- Noncovalent Functionalization of Single-walled Carbon Nanotubes Using Alkylated Zinc-phthalocyanine for the β-phase Formation of a Polyvinylidene Fluoride Matrix
Hoseung Son, Jae-Hoon Ji*, Jong Han Jeong, Seong-Hoon Han, Jung-Hyuk Koh*, and Jong S. Park†

Department of Organic Material Science and Engineering, Pusan National University, Busan 46241, Korea
*School of Electrical and Electronics Engineering, Chung-Ang University, Seoul 06974, Korea- 아연-프탈로시아닌에 의한 탄소나노튜브의 비공유 기능화와 이를 포함하는 폴리불화비닐리덴의 베타상 형성에 대한 연구
부산대학교 유기소재시스템공학과, *중앙대학교 전기전자공학부
We present the dispersion
properties of alkylated zinc-phthalocyanine (R-ZnPc) for single-walled carbon
nanotubes (SWCNTs), which finds useful for the β-phase formation of a
polyvinylidene fluoride (PVDF) matrix. The R-ZnPc was prepared by direct
tetramerization of alkylated phthalonitrile with zinc salts. Due to the
presence of long peripheral alkyl-substituents, the prepared R-ZnPc exhibited
controllable solubility in selected solvents. Taking advantage of the
hydrophobic interactions of alkyl chains and flat structural features of Pc
macrocycles, molecular wrapping of R-ZnPc around the SWCNTs was intended to
produce noncovalent supramolecular complexes. The prepared Pc-CNT complexes
were analyzed by various characterization methods, including UV-Vis, FTIR,
Raman, TGA, SEM, and XPS measurements. Furthermore, the Pc-CNT/PVDF composite
films were prepared using the Pc-CNT complex as effective nanofillers for the
PVDF matrix. The dielectric constants of the Pc-CNT/PVDF composite films were
significantly increased as a consequence of the promoted b-phase formation in the PVDF matrix, as evidenced by
FTIR and XRD measurements. Current results suggested that the R-ZnPc
functionalized SWCNTs have affected the crystalline structure of the PVDF
matrix, which can be a useful tool for producing nano-fillers for hybrid
polymer composites.
본 논문에서는 알킬기를 가지는 아연-프탈로시아닌(R-ZnPc)을 이용하여 단일벽 탄소나노튜브(single-walled carbon nanotube, SWCNT)의 분산 특성을 파악하고 이를 포함하는 폴리불화비닐리덴(polyvinylidene
fluoride, PVDF)의 베타상(b-phase) 형성에 대한 연구 결과를 제시한다. R-ZnPc는 알킬기를
가지는 프탈로니트릴(phthalonitrile) 중간체를 아연염과 함께 tetramerization 반응을 진행하여 합성하였다. 긴 알킬기가 외부에 존재하기 때문에 R-ZnPc는 다양한 유기 용매에
높은 용해성을 발휘하였다. 또한
알킬기의 소수성 상호작용과 편평한 분자구조의 특징을 이용하여 SWCNT 외벽에 R-ZnPc 분자 wrapping을 유도하여 비공유 결합에 기반한 초분자 복합체(supra-molecular
complex)를 제조하였다. Pc-CNT 복합체를 자외-가시광
흡수도, 퓨리에 적외선 분광법, 라만 분광법, 열중량 분석법, 주사전자현미경 측정 및 X선 광전자 분광법 등 다양한 측정 방법으로 분석하였다. 또한 Pc-CNT 복합체를 나노필러로 사용하여 Pc-CNT/PVDF 복합
필름(composite film)을 제조하였다. Pc-CNT/PVDF
복합 필름의 유전 상수는 큰 폭으로 증가하였으며 이는 PVDF의 베타상 형성이 촉진되었기
때문인 것으로 퓨리에 적외선 분광법과 X선 회절분석법 측정을 통해 확인하였다. 본 연구 결과는 R-ZnPc가
SWCNT 표면을 효과적으로 기능화할 수 있으며 제조된 Pc-CNT 복합체가 PVDF 매트릭스의 결정 구조 변화에 영향을 미쳤다는 것을 의미한다. 또한
본 연구에서 제시하는 방법은 하이브리드 복합 필름에 적합한 나노필러를
제조하는데 유용한 방법이 될 것으로 기대된다.
Noncovalent functionalization of single-walled
carbon nanotube (SWCNT) using alkylated zinc-phthalocyanine (R-ZnPc) is
presented, which finds useful for the β-phase formation of a
polyvinylidene fluoride (PVDF). Current result suggests that the presence of
R-ZnPc functionalized SWCNT has significantly affected the crystalline
structure of the PVDF matrix.
Keywords: polyvinylidene fluoride, zinc-phthalocyanine, single-walled carbon nanotube, carbon nanotube, composite, dielectric constant
This work was supported by the Technology Innovation
Program (10047756 and 10052838) funded by the Ministry of Trade, Industry, and
Energy (MOTIE, Korea).
Polyvinylidene fluoride (PVDF) and its copolymers have been widely used
in many application fields because of their superior piezoelectric,
ferroelectric properties and dielectric constants.1-4 The electrical
properties were closely related to various semi-crystalline polymorphs of PVDF.5,6
Among semi-crystalline phases, β-phase showed outstanding electrical properties
owing to its all-trans (TTT) configuration.7,8 The experimental
techniques and strategies to induce the β-phase structure have been
investigated in particular conditions, such as drawing films, applying an
extremely high electric field, and crystallization from a solution or melt.9,10
As a measure to enhance the electrical properties of PVDF and facilitate
the β-phase formation, the polymer nanocomposites combined with inorganic
nano-fillers have been widely investigated.11-13 Inorganic fillers,
such as BaTiO3, Ti4O12, and Pb(Zr,Ti)O3,
have been combined with PVDF, which effectively enhanced the physical,
mechanical and electrical properties.14-17 However, these
nanocomposites with inorganic fillers created processing problems because these
fillers made PVDF significantly dense and brittle. Meanwhile, organic fillers
are useful in maintaining the flexibility of PVDF nanocomposites. Notably,
carbon nanotubes (CNTs) have been extensively investigated because of their
electrical properties, low mass density, and large aspect ratio, which
effectively enhanced the electrical, ferroelectric, and piezoelectric
properties of the PVDF composites.18-22 However, since CNTs are
tightly entangled with each other by secure van der Waals forces, appropriate surface treatment and pre-milling procedures needed to be applied to
distribute CNTs uniformly inside the PVDF matrix.
In order to disperse CNT bundles
effectively inside the polymer matrix, numerous surface
modification methods have been employed. Covalent functionalization of CNTs
with a polymer matrix was tried, and, in this case, the polymer covering on the
CNT surface provided adhesion and dispersion to the polymer matrix so that the
prepared polymer composites had enhanced electrical and
mechanical performances.23-25 However, the covalent approach
mostly disrupted the well-ordered π-conjugated
system and deteriorated the electrical and mechanical properties of the CNTs.
To overcome the destruction of the structural nature, the
non-covalent approach, which is weak and long-range interactions, can be used
to maintain the inherent properties of CNTs. Among various methods, we have
been especially intrigued by noncovalent functionalization with metallophthalocyanines (MPcs).26 Due to excellent chemical,
thermal, and light stability, the polymer composites including MPc have provided high electrical conductivity and superior mechanical
and chemical stability, along with superb electrochemical activities.27-29
Meanwhile, several polymers have been employed as matrix materials for
preparing polymer-based nanocomposites.30-33 PVDF and its copolymers
have exhibited relatively high dielectric permittivity and breakdown
strength, compared to other commodity polymers. However, despite many
approaches, the
dielectric constant values are still low, and thus the practical applications
for energy storage capacitors are quite limited.
In this article we present the preparation and applications of alkylated
zinc-phthalocyanine (R-ZnPc) for noncovalent functionalization of single-walled
carbon nanotubes (SWCNTs), which we then use as a nanofiller for PVDF
composites. Peripherally substituted ZnPcs are suitable for SWCNT modification
due to hydrophobic interactions of alkyl chains and flat molecular structure of
the Pc macrocycles. Furthermore, alkyl chains at peripheral sites of ZnPc
allowed the molecular wrapping of Pcs around the SWCNTs. Thus, we expected that
a large amount of ZnPcs would firmly adhere to the CNTs surface and that the
enhanced compatibility would increase the dispersing nature of the SWCNTs
inside the PVDF polymer matrix. Furthermore, Pc-functionalized SWCNTs affected
the crystalline structure of the PVDF, inducing more β-phase during nucleation
inside the matrix. The prepared ZnPc and Pc-CNT complex was fully examined
using various characterization methods. The effect of the Pc-CNT filler on the β-phase
formation of the polymer matrix was examined, and the resulting dielectric
properties of ZnPc-CNT/PVDF composite film were presented.
Chemicals. Lithium metal, zinc(II) acetate,
1-hexanol, 1,8-diazabicyclo[5.4.0]undec-7-ene, polyvinylidene
fluoride (PVDF), tetrahydrofuran (THF),
dichloromethane (DCM), and N,N-dimethylacetamide (DMAc) were purchased from Sigma-Aldrich.
4-Nitrophthalonitrile was purchased from TCI, and vinyl terminated
polyisobutylene (HRPB1000) was provided by Daelim Corporation (Korea). All reagents were used without further purification. SWCNTs (>90 wt%, diameter 1.1 nm, length 1-3 µm) were purchased from US Research Nanomaterials.
Synthetic Procedures. Synthesis of Alkylated Phthalo-nitrile: PIB1000-alcohol was synthesized by
following the previous literature procedure.34 A mixture of 4-nitrophthalonitrile
(1.63 g, 8.89 mmol), PIB1000-alcohol (3.0 g, 2.96 mmol), and Cs2CO3 (4.81 g, 14.81 mmol) was dissolved
in 30 mL of THF and refluxed in the inert gas for 12 h. The residue was cooled
to room temperature, and the THF was evaporated under reduced pressure. The
crude products was extracted with hexane, and washed with a 90% ethanol-water
mixture three times, then dried over MgSO4. Hexane was removed under reduced
pressure using a rotary evaporator to afford alkylated
phthalonitrile as a viscous oil (2.89 g, 85.3% yield). 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J=8.5 Hz, 1H), 7.24 (d,
J=2.0 Hz, 1H), 7.16 (dd, J=8.5, 2.10 Hz, 1H), 3.85 (dd, J=8.5, 5.5 Hz, 1H),
3.74–3.71 (m, 1H), 1.61–0.85 (multiple peaks). MALDI-Mass (C79H148N2O) 1131.18 m/z.
Synthesis of Alkylated Zinc-phthalocyanine
(R-ZnPc): Alkylated phthalonitrile (1 g, 0.877 mmol) and ZnCl2 (44 mg, 0.323 mmol) were
added to the pressure tube, and the reaction mixture was heated to 160 oC for 4 h. After
cooled down to 25 oC. The alkylated zinc-phthalocyanine was extracted with hexane and
washed with acetonitrile and a 90% ethanol-water mixture three times. After
being dried over MgSO4, then filtered, hexane was removed under reduced pressure. The alkylated
zinc-phthalocyanine was isolated by column chromatography using silica gel and (DCM/hexane
4:6) as the eluent, and the product was a greenish viscous oil. 1H NMR (500 MHz, CDCl3) δ 9.05 (s, 3H), 8.54 (d, J=58.7 Hz,
4H), 7.62 (s, 5H), 4.39–4.22 (m, 8H), 2.50–0.70 (multiple peaks).
MALDI-mass (C316H584N8O4Zn) 4620 m/z.
Preparation of the Pc-CNT Complex: 1 mg SWCNT was sonicated in 10 mL CHCl3 for 30 min at 0 oC in a sonication bath (PowerSonic410, Hwashin, Korea). A solution of R-ZnPc 2 mg in
1 mL of CHCl3 was added dropwise to the above
SWCNT solution, and the mixed solution additionally was sonicated for 1 h.
The resulting ZnPc/SWCNT complex was isolated
with a PTFE membrane filter (pore size 200 nm). The residue
was washed successively with CHCl3 to remove excess ZnPc and proceed
until the filtrate was colorless. The complex was collected and dried in a
vacuum oven for 24 h.
Preparation of the Pc-CNT/PVDF Composite: 1 g of PVDF in 5 mL of DMAc was stirred at 50 oC until a homogeneous solution was obtained. Meanwhile, the
Pc-CNT dispersion in 1 mL DMAc was prepared by sonication of the solution
for 30 min at 0 oC in a sonication bath (PowerSonic410,
Hwashin, Korea). Then, the PVDF solution and Pc-CNT dispersion were
mixed in the desired quantity to make a suspension by sonication for
30 min. The suspensions were coated on a glass plate by the doctor blade
method, and the film thickness was controlled to 0.5 mm and dried in a vacuum oven at 80 oC.
Property Characterization: 1H NMR measurements were performed on an Inova Tech at 500 MHz using
CDCl3 as a solvent. The UV-Vis
absorption and photoluminescence spectra were measured by using Shimadzu
UV-1800 spectrophotometer and Perkin-Elmer LS-45 spectrofluorometer,
respectively. MALDI-TOF mass spectrometry, X-ray photoelectron spectroscopy,
and FTIR analysis were measured at the Korea Basic Science
Institute. Thermogravimetric analysis (TGA, STA6000, Perkin-Elmer) was performed in nitrogen at
a heating rate of 10 oC/min. Field emission scanning electron microscopy (FE-SEM) was
performed on a SUPRA25 (Carl Zeiss AG, Germany). Raman spectra were measured
using a NRS-5100 with an excitation laser of 532 nm. X-ray diffraction (XRD)
analysis was analyzed using Xpert 3. The dielectric
constant and dielectric loss values obtained on a HP 4194A impedance analyzer
at room temperature in the frequency range of 60 Hz to 10 MHz.
The alkylated phthalonitrile, an
intermediate, was synthesized by refluxing the
hydroxy-terminated polyisobutylene with 4-nitrophthalonitrile in the presence
of Cs2CO3. The cyclotetramerization of alkylated phthalonitrile with zinc(II)
chloride produced alkylated zinc phthalocyanine (R-ZnPc). (Scheme 1). The structure of R-ZnPc product was successfully identified by
MALDI-Mass spectroscopy.
Generally, in UV-Vis spectra, Pc
compounds have two absorption characteristics, one in the UV region (B- or
Soret band) and the other in the visible region (Q-band).35
As shown in Figure 1, R-ZnPc exhibited a sharp Q-band at 683 nm. R-ZnPc became highly soluble in THF and chloroform, but only a small amount
was soluble in nonpolar solvents like hexane.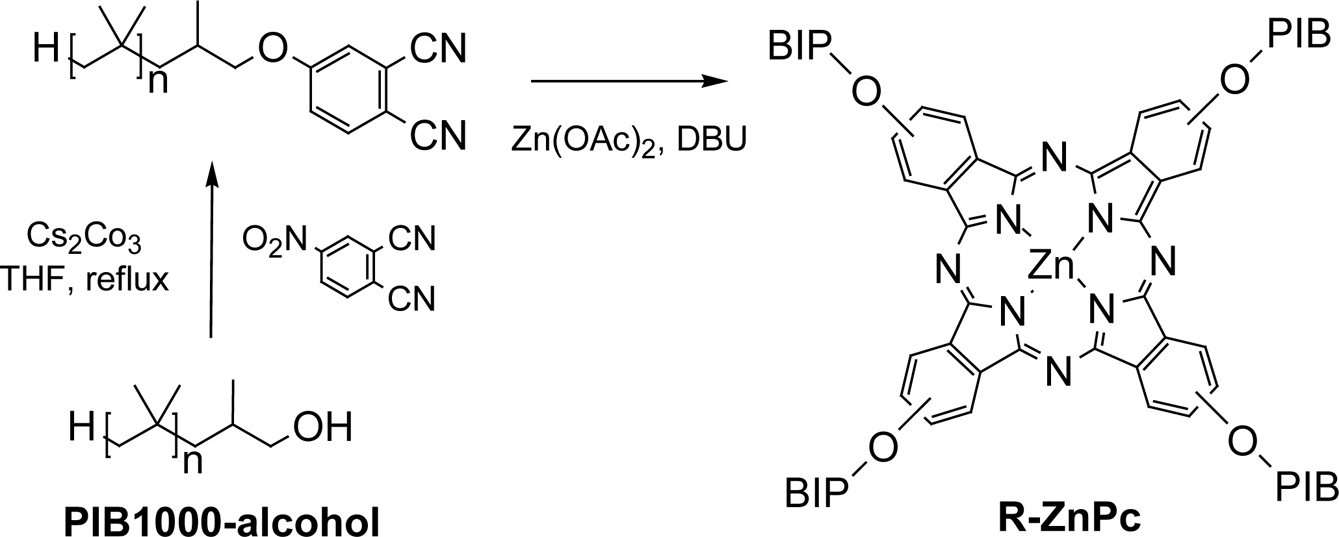
Scheme 1. Synthesis of alkylated zinc phthalocyanines (R-ZnPc).
The Pc-CNT complex was prepared
by sonication of R-ZnPc and SWCNTs in
chloroform for 1 h. Due to long, multiple peripheral
chains and the flat molecular structure of the phthalocyanine skeleton, the
R-ZnPc exhibited high affinity and strong adhesion to the surface of the
SWCNTs. The UV-Vis spectra of Pc-CNT complexes revealed
significant absorption and scattering effect of CNT bundles in the range of 300
and 600 nm. The Q-band of R-ZnPc in the Pc-CNT complex was also observed at 689
nm, with a slight red shift by 6 nm. This red shift in the Q-band after complex
formation is evidence of the existing noncovalent
interaction of ZnPcs with SWCNTs. In PL spectra, R-ZnPc exhibited the emission
maximum at 689 nm with excitation at 600 nm. After the complex formation, the
PL intensity was quenched entirely as a result of a facile electron transfer from ZnPc to SWCNT in the formed complex.
The morphological difference
between pristine and ZnPc-functionalized SWCNTs was noticed in the SEM images
(Figure 2). In pristine SWCNTs, the CNT bundles randomly adhered to each other, forming irregular aggregates. After the
complex formed, the surface of Pc-CNT complexes was mostly
separated into individual CNTs. Therefore, R-ZnPc induced the presence of
individual tubes as a result of effective de-bundling on the CNT surface.
In the FTIR analysis, a strong absorption band with a C=C stretch of SWCNTs was observed
at around 1600 cm-1 and a slight red shift at high frequencies after being complexed. It
was attributed to intramolecular electron transfer between noncovalently bonded
Pc-CNT complexes (Figure 3(a)). Compared to the
featureless spectrum of pristine SWCNTs, the Pc-CNT complex exhibited
characteristic peaks of the pyrrole backbone in the range of 1550-1200 cm-1. The characteristic absorption in the aromatic region of CNTs almost
disappeared due to the appearance of aliphatic
peaks in the Pc-CNT complex (about 2900-2800 cm-1). This result indicated that the polyisobutylene substituent
completely wrapped the aromatic structure of CNT surface (Figure 3(b)).
To investigate the structural
features of the functionalized SWCNTs with R-ZnPc, Raman spectroscopy was
employed. The Raman spectrum of pristine SWCNTs represented a broad D-band peak
at 1335 cm-1, attributed to the vibrations of sp3-hybridized carbon structures, and a G-band
peak at 1580 cm-1, attributed to in-plane vibration of sp2-hybridized graphitic carbon atoms,35,36
yielding an ID/IG
ratio of 0.46 (Figure 4(a)). After the CNT is functionalized with
R-ZnPc, the D- and G-bands of the complex shift slightly to higher frequencies,
indicating the extended electron delocalization. As a result, the ID/IG ratio of the Pc-CNT complex
increased significantly to 0.68. TGA measurements
were performed to analyze the thermal stability of the prepared complexes and
to estimate the amount of Pc stacked on the SWCNTs by non-covalent bonding
(Figure 4(b)). Over a wide temperature range, the pristine SWCNTs were stable,
with only a 10% weight loss above 650 oC, mainly due to the impurities
included in the nanotube preparation.28 R-ZnPc began to slowly degrade at
100-400 oC due to PIB chain breakage and showed a massive weight loss of about
400 oC, which corresponds to complete decomposition of
the Pc skeleton. The Pc-CNT complex showed degradation and weight-loss behavior
due to the detachments of surface decorating molecules.
XPS measurements were performed to analyze the ZnPc skeletons and
polyisobutylene, the characteristics of ZnPc-CNT. The deconvolution of C1s
peaks indicated two primary carbon atoms, C-C and C-O at 284.6 and
285.6 eV, respectively (Figure 5(a)).37,38
The N1s peak was deconvoluted into two separate 398.7 and 399.8 eV
peaks, due to the pyridinic and pyrrolic nitrogens of phthalocyanine (Figure 5(b)). The Zn2P
peaks were deconvoluted into two peaks at 1021 and 1044 eV,
corresponding to bond energy values of Zn2p1/2 and Zn2p3/2, respectively (Figure 5(c)).
The SEM images of the Pc-CNT/PVDF
composite were prepared in a film state, which revealed smaller
particles and fewer surface voids compared to pristine PVDF films (Figure 6(a) and 6(b)). Since the surface-modified SWCNTs were uniformly dispersed in PVDF, the morphology of the PVDF composite
was retained according to the filler content.
PVDF with various semi-crystalline
polymorphs have exhibited different molecular vibrations in FTIR spectroscopy.
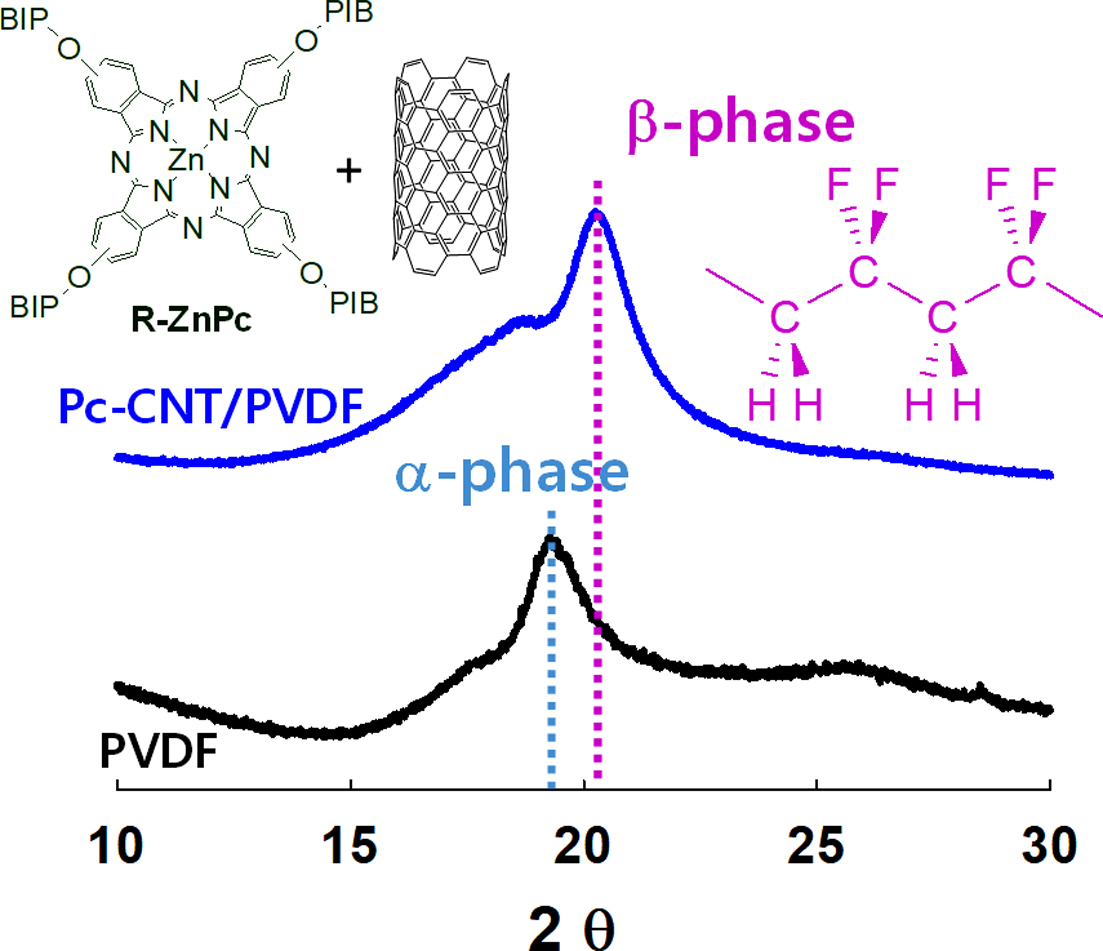
The absorption spectra at 613, 762, 795, and 975 cm-1 are the characteristic spectra for the α-phase crystalline, and the bands at 840 and 1275 cm-1 are assigned for β-phase
crystalline.39 In current measurements, the FTIR spectra were performed to analyze
the crystalline phase of PVDF composites with different amounts of Pc-CNT. All
these peaks were observed in the prepared PVDF films
(Figure 6(c)), indicating that PVDF formed both a- and β-phases simultaneously during the crystal formation. However, when the
content of R-ZnPc/SWCNTs was 0.5% in PVDF, the intensity of
the α-phase showed a decreasing tendency.
The a- and b-phases in the
PVDF matrix can also be distinguished through X-ray diffraction measurement
(Figure 6(d)). Generally, the a-phase crystalline of PVDF exhibits strong diffraction peaks at 2q=17.8o and 19.5o, respectively, assigned to the
lattice planes of (100) and (110). The a-phase can also be observed at 2q=26o corresponding to the (021) lattice plane. The peak at 2q=20.6o is assigned to (110) and (200) planes in the b-phase crystalline.40 The XRD pattern of PVDF clearly
showed two sharp diffraction peaks at 2q=17.8o and 19.5o, corresponding to α
phase (020) and (110) planes, respectively, and one broad peak at 2q=26.8 o of the a phase (021). In the PVDF nanocomposite film, the 0.5% Pc-CNTs were not different from those of
pristine PVDF. However, in the 1% Pc-CNTs, the intensity of the peaks at 2q=17.8o, 19.5o, and 26.8o was decreased and a new peak at 2q=19.5o corresponding to the b-phase (for 110 and 200 planes) was formed with higher intensity. These results indicate that the functionalized CNTs dispersed well inside the PVDF matrix and promoted b-phase formation.
The dielectric constant was investigated over a
wide range of frequencies from 100 Hz to 1 MHz for the Pc-CNT/PVDF
composite (Figure 7). When the amount of Pc-CNT
fillers was below 5 wt%, the change in the dielectric constant was small,
showing a slight increase in low-frequency regions, resulting in low
capacitance. When the fraction of the fillers increased to 10 wt%, the dielectric constant was significantly increased, which was attributed to the
formation of sizable interfacial polarization. The improvement of the
dielectric constant with the amount of functionalized SWCNT filler is explained
as a consequence of the promoted β-phase formation in the PVDF matrix,41-43 as evidenced by FTIR and XRD
measurements. Meanwhile, the dielectric loss of the Pc-CNT/PVDF composite films
was also measured. Similarly, the low content of the Pc-CNT complex in PVDF did
not alter the curve. In the case of the 10 wt% filler
content, the dielectric loss value showed a slight increase in low-frequency
regions. The behavior was attributed to the overlap of SWCNTs in the PVDF
matrix, contributing to the formation of conductive networks. However, an extreme increment in the dielectric loss was not observed in the
composite, while exhibiting the enhanced dielectric constant values. These
results suggest that noncovalent functionalization using substituted
metallophthalocyanines can be a practical method in
producing nanofillers that are useful for enhancement in dielectric properties
of PVDF based polymer composites.

|
Figure 1 UV spectra of (a) R-ZnPc; (b) Pc-CNT complex in dichloromethane. (c) Corresponding emission spectra of R-ZnPc and Pc-CNT complex in dichloromethane. |

|
Figure 2 SEM images of (a) pri stine SWCNTs; (b) the Pc-CNT complex. |

|
Figure 3 (a) FTIR spectra of R-ZnPc, the Pc-CNT complex, and pristine SWCNTs; (b) magnified spectra in the range from 600 to 2000 cm-1 of the corresponding samples. |

|
Figure 4 (a) Raman spectra of Pc-CNT complexes; (b) TGA thermograms of R-ZnPc and Pc-CNT complexes. |

|
Figure 5 XPS C1s spectra of Pc-CNT complexes; (b) XPS N1s spectra of the Pc-CNT complexes; (c) deconvoluted Zn2P XPS spectra of the Pc-CNT complexes. |

|
Figure 6 The surface morphology of (a) PVDF; (b) Pc-CNT/PVDF composite films. The concentrations of the fillers are 5 wt%. FTIR spectroscopy measurements showing the reduced -phase with the addition of (c) the Pc-CNT complex. X-ray diffraction measurement showing the enhancement of the -phase with the addition of (d) the Pc-CNT complex. |

|
Figure 7 Dielectric constant (a) and dielectric loss (b) of PC-CNT/PVDF composite films with different concentrations of the Pc-CNT complex. |
We have synthesized alkylated
zinc-phthalocyanine (R-ZnPc) by direct tetramerization of alkylated
phthalonitrile with zinc salt. Taking advantage of preferential
interactions of Pc macrocycles with single-walled carbon nanotube (SWCNT), we have produced supramolecular complexes from the molecular wrapping
of R-ZnPc around SWCNTs. The prepared Pc-CNT complexes were analyzed by various characterization techniques, including UV-Vis, FTIR, Raman, TGA,
SEM, and XPS measurements. Besides, the Pc-CNT/PVDF composite films were
prepared, and their dielectric properties were examined. The dielectric
constants were observed to be significantly increased, which was due to the
promoted β-phase formation in the PVDF
matrix, as evidenced by FTIR and XRD measurements.
- 1. Y. Liu, G. Tian, Y. Wang, J. Lin, Q. Zhang, and H. F. Hofmann, J. Intell. Mater. Syst. Struct., 20, 575 (2009).
-

- 2. J. B. Arochiam, H. S. Son, S. H. Han, G. Balamurugan, Y. H. Kim, and J. S. Park, ACS Appl. Energy Mater., 2, 8416 (2019).
-

- 3. T. W. Son, J. H. Kim, W. M. Choi, F. F. Han, and O. K. Kwon, Polym. Korea, 35, 130 (2011).
-

- 4. C. E. Chang, V. H. Tran, J. B. Wang, Y. K. Fuh, and L. W. Lin, Nano Lett., 10, 726 (2010).
-

- 5. Prateek, V. K. Thakur, and R. K. Gupta, Chem. Rev., 116, 4260 (2016).
-

- 6. E. Ozkazanc and H. Y. Guney, J. Appl. Polym. Sci., 112, 2482 (2009).
-

- 7. L. David, J. I. Winsor, and B. Scheinbeim, J. Polym. Sci., Part B: Polym. Phys., 34, 2967 (1996).
-

- 8. P. Sukitpaneenit and T. S. Chung, J. Membr. Sci., 340, 192 (2009).
-

- 9. A. J. Lovinger, Polymer, 22, 412 (1981).
-

- 10. H. Rekik, Z. Ghallabi, I. Royaud, M. Arous, G. Seytre, G. Boiteux, and A. Kallel, Compos. Part B-Eng., 45, 1199 (2013).
-

- 11. P. Martins, A. C. Lopes, and S. Lanceros-Mendez, Prog. Polym. Sci., 39, 683 (2014).
-

- 12. M. Arbatti, X. Shan, and Z. Cheng, Adv. Mater., 19, 1369 (2007).
-

- 13. P. Thomas, S. Satapathy, K. Dwarakanath, and K. B. R. Varma, Polym. Lett., 4, 621 (2010).
-

- 14. Z. H. Mbhele, M. G. Salemane, C. G. C. E. van Sittert, J. M. Nedeljkovic, V. Djokovic, and A. S. Luyt, Chem. Mater., 15, 5019 (2003).
-

- 15. I. Hussain, M. Brust, A. J. Papworth, and A. I. Cooper, Langmuir, 19, 4831 (2003).
-

- 16. G. A. Gaddy, J. L. McLain, A. S. Korchev, B. L. Slaten, and G. Mills, J. Phys. Chem. B, 108, 14858 (2004).
-

- 17. H. D. Yoon, S. Nam, N. D. K. Tu, D. Kim, and H. Kim, Polym. Korea, 37, 638 (2013).
-

- 18. R. H. Baughman, A. A. Zakhidov, and W. A. Heer, Science, 297, 787 (2002).
-

- 19. B. K. Kuila, S. Malik, S. K. Batabyal, and A. K. Nandi, Macromolecules, 40, 278 (2007).
-

- 20. F. R. Fan, W. Tang, and Z. L. Wang, Adv. Mater., 28, 4283 (2016).
-

- 21. J. Eom, Y. R. Lee, J. H. Lee, S. K. Park, J. S. Park, and Y. H. Kim, Compos. Sci. Technol., 169, 1 (2019).
-

- 22. Y. R. Lee, J. Park, Y. Jeong, and J. S. Park, Fiber. Polym., 19, 2478 (2018).
-

- 23. P. Miaudet, C. Bartholome, A. Derre, M. Maugey, G. Sigaud, C. Zakri, and P. Poulin, Polymer, 48, 4068 (2007).
-

- 24. R. Sen, B. Zhao, D. Perea, M. E. Itkis, H. Hu, J. Love, E. Bekyarova, and R. C. Haddon, Nano Lett., 4, 459 (2004).
-

- 25. H. H. Lee, U. S. Shin, G. Z. Jin, and H. W. Kim, Bull. Korean Chem. Soc., 32, 157 (2011).
-

- 26. H. S. Son, M. H. Yang, A. K. Mutyala, D. W. Chang, and J. S. Park, Dyes Pigments, 162, 662 (2019).
-

- 27. A. Ndiaye, P. Bonnet, A. Pauly, M. Dubois, J. Brunet, C. Varenne, K. Guerin, and B. Lauron, J. Phys. Chem. C, 117, 20217 (2013).
-

- 28. M. Li, X. Bo, Y. Zhang, C. Han, and L. Guo, J. Power Sources, 264, 114 (2014).
-

- 29. P. J. Wei, G. Q. Yu, Y. Naruta, and J. G. Liu, Angew. Chem. Int. Ed., 53, 6659 (2014).
-

- 30. X. W. Liang, X. C. Yu, L. L. Lv, T. Zhao, S. B. Luo, S. H. Yu, R. Sun, C. P. Wong, and P. L. Zhu, Nano Enery, 68, 104351 (2020).
-

- 31. K. Meeporn and P. Thongbai, Compos. Part B–Eng., 184, 107738 (2020).
-

- 32. L. Y. Xie, X. Y. Huang, Y. H. Huang, K. Yang, and P. K. Jiang, J. Phys. Chem. C, 117, 22525 (2013).
-

- 33. L. M. Clayton, A. K. Sikder, A. Kumar, M. Cinke, M. Meyyappan, T. G. Gerasimov, and J. P. Harmon, Adv. Funct. Mater., 15, 101 (2005).
-

- 34. J. Li, S. Sung, J. Tian, and D. E. Bergbreiter, Tetrahedron, 61, 12081 (2005).
-

- 35. A. Shaabani, R. Maleki-Moghaddam, A. Maleki, and A. H. Rezayan, Dyes Pigments, 74, 279 (2007).
-

- 36. Y. Jiang, Y. Lu, X. Lv, D. Han, O. Zhang, L. Niu, and W. Chen, ACS Catal., 3, 1263 (2013).
-

- 37. C. Yang, S.-J. Hao, S.-L. Dai, and X.-Y. Zhang, Carbon, 117 301e312 (2017).
-

- 38. B. Wang, Y. Wu, X. Wang, Z. Chen, and C. He, Sensor. Actuat. B-Chem., 190, 157 (2014).
-

- 39. Y. Wang, A. D. Zhou, Y. Jiang, X. Y. Chen, and J. B. He, RSC Adv., 5, 37823 (2015).
-

- 40. S. X. Song, S. Xia, S. K. Jiang, X. Lv, S. L. Sun, and Q. M. Li, Materials, 11, 347 (2018).
-

- 41. J. M. Zhu, X. Y. Ji, M. Yin, S. Y. Guo, and J. B. Shen, Compos. Sci. Technol., 144, 79 (2017).
-

- 42. R. Costa, J. Silva, and S. L. Mendez, Compos. Part B-Eng., 93, 310 (2016).
-

- 43. Y. H. Zhang, D. Xu, W. H. Xu, W. W. Wei, S. W. Guan, and Z. H. Jiang, Compos. Sci. Technol., 104, 89 (2014).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(3): 301-308
Published online May 25, 2020
- 10.7317/pk.2020.44.3.301
- Received on Jan 21, 2020
- Revised on Mar 16, 2020
- Accepted on Mar 16, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jong S. Park
-
Department of Organic Material Science and Engineering, Pusan National University, Busan 46241, Korea
- E-mail: jongpark@pusan.ac.kr
- ORCID:
0000-0002-7624-9502








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.