- Preparation of Thermally Expandable Poly(acrylonitrile-co-methacrylonitrile) Microcapsules Using β-Cyclodextrin as Pickering Emulsifier
Department of Chemistry & Chemical Engineering, Inha University, 100 Inha-ro, Michuhol-gu, Incheon 22212, Korea
- β-Cyclodextrin을 피커링 유화제로 이용한 열팽창 Poly(acrylonitrile-co-methacrylonitrile) 마이크로캡슐의 제조
인하대학교 화학 및 화학공학 융합대학원
Thermally expandable
poly(acrylonitrile-co-methacrylonitrile) microcapsules were prepared by
Pickering suspension polymerization. β-Cyclodextrin (β-CD) and
polyvinylpyrrolidone (PVP) pair as the Pickering emulsifier was used as picking
emulsifiers for the first time. β-CD is low in toxicity, cheap, and can produce
particles with a relatively narrow particle size distribution. Acrylonitrile
(AN) and methacrylonitrile (MAN) were used as monomers. The effects of β-CD content, the ratio of β-CD/PVP
relative to the amount of monomer, AN/MAN ratio, and the various blowing agents were investigated for the
preparation and properties of microcapsules. Thermally expandable
microcapsules having a size of about 125 µm were produced. When β-CD : PVP was
2 : 1 at a total dispersant content of 3.5 wt%, the microcapsules had the
best expansion performance. TGA analysis confirmed that about 35% of the
hydrocarbons were encapsulated in the microcapsules. Finally, it was found that
β-CD serves as a good Pickering emulsifier in suspension polymerization
피커링 현탁 중합법을 통하여 열팽창성 poly(acrylonitrile-co-methacrylonitrile) 마이크로 캡슐을 제조하였다. 피커링 유화제로서 β-cyclodextrin(β-CD)과 polyvinylpyrrolidone(PVP)의 조합이 최초로
사용되었다. β-CD는 독성이 낮으며 경제적이며 상대적으로
좁은 입자 크기 분포도를 제조하는 것이 가능하다고 알려져 있다. Acrylonitrile(AN)과 methacrylonitrile(MAN)이 단량체로 사용되었으며, β-CD 함량, β-CD/PVP의
단량체에 대한 비율, AN/MAN 비율, 다양한 발포제의
효과에 대하여 입자의 제조 특성 및 마이크로캡슐의 물성에 대하여 조사하였다. 약 125 μm 입경의 캡슐이 제조되었으며, 2 : 1 β-CD : PVP 비율, 3.5 wt%의 피커링 분산제의 함량에서 제조된 캡슐이 가장 우수한 열팽창 성능을 보였다. TGA 분석 결과 약 35%의 발포제가 캡슐화되었다. 결과적으로 본 연구에서 사용된 β-CD는 현탁중합에 있어 우수한 피커링 유화제로서의 성능을 보였다
Thermally expandable microcapsules were prepared by Pickering suspension polymerization. β-Cyclodextrin (β-CD) and polyvinylpyrrolidone (PVP) pair as the Pickering emulsifier was used as picking emulsifiers for the first time. The effects of β-CD content, the ratio of β-CD/PVP relative to the amount of monomer, monomer ratio, the various blowing agents were investigated for the preparation and properties of microcapsules.
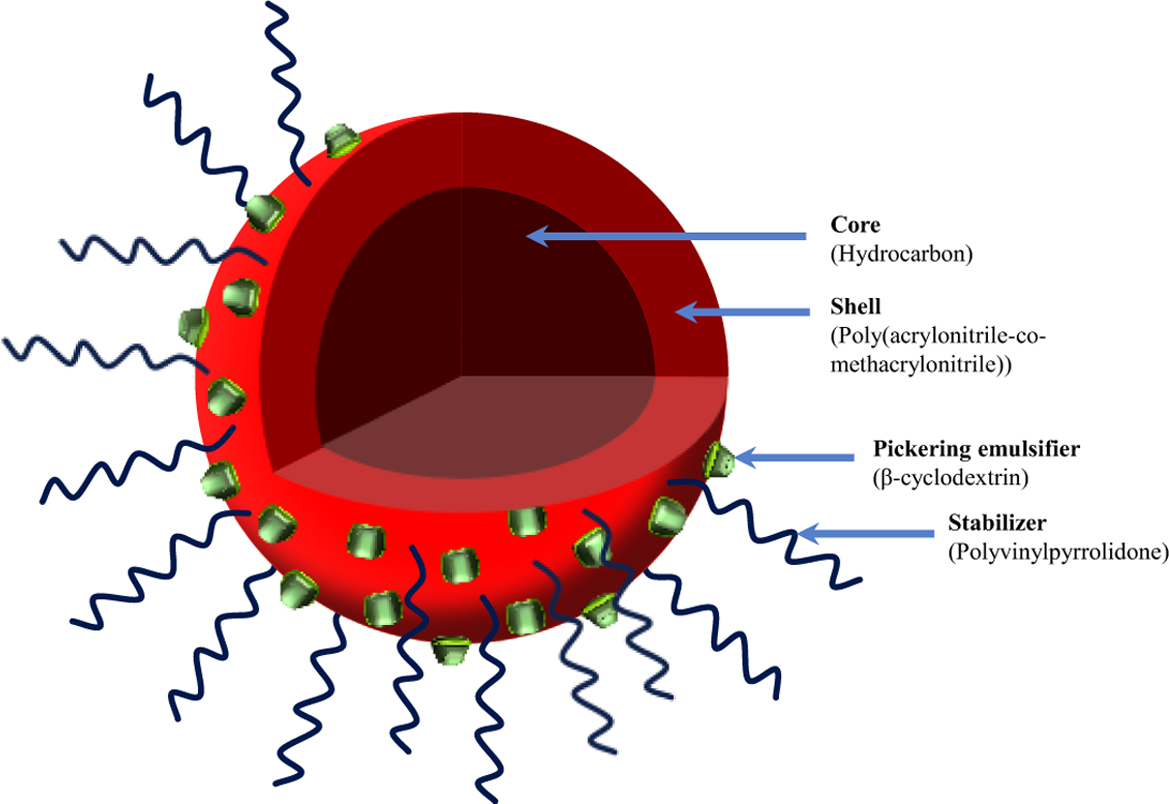
Keywords: expandable microcapsule, β-cyclodextrin, pickering emulsifier, suspension polymerization, poly(acrylonitrile-co-methacrylonitrile).
This work was financially supported by Inha University.
Microencapsulation is a procedure in which solids, liquids, or gasses are
surrounded by a layer of natural or synthetic polymers to provide
microcapsules.1 It is principally used to preserve unsettled or
sensitive functional materials, segregate a core from its surroundings, impede
leaching or volatilization risks of a volatile core, and enhance the handling
properties of an adhesive material.2-5 When the blowing agent is
encapsulated through in this process, the resulting product is called thermally
expandable microcapsule.6 When the temperature exceeds the
glass-transition temperature (Tg) of the shell composed of a
polymer, the shell is softened and the hydrocarbon is vaporized, causing the
microcapsule to expand.7,8 Thermally expandable microspheres are
used for the printing ink industries to improve the surface texture of textile
and wallpaper, and they are used in the automobile industry in underbody
coating, tires, gap filler, and adhesive debonding applications.9
In various heterogenous polymerizations such as emulsion and suspension
polymerization, Pickering emulsions are more stable opposed to coalescence than
conventional surfactants or protective colloids and can gain many useful
properties. The existence of Pickering emulsifiers at the interface between the
oil and aqueous phases affects not only the preparation of microcapsules, but
also the properties of resulting microcapsules, affording superior stability,
low toxicity, and stimuli-responsiveness compared to classical emulsions
stabilized by surfactants.10 Various inorganic and organic colloidal
particles with different sizes, shapes and surface chemistry have been studied
to prepare Pickering emulsions, including silica, clays, wax, layered double
hydroxides particles, carbon nanotubes, magnetic particles, and thermosensitive
particles like poly(N-isopropylacrylamide).11-18
Cyclodextrins (CDs), which can make surface active compositions at
oil-water interfaces, are attractive alternative emulsion stabilizers due to
the capability to form a host-guest inclusion complex.19-21 It was
suggested that such emulsions are a type of Pickering emulsion stabilized by
the self-assembled insertion complexes of CD–oil.22 CDs can be
tempting alternative emulsion stabilizers with probable use in food,
pharmaceutical and skin care due to their host-guest inclusion complex ability.23
CDs are biocompatible and nontoxic and previous studies have shown that CDs can
form surface active complexes at the o/w interfaces that can stabilize
emulsions.24
In this study, thermally expandable microcapsules were prepared by
suspension polymerization using β-CD as a Pickering emulsifier to stabilize o/w
emulsions for the first time. This study reports the preparation and properties
of thermally expandable microcapsules with a poly(acrylonitrile-co-methacrylonitrile)
(poly(AN-co-MAN)) using β-CD as a Pickering emulsifier. The effects of β-CD
ratio, stabilizer concentration, AN/MAN ratio, and several liquid hydrocarbons
were investigated for the preparation of the microcapsules.
Materials. Acrylonitrile (AN,
Aldrich, USA) and methacrylonitrile (MAN, TCI, Japan) were used as monomers.
1,4-Butanediol dimethacrylate (BDDMA, Aldrich, USA) was used as a crosslinking
agent. β-Cyclodextrin hydrate (β-CD; 99%, Samchun Chemicals, Korea) was used as
Pickering emulsifier. Polyvinylpyrrolidone (PVP K-30, Junsei, Japan) was
obtained. n-Octane, iso-octane, n-pentane, iso-hexane
from Daejung Chemicals (Korea) were used as blowing agents.
Azobis-isobutyronitrile (AIBN) was purchased from Junsei Chemical (Japan).
Sodium chloride (NaCl; 99.5%) and sodium nitrite (NaNO2; 98.5%) were
purchased from Samchun Chemicals (Korea).
Polymerization. To prepare the continues
phase, the β-CD powder was dissolved in deionized water with vigorous agitating
at 80 ℃. Subsequently, 0.5 g NaCl and 0.5 g NaNO2
were added to this mixture. The dispersion phase was prepared with monomers
consisting of AN and MAN, which was followed by the addition of hydrocarbon.
The dispersion phase mixture was added to the continuous phase and homogenized
with IKA Ultra Turrax T25 Basic Instruments at 9500 rpm for 2 min. The
homogenized mixture was polymerized in a high pressure reactor made of
stainless steel under agitation and nitrogen purging. The pressure was
regulated to the proper value and the reaction was carried out for 8 h.
After finishing polymerization, the particles produced were filtered and
rinsed, and then dried in a vacuum oven at ambient temperature for 24 h.
Characterization. The particle morphology was
observed by scanning electron microscopy (SEM, Hitachi, S-4300, Japan). The
particle size and particle size distribution were investigated using a laser
scattering particle size analyzer (PSA, Mastersizer2000, Malvern Panalytical,
USA). Prior to analysis, the particles were sonicated with distilled water in
small bottles for 5 min. Thermogravimetric analysis (TGA, TA Q50, TA
instruments, USA) was used to measure the encapsulated content of the blowing
agent. The samples were heated from 30 to 600 °C with 10 °C/min under
a nitrogen atmosphere. Thermomechanical analysis (TMA, TMA 800 system,
Instrument Specialists Incorporated, USA) was performed to monitor the
expansion properties such as the temperature of maximum expansion (Tmax)
and the displacement of microspheres volume upon heating. The samples were
heated from 25 to 250 °C with 10 °C/min under nitrogen.
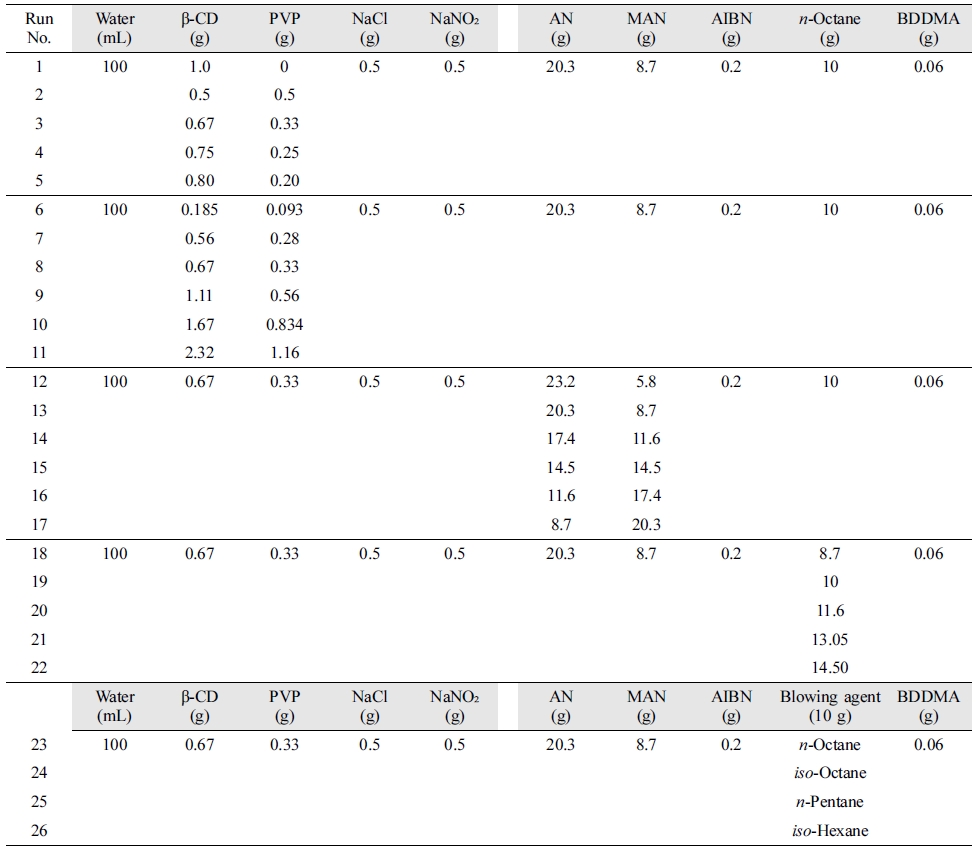
Table 1 shows the composition of thermally expandable microcapsules
prepared via Pickering suspension polymerization. To distinctly observe the
change of morphology and confirm the influence of β-CD/PVP ratio relative to
monomer on the microcapsules, the β-CD/PVP ratio relative to monomers was
investigated first (Table 1, sample 1-5). Then, the effects of the stabilizer
content were investigated at fixed β-CD/PVP ratio relative to monomers (Table
1, sample 6-11). MAN was used as co-monomer since it has a high coefficient of
thermal expansion.9 To enhance the expansion performance of the
microcapsules, AN/MAN ratio was investigated at a fixed stabilizer
concentration (Table 1, sample 12-17). After investigating the optimal amount
of blowing agent using n-octane (Table 1, sample 18-22), four
hydrocarbons were used to obtain thermally expandable microcapsules with
optimal expansion performance (Table 1, sample 23-26).
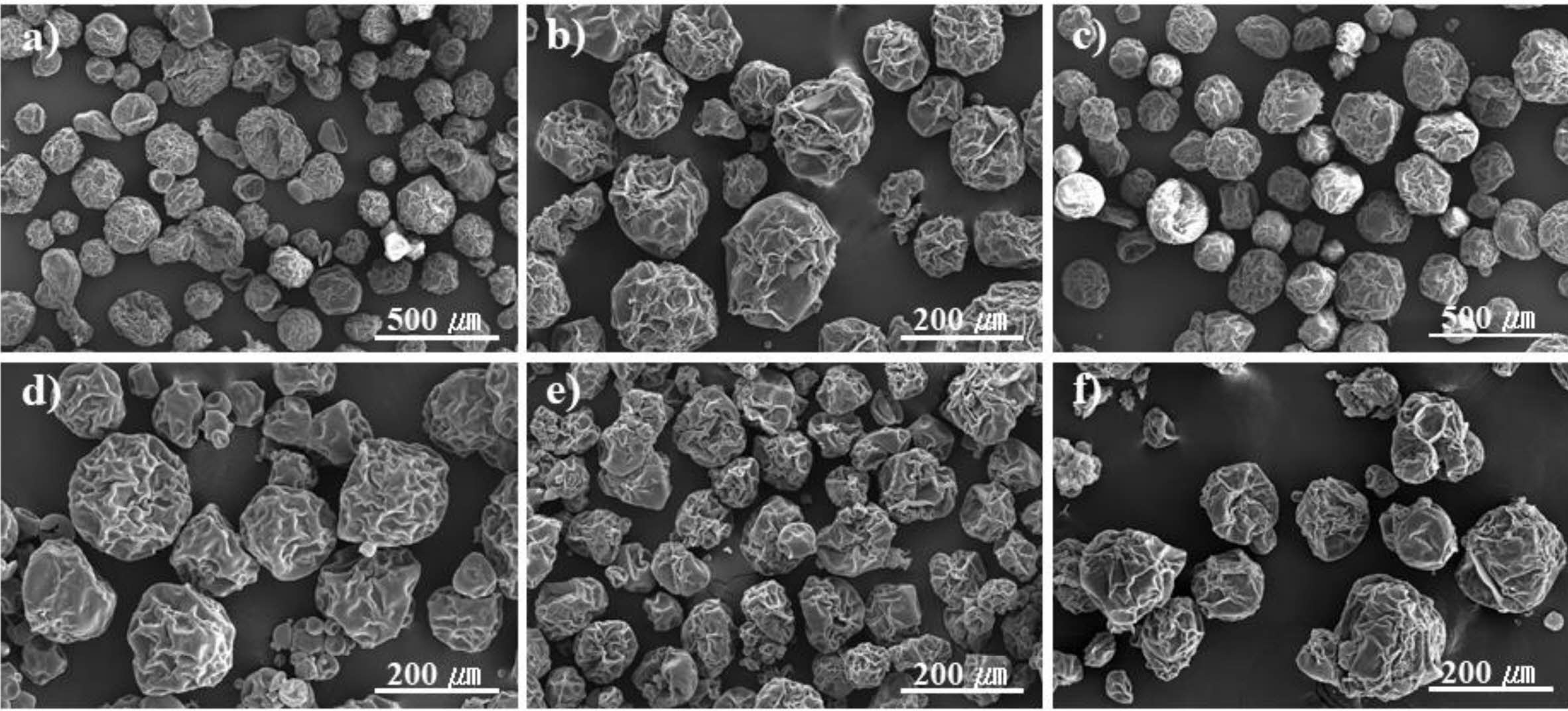
SEM morphology of thermally expandable microcapsule particles with
different β-CD and PVP concentrations is shown in Figure 1. After 8 h
polymerization, microcapsules are formed by using β-CD and PVP. The
size of the particles becomes smaller and tends to aggregate together as the β-CD
content increases. Above 2:1 wt% of β-CD/PVP relative to the amount of monomer,
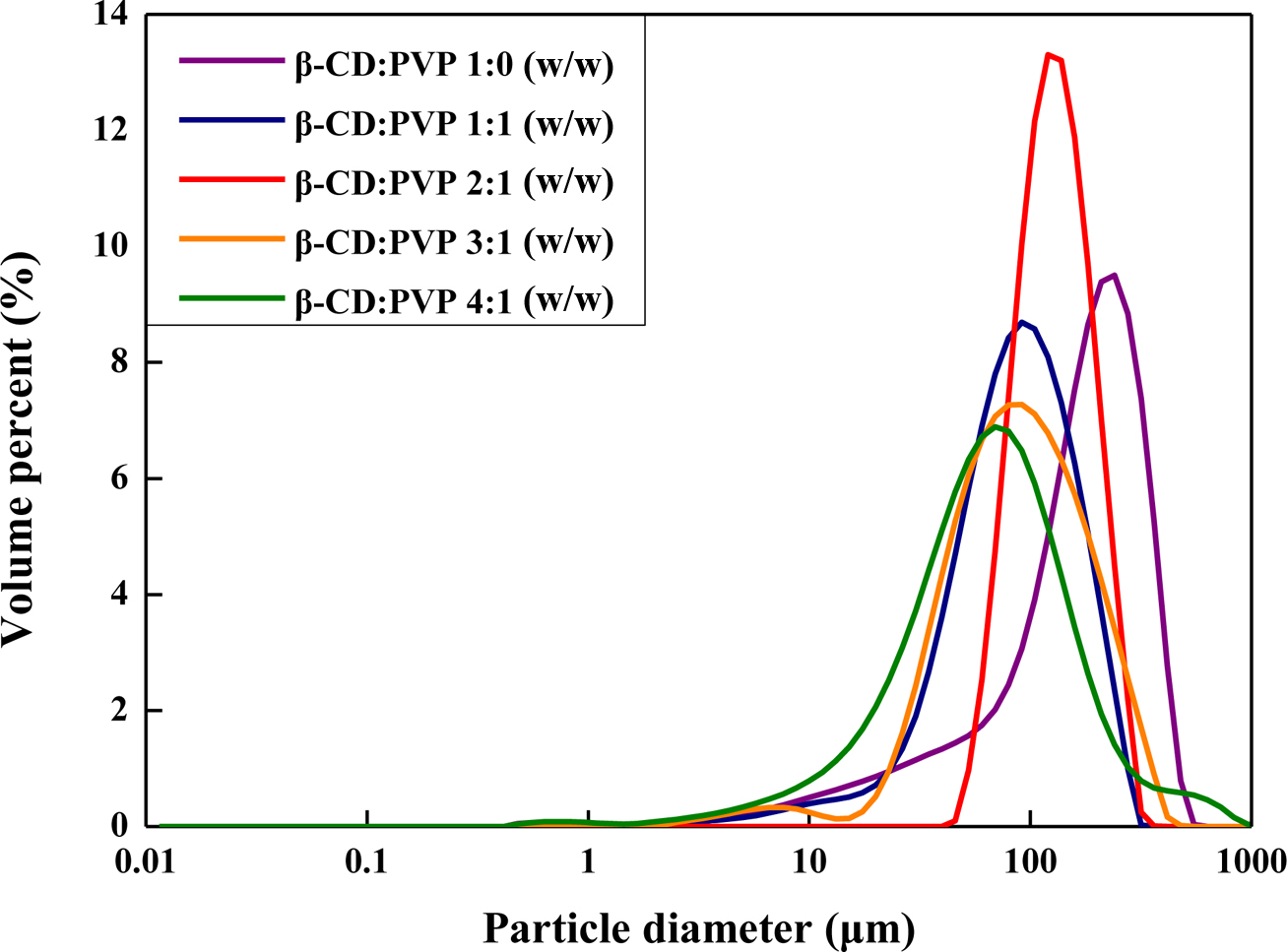
capsules begin to clump (Figure 1(c) and 1(d)). Figure 2 shows the particle
size distribution of expandable microcapsules prepared at different ratios of β-CD/PVP.
It indicates that the particle size of microcapsule decreases with increasing β-CD
contents. In this experiment, Pickering emulsion droplets are used as reaction
receptacle during suspension polymerization. Therefore, the initial emulsion
droplet leads to thermally expandable microcapsule particles.25
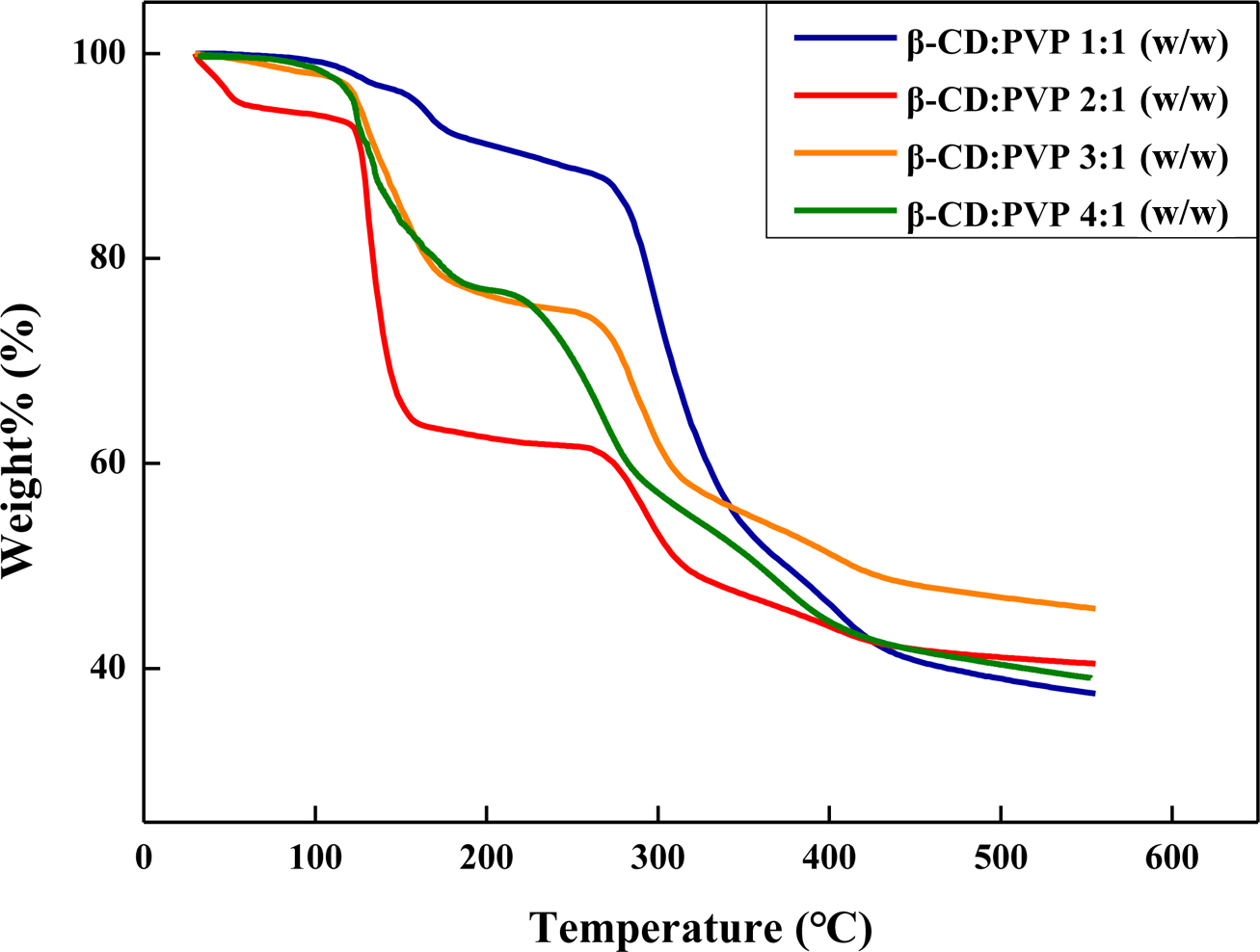
Figure 3 shows the TGA thermograms of the microcapsules prepared with a
different ratio of β-CD/PVP. Thermally expandable microcapsules have two-step
decompositions process. The first weight loss at around 125 ℃ is due to
the vaporization of the hydrocarbon blowing agent. And second weight loss is
ascribed to the thermal decomposition of the polymeric shell. When the
microcapsules are heated above the boiling point of n-octane, 125 ℃,
the polymeric shell starts to expand and finally blows up because the polymeric
shell cannot endure the internal pressure by vaporization of the hydrocarbon.26
When the ratio of β-CD/PVP was 2:1 wt/wt%, the largest amounts of
hydrocarbons was encapsulated. At low β-CD concentrations, the o/w emulsions
were so precarious that the droplets can be broken easily, and high β-CD
concentration resulted in the low encapsulation of the blowing agents. It seems
that as great amount of stabilizers cover the surface of the emulsion droplets,
they prevent the hydrocarbon from entering the core of the particles.
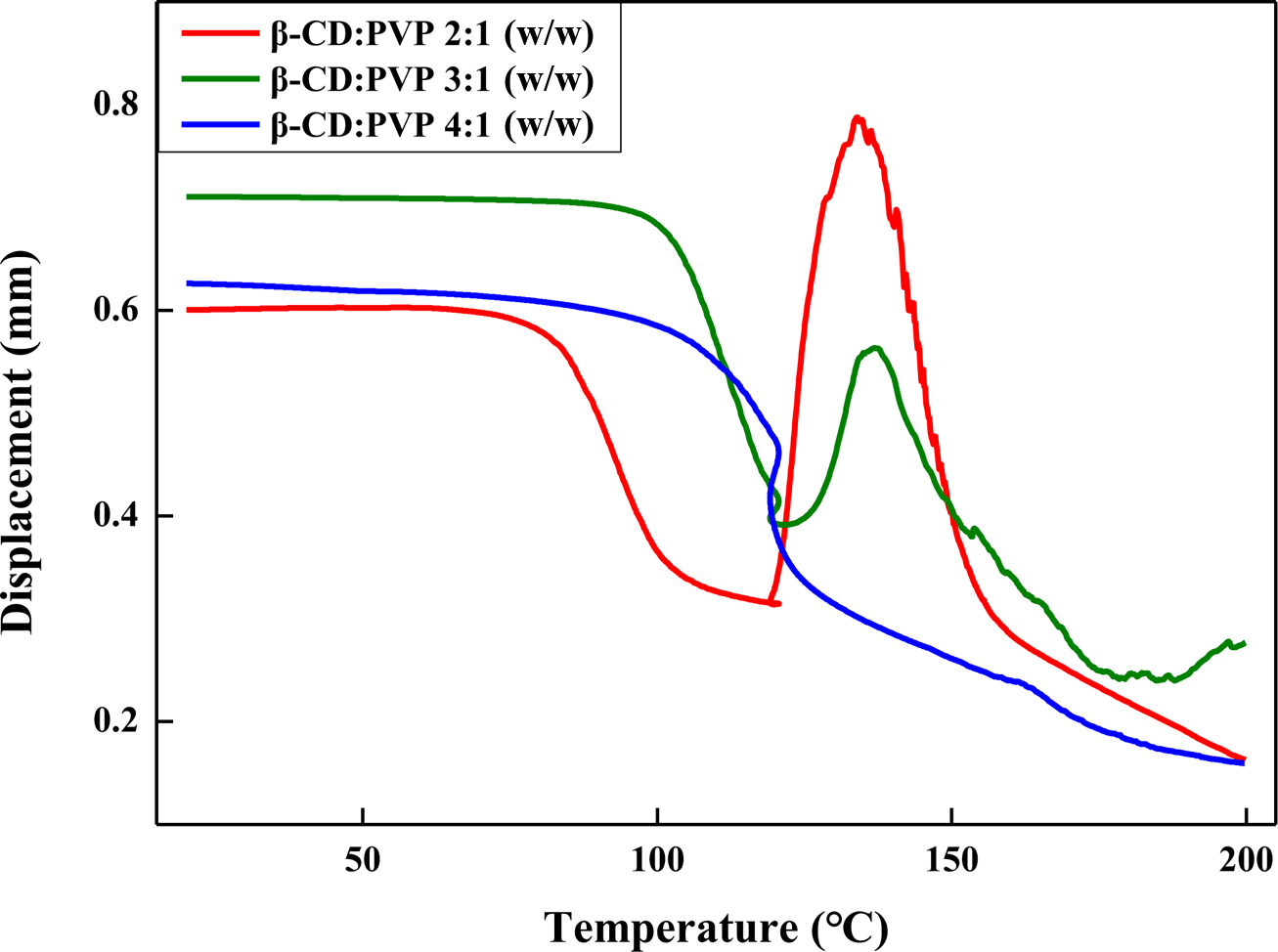
TMA thermograms indicate the expansion properties of microcapsules
prepared with different ratio of β-CD/PVP relative to the amount of monomer,
which show the individual characteristic for thermally expandable microcapsules
as the expansion performance. During heating the microcapsules, the polymeric
shells should endure the pressure of vaporized hydrocarbons. The start point of
expansion temperatures is between 100 ℃ and 150 ℃, which is associated
with the degradation starting temperatures obtained from TGA. As shown in
Figure 4, the TMA results have good expansion performance with microcapsules
composed of 2:1 β-CD/PVP. Therefore, subsequent experiments were studied with a
fixed 2:1 ratio of β-CD/PVP relative to the amount of monomer.
The effects of the stabilizer contents on thermally expandable
microcapsules were investigated at a 2:1 β-CD/PVP ratio. Different recipes for
this set of experiment are shown in Table 1 (sample 6-11). The morphology of
the particles in Figure 5 was not completely spherical in structure. As seen in
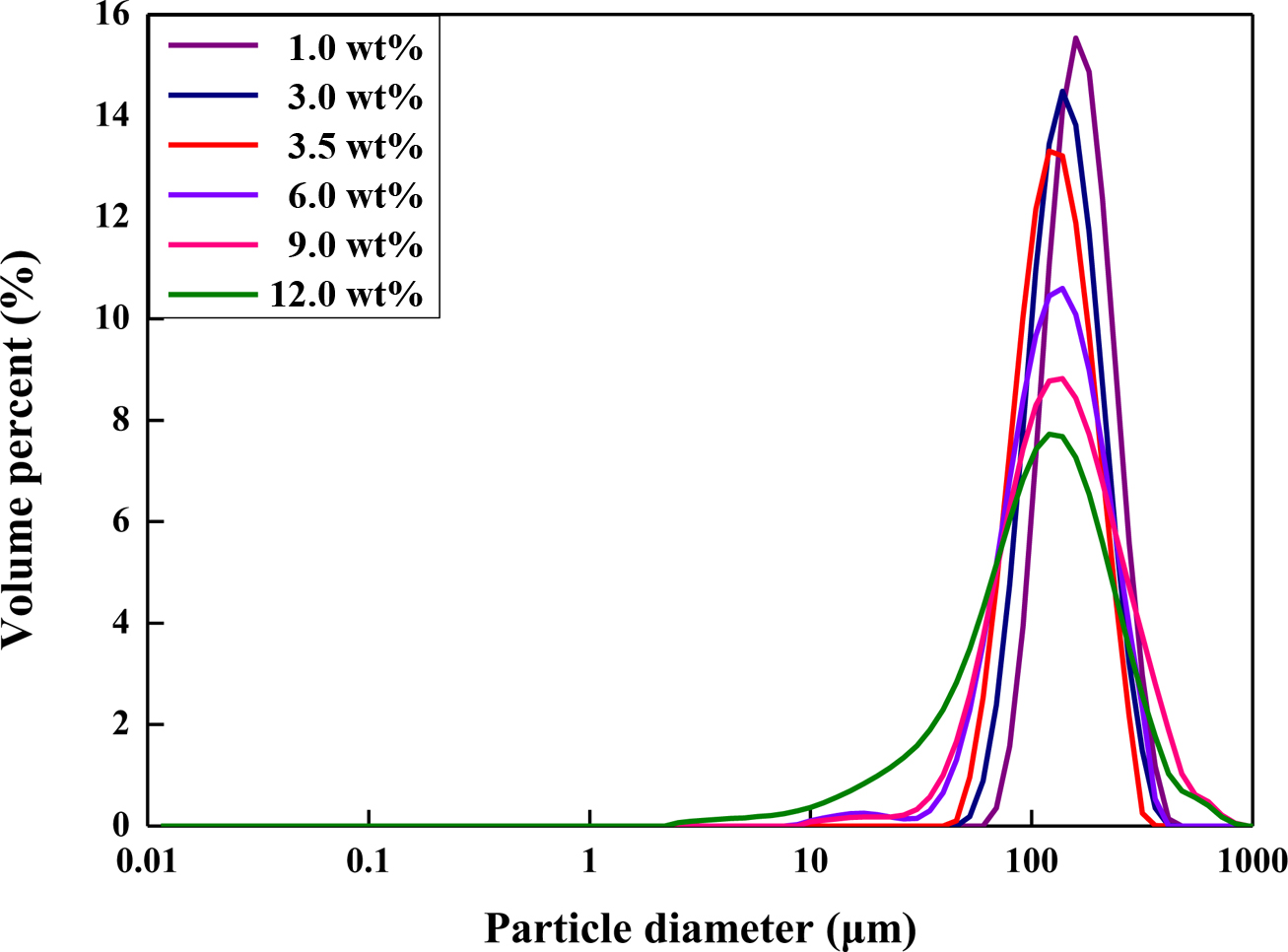
Figure 6, the range of particle diameters becomes broad. Also, the particle
size distribution peaks were shifted to the left with increasing amounts of
stabilizers, indicating that the decrease in particle sizes of microcapsules.
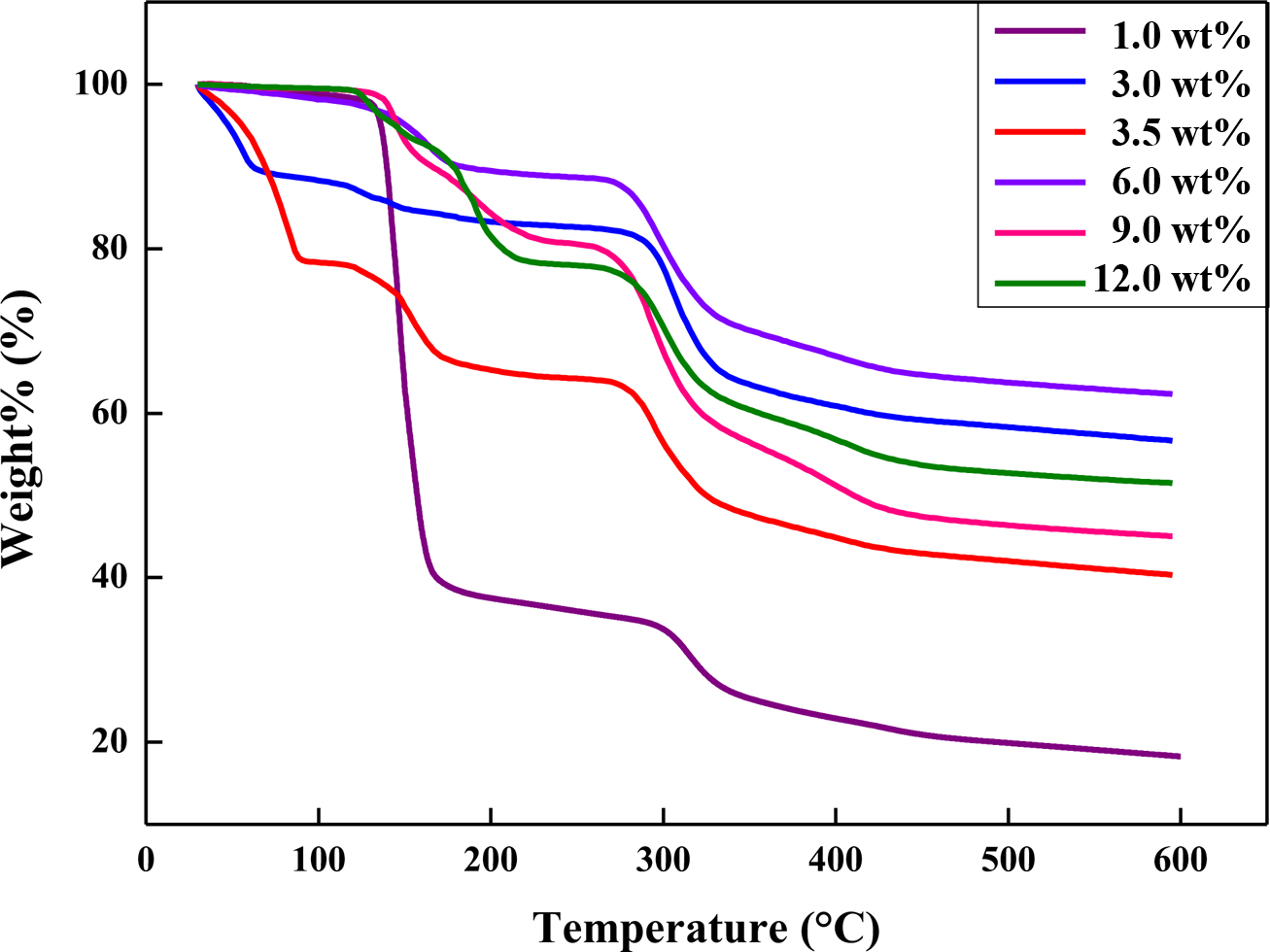
Figure 7 shows the TGA thermograms of the microcapsules prepared at different
contents of the stabilizer. When the contents of stabilizer were 1 wt%,
the amounts of the blowing agent encapsulated in the microcapsules were the
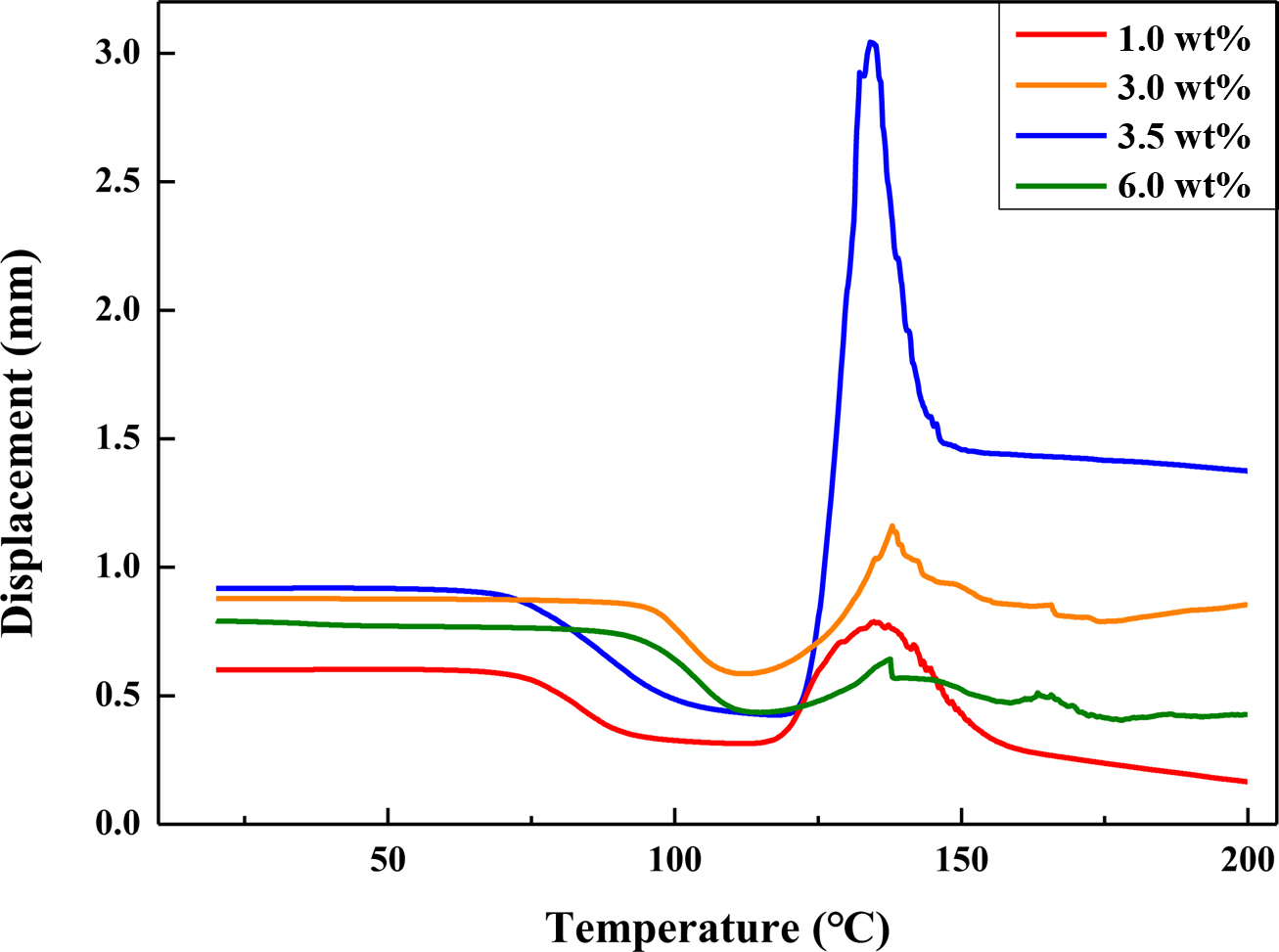
highest at about 60%. However, the TMA thermograms shows that the expansion
properties of microcapsules were dramatically increased by using 3.5 wt%
of stabilizer relative to the amount of monomer, which indicated the maximum displacement
of about 3.1 mm in Figure 8. Therefore, the optimum amount of stabilizer
for expansion performance was 3.5 wt%.
The influence of AN/MAN ratio was investigated at a fixed stabilizer
concentration, 3.5 wt% relative to the amount of monomer. The various
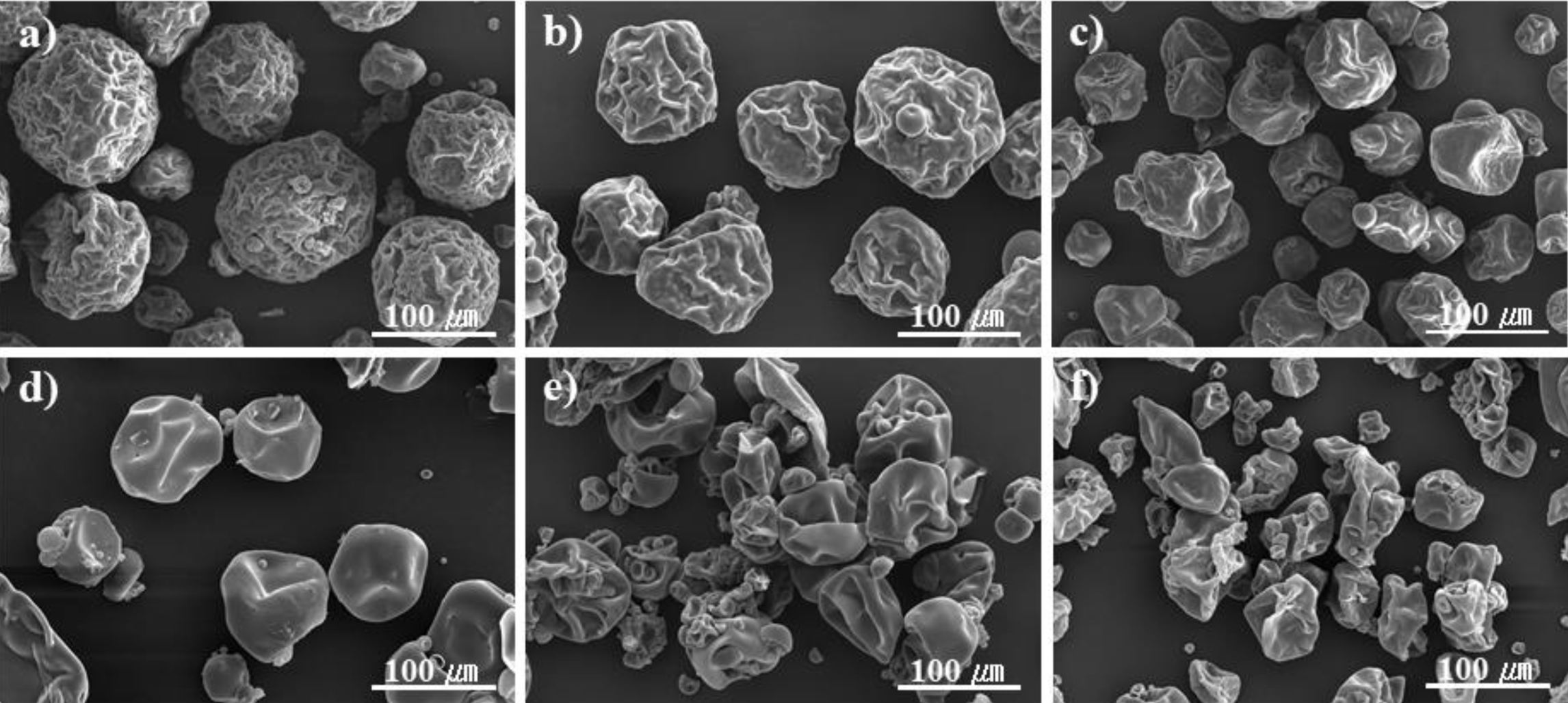
ratios are shown in Table 1 (sample 12-17). As shown in Figure 9, when the
ratio of AN is higher, it can be observed that small grains are attached to the
surface. This formation of small particles is due to the fact that monomers,
such as AN, which has low solubility in water would enter the continuous phase,
and secondary particles would form on the droplet surface during
polymerization.26 Methacrylonitrile (MAN) has a high thermal
expansion coefficient, and polyacrylonitrile (PAN) is famous for its excellent
gas barrier properties.27 However, adding an excess of MAN will not
encapsulate the hydrocarbons (Figure 9(e) and 9(f)). The diameter of the
microspheres increased as the AN portion became primary. Figure 10 shows the
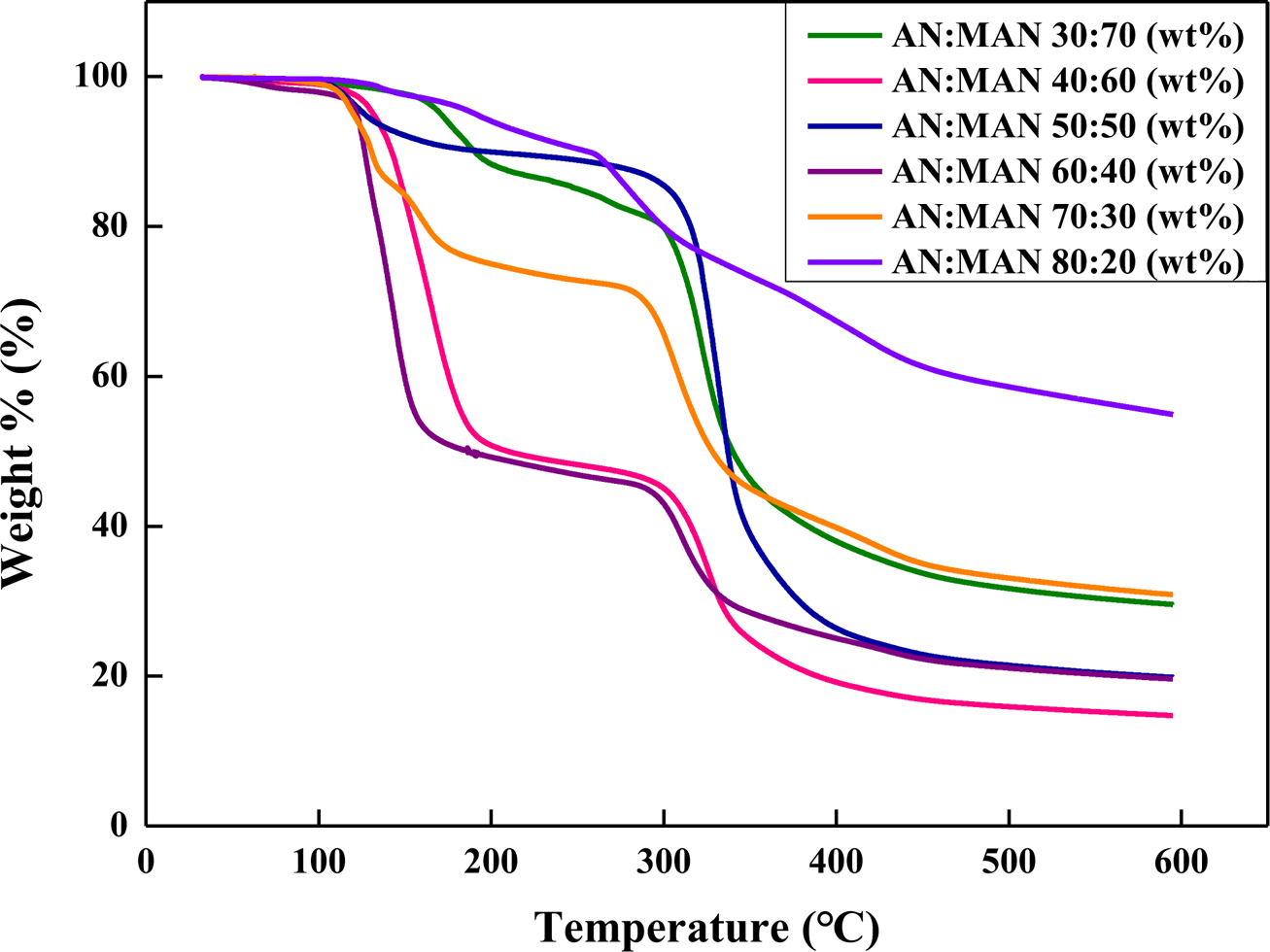
thermal decomposition phenomena of the microcapsules prepared at various AN/MAN
ratios. As the amount of AN decreases, the second decomposition temperature
decreases. The blowing agent vaporizes and increases in volume of the
microcapsules. The microcapsule expands because it pushes the shell out. The
optimum proportion of monomers can be found at AN:MAN 70:30 w/w.
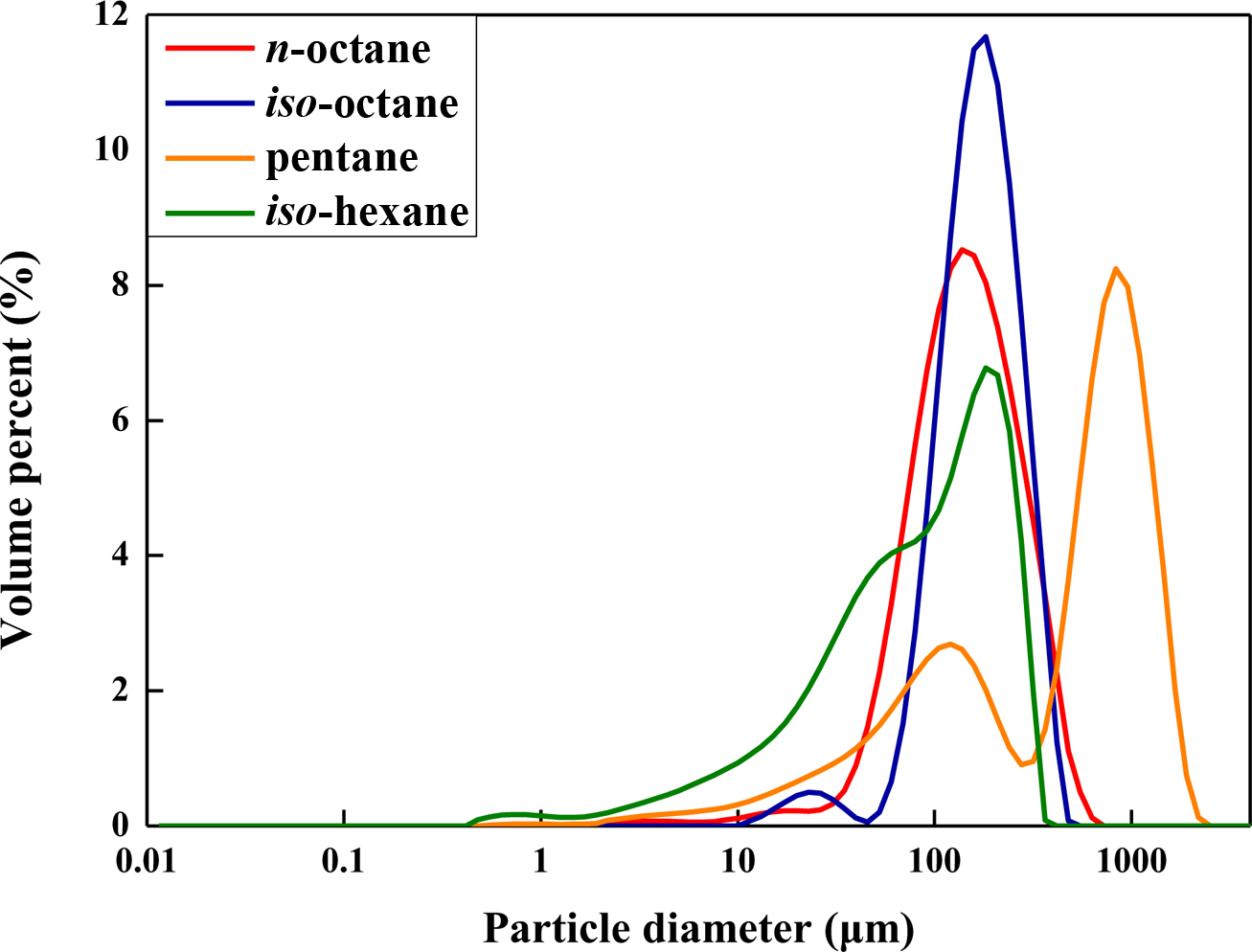
Finally, the microcapsules were compared by changing blowing agents. The
blowing agents used are n-octane, iso-octane, n-pentane,
and iso-hexane, respectively. Figure 11 shows the particle size
distribution according to the type of blowing agent. When using n-pentane
and iso-hexane, the capsules with unimodal size distribution were not
formed. Microcapsules with unimodal particle distribution were prepared using iso-octane
and n-octane. The average particle size of the microcapsules with n-octane
and iso-octane was about 125 and 156 µm, respectively. It is thought
that the different solubilities of core hydrocarbons into water and monomer
phases would cause the difference in particle size distribution. Figure 12
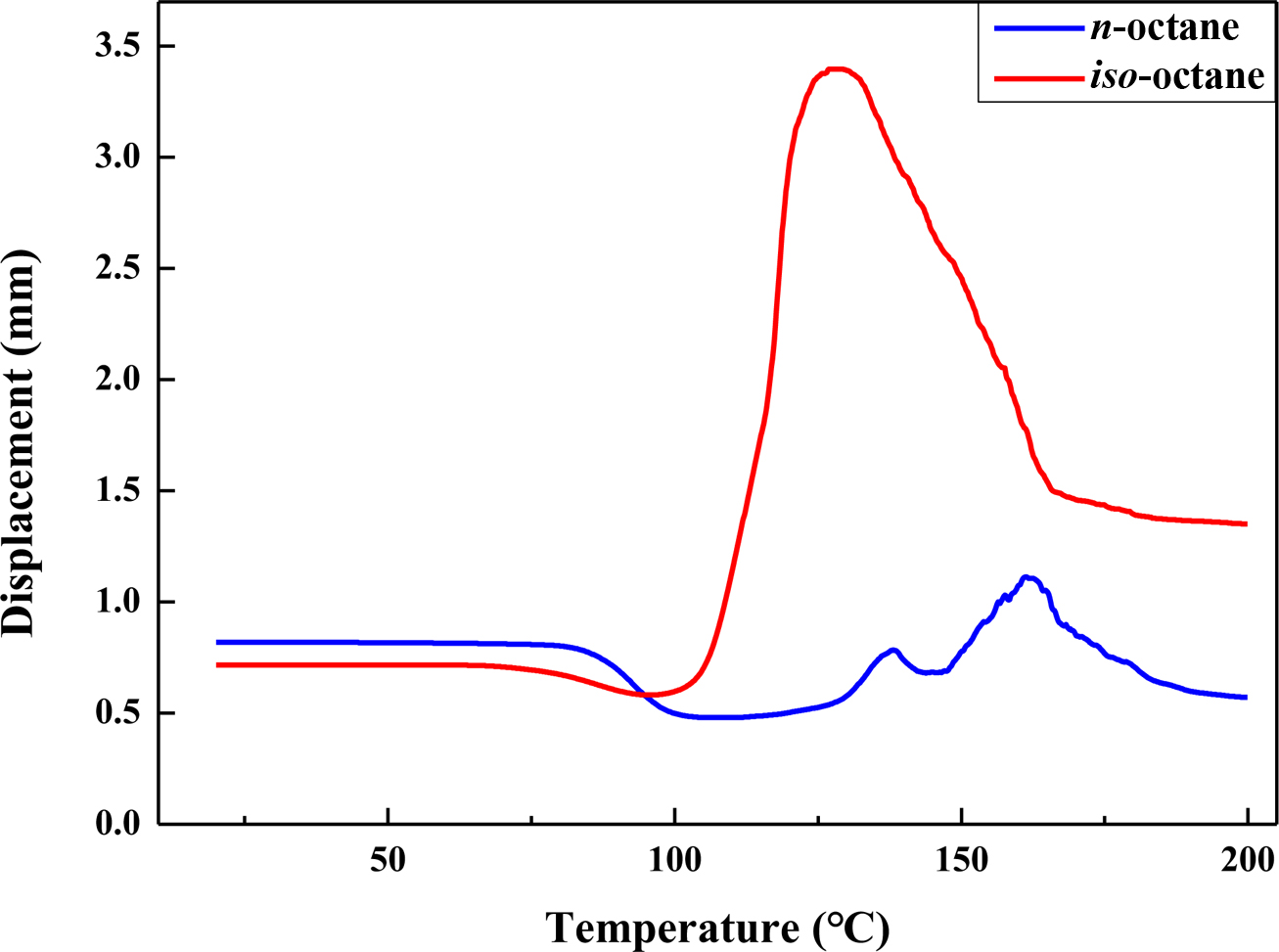
shows the maximum expansion temperature (Tmax) of the
microcapsules and the size after heating using iso-octane and n-octane.
As shown in Figure 12, the expansion performance of microcapsules using iso-octane
was the best. Figure 13 visually shows the expansion of the microcapsules
before and after expansion at 130 °C in which the microcapsules were
prepared using 2 : 1 (β-CD : PVP) ratio and total dispersant content of
3.5 wt% (Table 1, sample 24). The microcapsules have the best expansion
performance and the volume of the microcapsule after expansion is about 4 times
greater than that before expansion.

|
Figure 1 SEM microphotographs of expandable microcapsules prepared at different β-CD/PVP ratios: (a) 1:1; (b) 2:1; (c) 3:1; (d) 4:1 w/w. |
|
Figure 2 Particle size analysis of the expandable microcapsules prepared at different β-CD/PVP ratios. |
|
Figure 3 TGA thermograms of expandable microcapsules prepared at different β-CD/PVP ratios. |
|
Figure 4 TMA thermograms of the expandable microcapsules prepared at different β-CD/PVP ratios. |
|
Figure 5 SEM microphotographs of expandable microcapsules prepared at different contents of stabilizer. (a) 1.0; (b) 3.0; (c) 3.5; (d) 6.0; (e) 9.0; (f) 12.0 wt%. |
|
Figure 6 Particle size analysis of the expandable microcapsules prepared with different contents of stabilizer. |
|
Figure 7 TGA thermograms of expandable microcapsules prepared with different contents of stabilizer. |
|
Figure 8 TMA thermograms of the expandable microcapsules prepared with different contents of stabilizer. |
|
Figure 9 SEM microphotographs of expandable microcapsules prepared at different AN/MAN ratios: (a) 80:20; (b) 70:30; (c) 60:40; (d) 50:50; (e) 40:60; (f) 30:70 w/w. |
|
Figure 10 TGA thermograms of expandable microcapsules prepared at different AN/MAN ratios. |
|
Figure 11 Particle size analysis of the expandable microcapsules prepared at different type of blowing agent. |
|
Figure 12 TMA thermograms of the expandable microcapsules prepared with different type of blowing agent. |

|
Figure 13 Digital photographs of (a) unexpanded microcapsules; (b) expanded microcapsules (Table 1, sample 24) at 130℃. |
|
Table 1 Composition of Thermally Expandable Microcapsules via Pickering Suspension Polymerization |
In this study, we have succeeded in manufacturing thermally expandable
microcapsules of core-shell structure using Pickering suspension
polymerization. β-CD was used as Pickering emulsifier and PVP was used as an
auxiliary dispersant. Since the use of β-CD in the preparation of thermally
expandable microcapsules has not been reported to date, various experimental
parameters were investigated for the formation of microcapsules, including β-CD
: PVP ratio, stabilizer concentration, AN : MAN ratio, and types of blowing
agents. As the content of β-CD increased, the particle size decreased. At 2 : 1
(CD: PVP) ratio, the encapsulation was the most successful. When the total
dispersant content was 3.5 wt%, the microcapsules showed the best
expansion performance. The shape of the particles formed uniformly when the
ratio of AN/MAN was 70/30 w/w. The microcapsules were most suitably formed when
35 wt% of the blowing agent was added. The expansion performance also
varied depending on the type of blowing agent. Among the various blowing
agents, iso-octane and n-octane showed the excellent expansion
properties.
- 1. A. Kondo, Microcapsule Processing and Technology, Marcel Dekker Inc., New York, 1979.
- 2. S. Benita, Microencapsulation: Methods and Industrial Applications, CRC Press, Boca Raton, 2006.
- 3. R. Lu, H. Dou, Y. Qiu, D. Zhang, K. Sun, and Y. Zhang, Colloid Polym. Sci., 287, 683 (2009).
-

- 4. P. Griss, H. Andersson, and G. Stemme, Lab Chip., 2, 117 (2002).
-

- 5. Y.-D. Kim and C.-V. Morr, J. Agr. Food Chem., 44, 1314 (1996).
-

- 6. M. Jonsson, O. Nordin, A.-L. Kron, and E. Malmström, J. Appl. Polym. Sci., 117, 384 (2010).
-

- 7. Y. Kawaguchi and T. Oishi, J. Appl. Polym. Sci., 93, 505 (2004).
-

- 8. G. Vamvounis, M. Jonsson, E. Malmström, and A. Hult, Eur. Polym. J., 49, 1503 (2013).
-

- 9. M.-J. Rheem, H. Jung, J.-U. Ha, S.-H. Baeck, and S.-E. Shim, Colloid Polym. Sci., 295, 171 (2017).
-

- 10. J. Wu and G.-H. Ma, Small, 12, 4633 (2016).
-

- 11. A. Sadeghpour, F. Pirolt, and O. Glatter, Langmuir, 29, 6004 (2013).
-

- 12. R. Pichot, F. Spyropoulos, and I.-T. Norton, J. Colloid Interf. Sci., 377, 396 (2012).
-

- 13. C.-P. Whitby, D. Fornasiero, and J. Ralston, J. Colloid Interf. Sci., 323, 410 (2008).
-

- 14. C. Li, Q. Liu, Z. Mei, J. Wang, J. Xu, and D. Sun, J. Colloid Interf. Sci., 336, 314 (2009).
-

- 15. F. Yang, S. Liu, J. Xu, Q. Lan, F. Wei, and D. Sun, J. Colloid Interf. Sci., 302, 159 (2006).
-

- 16. H. Wang and E.-K. Hobbie, Langmuir, 19, 3091 (2003).
-

- 17. J. Zhou, L. Wang, X. Qiao, B.-P. Binks, and K. Sun, J. Colloid Interf. Sci., 367, 213 (2012).
-

- 18. S. Tsuji and H. Kawaguchi, Langmuir, 24, 3300 (2008).
-

- 19. M. Inoue, K, Hashizaki, H. Taguchi, and Y. Saito, J. Oleo Sci., 58, 85 (2009).
-

- 20. D. Duchêne, A. Bochot, S.-C. Yu, C. Pépin, and M. Seiller, Int. J. Pharm., 266, 85 (2003).
-

- 21. M. Inoue, K. Hashizaki, H. Taguchi, and Y. Saito, J. Dispers. Sci. Technol., 31, 1648 (2010).
-

- 22. K.-A. Shimada, K.-I. Kawano, J.-U. Ishii, and T.-A. Nakamura, J. Food Sci., 57, 655 (1992).
-

- 23. B.-G. Mathapa and V.-N. Paunov, Phys. Chem. Chem. Phys., 15, 17903 (2013).
-

- 24. J.-G. Kim, J.-U. Ha, S.-K. Jeoung, K. Lee, S.-H. Baeck, and S.-E. Shim, Colloid Polym. Sci., 293, 3595 (2015).
-

- 25. J.-W. Kim and K.-D. Suh, Polymer, 41, 6181 (2000).
-

- 26. G.-H. Ma, H. Sone, and S. Omi, Macromolecules, 37, 2954 (2004).
-

- 27. S.-M. Allen, M. Fujii, V. Stannett, H.-B. Hopfenberg, and J.-L. Williams, J. Membr. Sci., 2, 153 (1977).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(2): 240-247
Published online Mar 25, 2020
- 10.7317/pk.2020.44.2.240
- Received on Jan 20, 2020
- Revised on Jan 31, 2020
- Accepted on Jan 31, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sang Eun Shim
-
Department of Chemistry & Chemical Engineering, Inha University, 100 Inha-ro, Michuhol-gu, Incheon 22212, Korea
- E-mail: seshim@inha.ac.kr
- ORCID:
0000-0002-3678-6856









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.