is required to be greater than approximately 4 MPa in most soft PVC products.
|
Figure 2 GC-FID chromatogram of (A) the RBD coconut oil; (B) the coconut oil after bioconversion; (C) the COBP after distillation. |
|
Figure 3 FTIR spectra of RBD coconut oil and COBP. |
|
Figure 4 1H NMR spectra of (A) coconut oil; (B) COBP. |
|
Figure 5 Rheological behavior of the plastisols according to processing temperatures: (A) 25 ℃; (B) 35 ℃; (C) 45 ℃; (D) 55 ℃. |
|
Figure 6 Viscosity changes of the plastisols over a long period of time. |
|
Figure 7 (A) Viscoelastic behavior of the plastisols and the correlated processing properties; (B) The measurement of Tg using DSC analysis; (C) The measurement of the mechanical properties using UTM; (D) The measurement of the thermal stability using TGA analysis. |
The COBP, as a new bioplasticizer, was successfully synthesized by the bioconversion of coconut oil with an enzyme catalyst. The rheological and fusion effects of the COBP on PVC resin were compared with those of commercially available phthalate plasticizers in terms of processability, and a predictive model for correlating the mechanical and thermal properties was suggested. The COBP has an outstanding rheological flow compared to that of DINP and DOTP. The plastisol with the COBP demonstrates Newtonian flow and excellent short- and long-term viscosity stability compared to those with DINP and DOTP; thus, it is more suitable for coating processes and dipping processes. The COBP also exhibits a fusion behavior that is superior to that of DINP and DOTP. The COBP demonstrates faster gelation and fusion characteristics and thus enhances thermal and elongation properties. As a result, the COBP is an appropriate material that can be used as an eco-friendly bioplasticizer to replace phthalate plasticizers, and its rheological and fusion behaviors can be used as a model for predicting the mechanical and thermal properties of plasticized PVC systems.
- 1. J. K. Sears and J. R. Darby, The Technology of Plasticizers, A Wiley-Interscience Publication, New York, 1982.
-

- 2. M. G. A. Vieira, M. A. da Silva, L. O. dos Santos, and M. M. Beppu, Eur. Polym. J., 47, 254 (2011).
-

- 3. H. Hosney, B. Nadiem, I. Ashour, I. Mustafa, and A. EI-Shibiny, J. Appl. Polym. Sci., 135, 46270 (2018).
-

- 4. 2015 IHS & ECPI Estimates (2015).
- 5. W. M. Kluwe, J. K. Haseman, J. F. Douglas, and J. E. Huff, J. Toxicol. Environ. Health, 10, 797 (1982).
-

- 6. J. D. Meeker, S. Sathyanarayana, and S. H. Swan, Philos. Trans. R. Soc. B Biol. Sci., 364, 2097 (2009).
-

- 7. R. Hauser and A. M. Calafat, J. Occup. Environ. Med., 62, 806 (2005).
-

- 8. G. Latin, Clin. Chim. Acta, 361, 20 (2005).
-

- 9. T. Schettler, Int. J. Androl., 29, 134 (2006).
-

- 10. Y. W. Kim, D. H. Kim, and J. Y. Bae, Polym. Korea, 42, 654 (2018).
-

- 11. T. Otake, J. Yoshinaga, and Y. Yanagisawa, J. Expo. Sci. Environ. Epidemiol., 14, 524 (2004).
-

- 12. I. Colón, D. Caro, C. J. Bourdony, and O. Rosario, Environ. Health Perspect., 108, 895 (2000).
-

- 13. Y. Zhao, H. Liang, D. Wu, J. Bian, Y. Hao, G. Zhang, S. Liu, H. Zhang, and L. Dong, Polym. Korea, 39, 247 (2015).
-

- 14. J. J. Adibi, F. P. Perera, W. Jedrychowski, D. E. Camann, D. Barr, R. Jacek, and R. M. Whyatt, Environ. Health Perspect., 111, 1719 (2003).
-

- 15. L. Oie, L. G. Hersoug, and J. O. Madsen, Environ. Health Perspect., 105, 972 (1997).
-

- 16. C. G. Bornehag, J. Sundell, C. J. Weschler, T. Sigsgaard, B. Lundgren, M. Hasselgren, and L. Hägerhed-Engman, Environ. Health Perspect., 112, 1393 (2004).
-

- 17. G. A. Pedersen, L. K. Jensen, A. Fankhauser, S. Biedermann, J. H. Petersen, and B. Fabech, Food Addit. Contam. Part A, 25, 503 (2008).
-

- 18. H. Baltacioglu and D. Balkose, J. Appl. Polym. Sci., 74, 2488 (1999).
-

- 19. J. S. Choi and W. H. Park, Polym. Test., 23, 455 (2004).
-

- 20. P. Li, Y. Wang, X. Mao, Y. Jiang, J. Liu, J. Li, J. Wang, R. Wang, J. She, J. Zhang, J. Yang, Y. Liu, and P. Liu, Polym. Test., 6, e322 (2017).
-

- 21. H. Pyeon, J. Park, and D. Suh, Polym. Test., 63, 375 (2017).
-

- 22. M. C. Sin, I. K. P. Tan, M. S. M. Annuar, and S. N. Gan, Int. J. Polym. Sci., Article ID 846189 (2014).
-

- 23. B. Sun, B. I. Chaudhary, C. Y. Shen, D. Mao, D. M. Yuan, G. C. Dai, B. Li, and J. M. Cogen, Polym. Eng. Sci., 53, 1645 (2013).
-

- 24. O. Fenollar, D. Garcia-Sanoguera, L. Sanchez-Nacher, T. Boronat, J. López, and R. Balart, Polym. Plast. Technol. Eng., 52, 761 (2013).
-

- 25. B. Yin, N. Aminlashgari, X. Yang, and M. Hakkarainen, Eur. Polym. J., 58, 34 (2014).
-

- 26. M. Tsimidou and R. Macrae, Food Chem., 25, 251 (1987).
-

- 27. M. Salivaras and A. R. McCurdy, in Food Flavors, Ingredients and Composition, Elsevier Science Publishers, Amsterdam, Netherlands, p 279 (1993).
- 28. T. Rezanka and H. Rezankova, Anal. Chim. Acta, 398, 253 (1999).
-

- 29. N. Vlachos, Y. Skopelitis, M. Psaroudaki, V. Konstantinidou, A. Chatzilazarou, and E. Tegou, Anal. Chim. Acta, 573, 459 (2006).
-

- 30. M. A. Moharam and L. M. Abbas, Afr. J. Microbilol. Res., 4, 1921 (2010).
- 31. O. Jović, T. Smolić, Z. Jurišić, Z. Meić, and T. Hrenara, Croat. Chem. Acta, 86, 335 (2013).
-

- 32. C. E. Wilkes, J. W. Summers, and C. A. Daniels, PVC Handbook, Hanser Publications, München, 2005.
-

- 33. B. Y. Yu and S. Y. Kwak, Annual Transactions the Nordic Rheology Society, 19 (2011).
- 34. D. Fenollar, D. Garcia-Sanoguera, L. Sanchez-Nacher, J. Lopez, and R. Balart, J. Mater. Sci., 45, 4406 (2010).
-

- 35. N. Nakajima and C. A. Daniels, J. Appl. Polym. Sci., 25, 2019 (1980).
-

- 36. N. Nakajima and D. W. Ward, J. Appl. Polym. Sci., 28 807 (1983).
-

- 37. N. Nakajima and E. R. Harrell, J. Colloid Interface Sci., 238, 105 (2001).
-

- 38. B. Y. Yu, A. R. Lee, and S. Y. Kwak, Eur. Polym. J., 48, 885 (2012).
-

- 39. J. Verdu, A. Zoller, and A. Marcilla, J. Appl. Polym. Sci., 129, 2840 (2013).
-

- 40. R. Jamarani, H. C. Erythropel, D. Burkat, J. A. Nicell, R. L. Leask, and M. Maric, Processes, 5, 43 (2017).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(5): 778-786
Published online Sep 25, 2019
- 10.7317/pk.2019.43.5.778
- Received on Jun 7, 2019
- Revised on Jul 19, 2019
- Accepted on Jul 21, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Kang Moo Huh
-
Department of Polymer Science and Engineering, Chungnam National University, 99, Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
- E-mail: khuh@cnu.ac.kr
- ORCID:
0000-0002-2406-6659










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.