- Effect of Interchain Hydrogen Bond on the Water Solubility and Mechanical Properties of PLGA/PEO Blend
School of Material Science and Engineering, East China University of Science and Technology, Shanghai, 200237, China
- PLGA/PEO 블렌드의 수용성 및 기계적 특성에 미치는 사슬간 수소결합의 영향
Polyethylene oxide (PEO)
possesses poor mechanical properties, which limits its application. For the
purpose of improving the mechanical properties of water-soluble films, blend of
polylactic glycolic acid (PLGA) with PEO was prepared by melt blending. The
hydrogen bonding between PEO and PLGA was investigated by Fourier transform
infrared spectrometer. The crystallization behavior, water solubility, and
mechanical properties were evaluated. The results showed that there was a
hydrogen bonding force between PLGA and PEO, and this force can improve the
mechanical strength and water absorption of the film. When the PLGA content reached
20 wt%, the tensile strength and the elongation at break achieved a
maximum 26.1 MPa and 771.2%, respectively. Compared to neat PEO, blend
with 10 wt% of PLGA had considerably higher water absorption capacity.
When the PLGA content was 40 wt%, the film would not disperse in water.
Blend of poly(lactic
glycolic acid) (PLGA) with
PEO was prepared by melt blending. The results showed that there was a hydrogen
bonding force between PLGA and PEO, and this force can improve the mechanical
strength and water absorption of the film.
Keywords: polylactic glycolic acid, polyethylene oxide, crystallization, water solubility, mechanical properties
Polyethylene oxide (PEO) is a degradable biocompatible polymer material.
It is soluble in water and most organic solvents. It can be absorbed by the
body and does not produce any toxic side effects.1 Therefore, PEO
can be applied to biomedical area, such as preventing protein adhesion
(biological contamination), controlling drug delivery, tissue scaffolds and
hydrogel nanocomposites.2 But, PEO has poor thermal stability and
poor mechanical strength, so it needs to be modified. Modification methods
mainly include blending, copolymerization, branching and interpenetrating
polymer network structure.3-7 Among them, blending is the most
economical and simple modification method. However, most studies focus on
modifying PEO with small molecules.3-7 This paper attempted to
modify it with degradable polylactic glycolic acid (PLGA) via melt blending.
PLGA is also an important biodegradable material produced by the
copolymerization of lactic acid and glycolic acid. It has good biocompatibility
and non-toxicity. It can be decomposed normally in the human body, so it is
also widely used in medical materials, drug delivery and so on.8,9
The main methods for synthesizing PLGA include ring-opening polymerization and
melt polymerization. Compared with ring-opening polymerization, melt
polymerization is simpler, more cost-effective and less toxic.10-13
Studies have shown that the higher the glycolic acid content of PLGA, the
faster the degradation rate of PLGA. But there is a special case when the molar
ratio of lactic acid to glycolic acid reaches 1 : 1, the degradation
rate of PLGA is faster, which only is two months.14 And the water
absorption of PEO can make PLGA more easily degraded in humid environment. So,
PLGA/PEO will be a better biodegradable material.
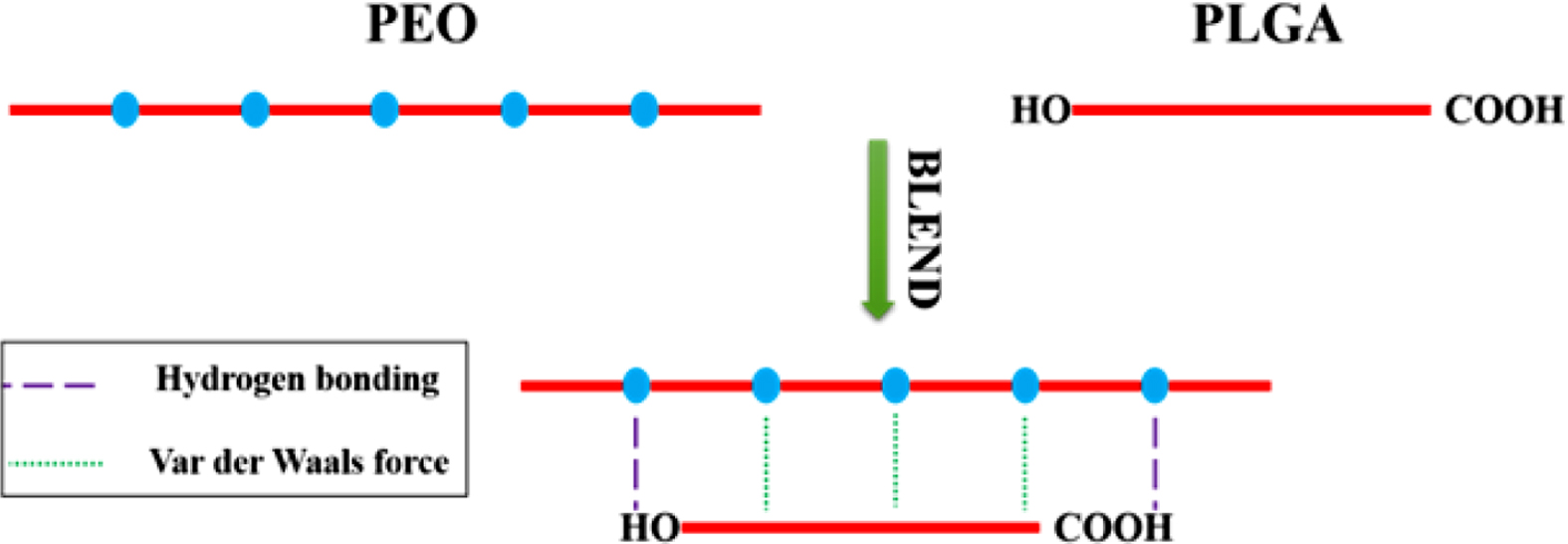
It has been reported that two macromolecules can form hydrogen bonding
complexes through hydrogen bonding interactions between polymer chains.15,16
The formation of hydrogen bonds will change the aggregated structure of the
material, enhance the mechanical properties of the material, improve the
compatibility between different components of the material, and give the
material special properties such as shape memory and self-healing properties.17-19
Therefore, it can improve the properties of materials by regulating the
hydrogen bonding between end groups in PLGA and ether bonds in PEO.
PLGA/PEO materials have high application value in the medical field. Most
of previous studies focus on the effects of PLGA/PEO on biological activity of
microencapsulated proteins.20 In this paper, The modification effect of PLGA to PEO has been discussed in terms of crystallization
behavior, water solubility and mechanical properties.
Materials. Polyethylene oxide
(Mw=3000000) was supplied by Shanghai Liansheng Company (Shanghai, China).
Glycolic acid was supplied by Jiangsu Taixing Water Chemical Co., Ltd
(Shanghai, China). Toluene-4-sulfonic acid was purchased from Acmec Biochemical
Co., Ltd (Shanghai, China). Lactic acid and tin chloride were both purchased
from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China).
Synthesis
of PLGA. Lactic acid and glycolic acid (166 g in total) were
weighed at a molar ratio of 1 : 1 and added to a 250 mL
three-necked flask, which was heated in an oil bath with magnetic stirring.
Firstly, the mixture was heated at 100 °C to remove water for 1 h,
then heated at 120 °C for 2 h. Secondly, it was mixed with catalyst
stannous chloride (0.5 g) and catalyst toluene-4-sulfonic acid
(0.5 g), heated at 140 °C for 2 h with an absolute pressure of
400 Pa. Finally, it was heated at 175 °C for 7 h with an
absolute pressure of 100 Pa.
Preparation
of PLGA/PEO Blend. The PLGA and PEO
were added to a KCCK XSS-300 rheometer equipped with a 60-mL mixing chamber (KCCK, China) at a mass ratio of 10:90
(5 g/45 g), 20:80 (10 g/40 g), 30:70 (15 g/35 g),
and 40:60 (20 g/30 g) for 6 min. The three sections of the mixing chamber
are all 130 °C, the rotor speed is 100 r/min. Then, the blends were
moulded via hot-press into a 0.5-mm-thick film at 130 °C for 10 min,
followed by water cooling for 1 min to 25 °C with a
pressure of 10 MPa.
Measurements. Gel Permeation Chromatograph (GPC): Determination of
sample molecular weight were examined by gel permeation chromatograph
(GPC, 1515, USA), and rinse speed is 1.0 mL/min with polystyrene as
standard at 35 °C. The solvent is THF.
Fourier
Transform Infrared Spectroscopy (FTIR): To determine the
functional group of a sample, the Nicolet 6700 spectrometer (Thermo Fisher,
USA) were used to perform the FTIR spectra of blends. The measurement range is
4000~ 600 cm−1, the resolution is 4 cm−1, and
the number of scans is 32.
Nuclear Magnetic Resonance Spectrometer (NMR): Nuclear magnetic resonance spectrometer
(NMR, AVANCE 500, Germany) was used to measure the structure of the samples at
600 MHz with CDCl3 as solvent, using tetramethylsilane (TMS) as
an internal standard, chemical shifts (d) were given in
ppm.
Differential
Scanning Calorimetry (DSC): To test the
thermal properties of the sample and analyze the crystallinity, differential
scanning calorimetry (DSC) measurements were recorded on a modulated DSC 2910
(TA Instruments, USA). The pan contained 10 mg sample, and a blank aluminum pan
was used as the reference. All the
samples were heated from 25 to 140 °C, hold at 140 °C for
5 min, cooled to -20 °C, hold at -20 °C for 5 min, and heated to
140 °C. Heating and cooling rates were always 10 °C/min. The
crystallinity is calculated using the following formula:

ΔHm: melting enthalpy. ΔHm: enthalpy change when PEO is completely
crystallized (213.7 J/g).21 W: mass fraction of PEO in
blend.
Weight
Change in Water: The water absorption and water solubility of the sample
were reflected by weight change in water, and tested by using a constant
temperature water bath instrument. The dried film was cut into a size of
2 × 2 cm2, immersed in 50 mL of distilled water
and taken out at regular intervals. The surface water was dried slightly by
filter paper. and the weight of the films was measured. The
water absorption (WS) is calculated using the following formula:22

where W1 is the weight of PLGA/PEO composite film when
it absorbs water (g); W0 is the dried weight of PLGA/PEO
composite film (g).
PLGA
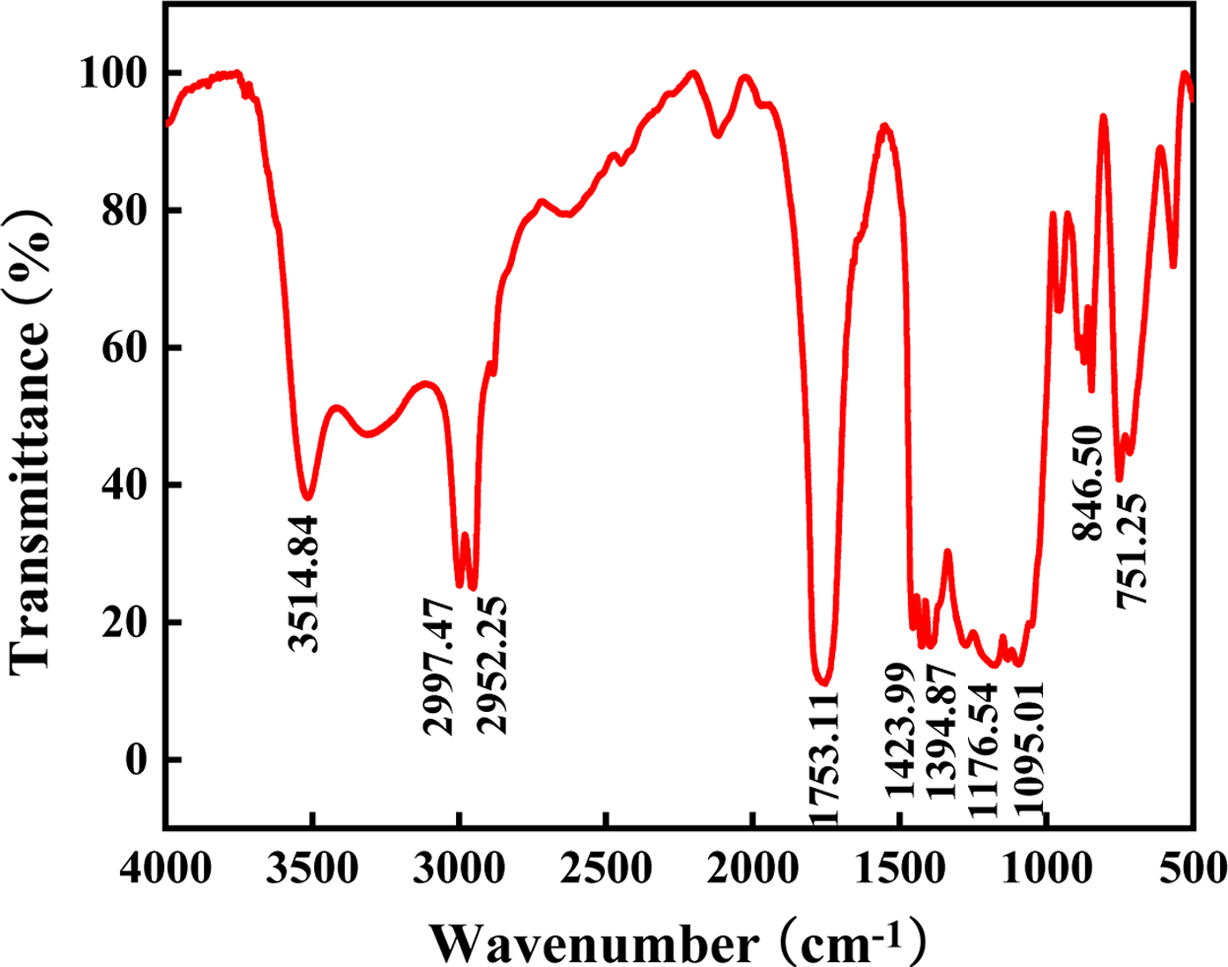
Structure Analysis. Figure 1 is the infrared spectrum of PLGA. There is a
strong absorption peak at 1751.11 cm-1, which corresponds to
the stretching vibration of the C=O bond. The absorption peaks at
2997.47 cm-1 and 2952.25 cm-1 are the C-H bonds
correspond to stretching vibrations. The absorption peaks at 1423.99 and
1394.87 cm-1 correspond to the bending vibrations of the C-H
bond. The broad absorption peaks at 1176.54 and 1095.01 cm-1
corresponding to the bending vibration of the C-O-C bond, indicate the presence
of an ester group. The absorption peak at 3514.84 cm-1 is the
stretching vibration of the hydroxyl group.
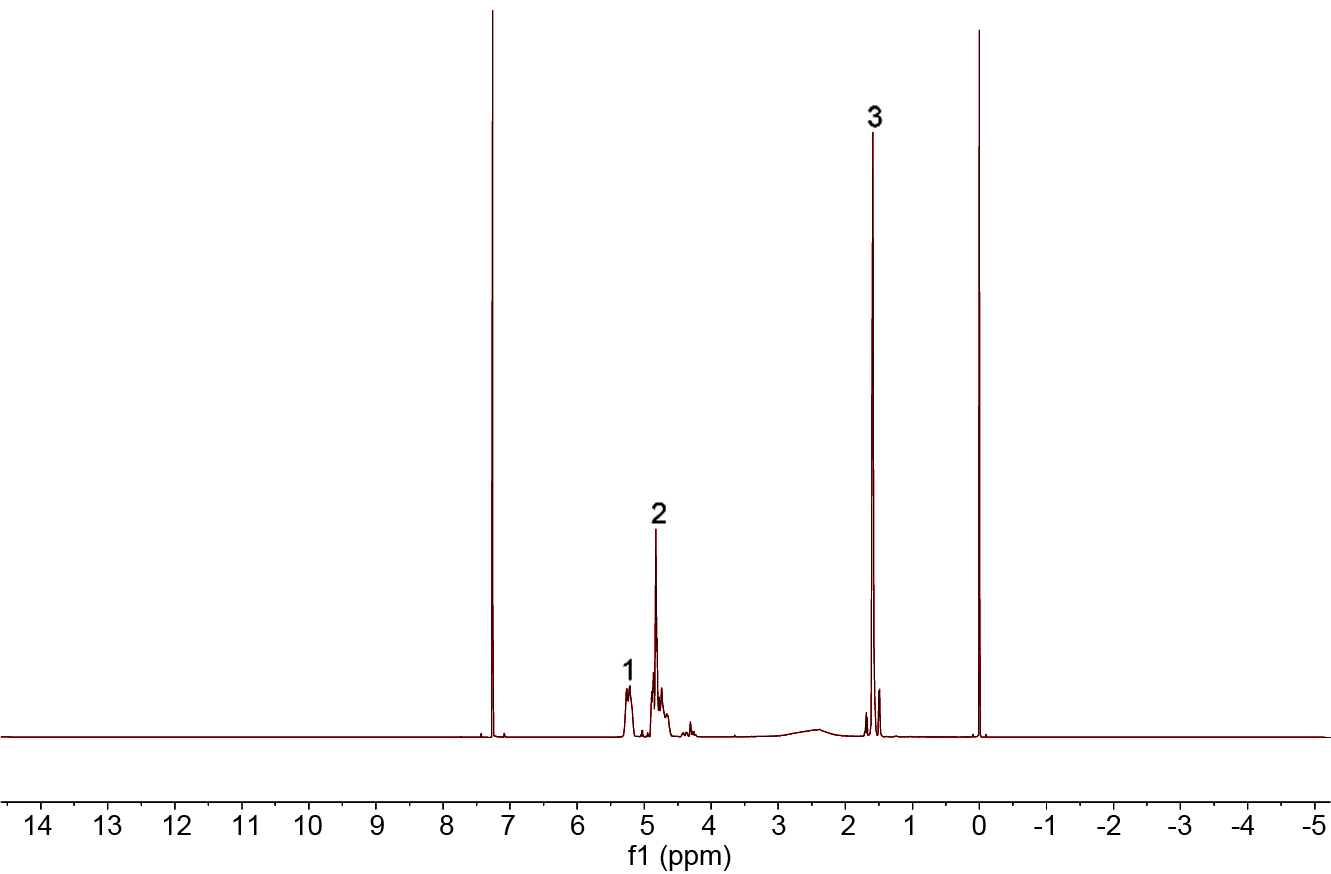
Figure 2 is the 1H NMR spectrum of PLGA. The peak 1 (δ=5.24) is the proton resonance peak of the methine group
of the lactic acid unit, and the peak 2 (δ=4.84) is a proton
resonance peak of the methine group of the glycolic acid unit. The peak 3 (δ=1.49) is the methylene peaks of the lactic acid unit.
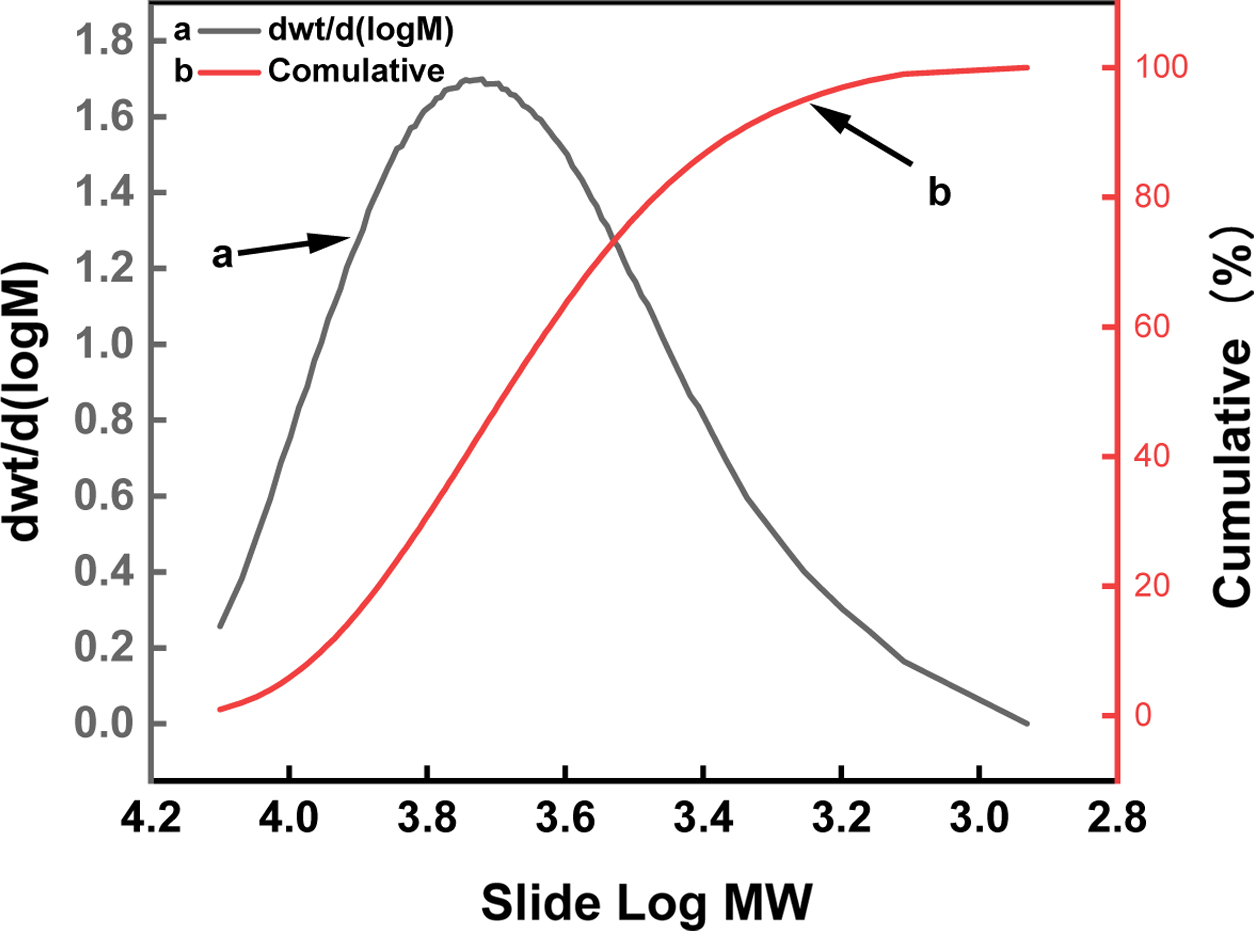
Figure 3 is a molecular weight distribution diagram of PLGA. The
polydispersity index is 1.31. It can be considered that the molecular weight
distribution is narrow and concentrate in the high molecular weight region. The
relative molecular weight (Mw) of the polymer obtained by GPC was 5269.
Hydrogen
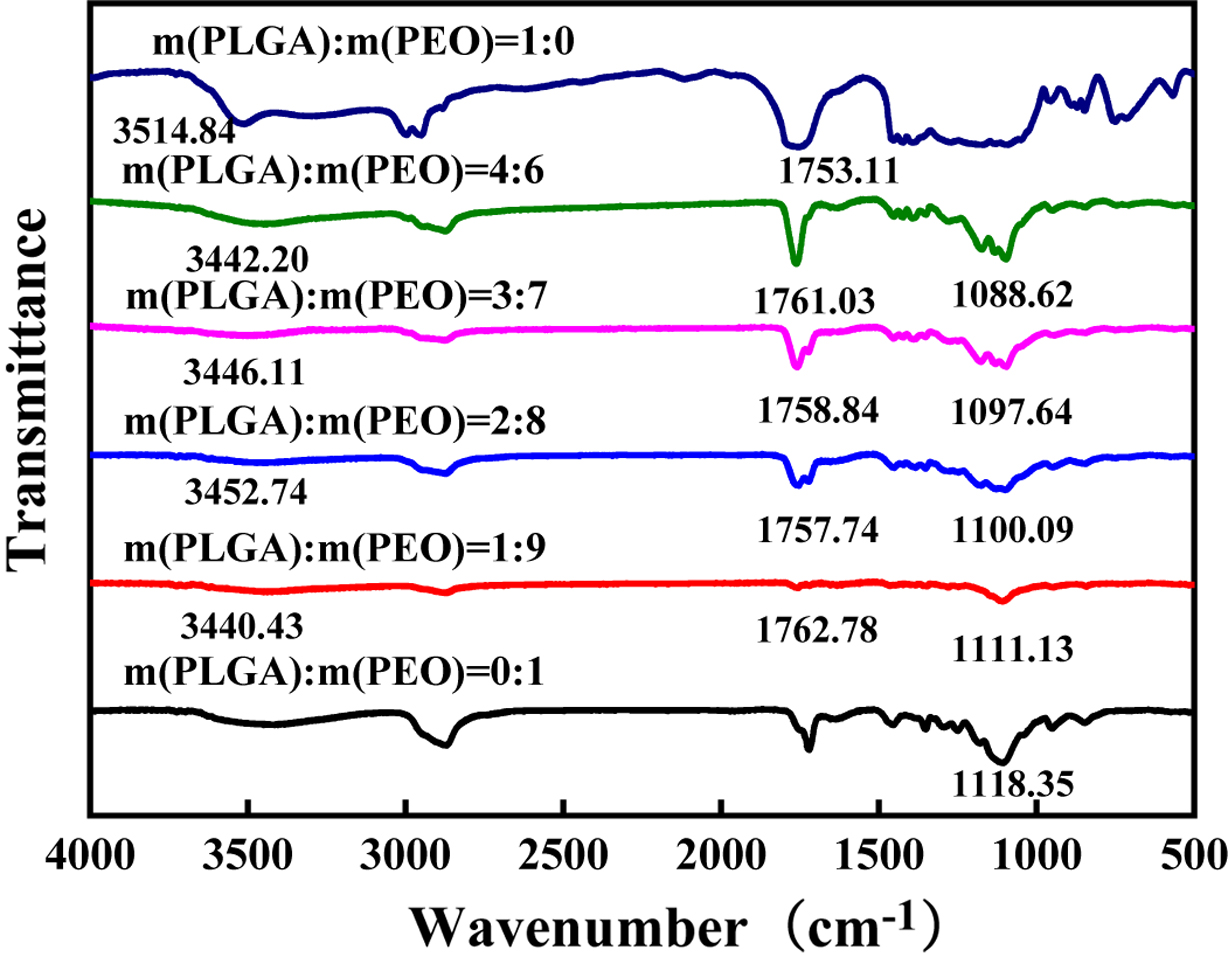
Bond Analysis. Infrared spectroscopy was used to study the
hydrogen bonding in PLGA and PEO. As shown in Figure 4. The absorption peak of
the ether-oxygen bond stretching vibration of pure PEO is 1118.35 cm-1.
Ozaki et al. analyzed the main components and found that the carbonyl
characteristic peaks were broken down into 4 parts: 1686, 1705, 1723, and
1745 cm-1.23 The components at 1705 and 1745 cm-1
may be assigned to C=O stretching modes of the cyclic hydrogen-bonded COOH
group in dimeric form and free (non-hydrogen bonded)-COOH group, respectively.
1686 cm-1 is attributed to the carbonyl stretching vibration of
the oligomer. 1723 cm-1 is attributed to the terminal
carboxyl group of the oligomer group. As shown in Figure 1, in the PLGA body,
the stretching vibration absorption peak of the carbonyl group is broad and
asymmetric, with the peak position at 1751.11 cm-1; the stretching
vibration absorption peak of the hydroxyl group is at the position of
3354.84 cm-1.
When PLGA and PEO form a hydrogen bonding complex, hydrogen bonds between
carboxyl groups, especially hydrogen bonds between ring-forming carboxyl
groups, will dissociate.24,25 When -OH of the -COOH in PLGA forms
hydrogen bonds with C-O-C in PEO, C=O in COOH will be released. So, the number
of free C=O will increase. Therefore, as shown in Figure 4, compared with pure
PLGA, the carbonyl peak of PLGA in the blend shifts to a high wavenumber, and
the hydroxyl peak of PLGA and the ether-oxygen bond of PEO move to a low
wavenumber. Because the number of ether oxygen bonds in PEO is much larger than
the number of carboxyl groups and hydroxyl groups in PLGA. Carboxyl groups and
hydroxyl groups of PLGA will fully form hydrogen bonds with ether oxygen bonds.
Therefore, with the increase of PLGA content, the positions of hydroxyl peak
and carboxyl peak do not change much. However, with the increase of PLGA, the
number of ether-oxygen bonds forming hydrogen bonds also increase, and the
peaks of ether-oxygen bonds gradually shift to lower wavenumbers.
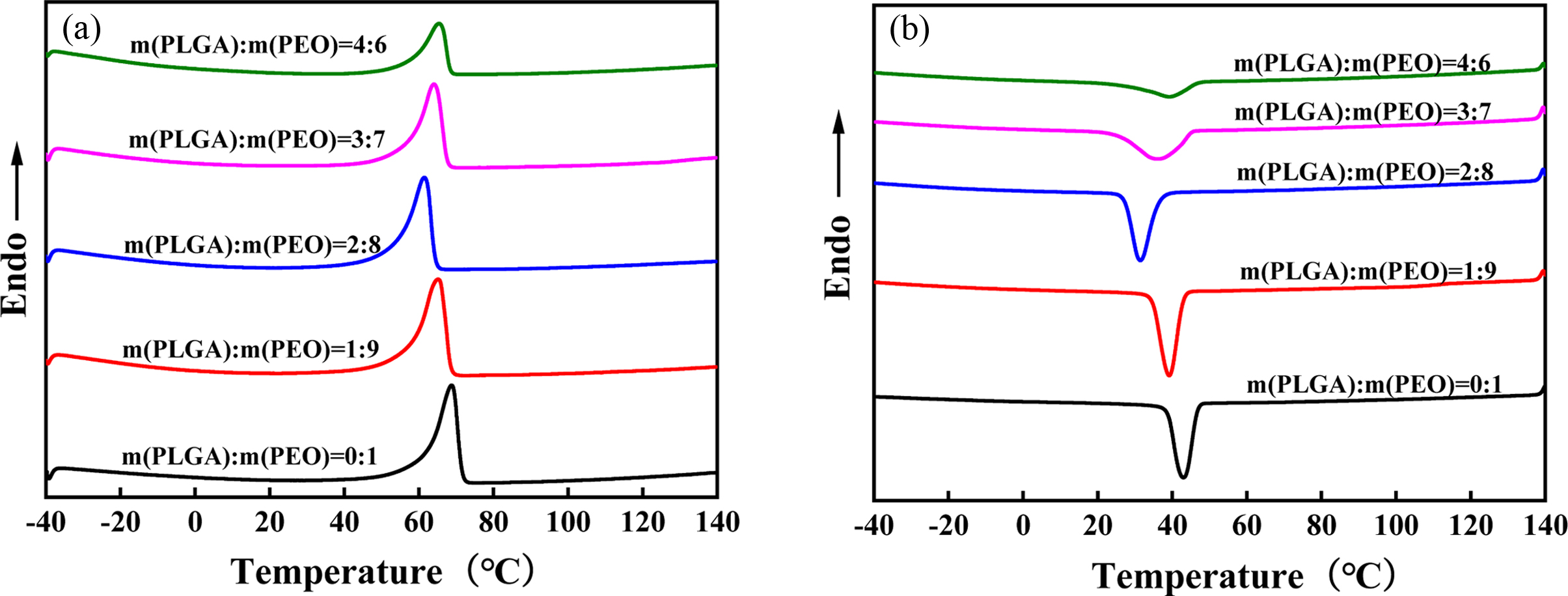
Crystallinity. DSC is a very good
thermal analysis method. Figure 5 shows the second heating curve (a) and
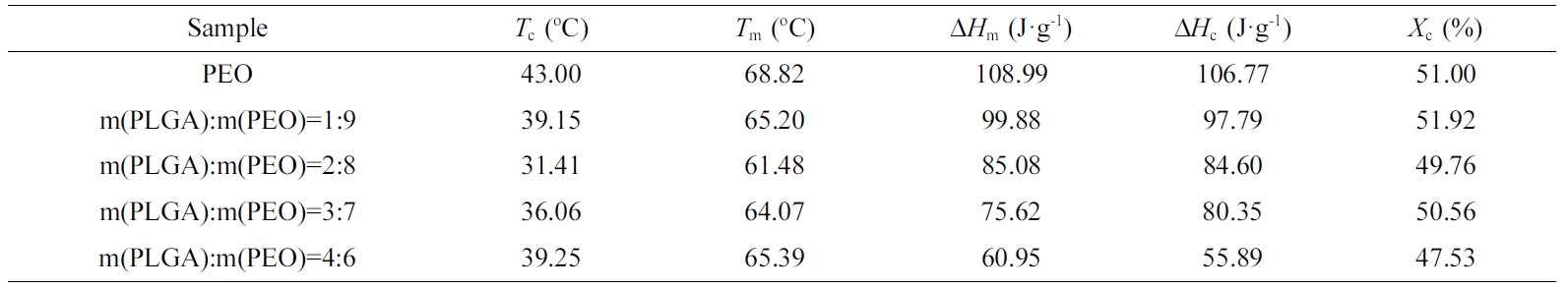
cooling curve (b) of PLGA/PEO blends with different mass ratios. Table 1 shows
the results obtained by DSC. As the PLGA content in blend increases, both the
melting enthalpy (ΔHm) and the crystallization enthalpy (ΔHc)
gradually decrease, because PLGA is a random copolymer and amorphous. But the
crystallinity of PEO does not change much, and it is always maintained at about
50%. It shows that the hydrogen bonding between PLGA and PEO has little effect
on the crystallinity of PEO. With the increase of PLGA content, the melting point
(Tm) and crystallization temperature (Tc)
of the blend decrease. It shows that PLGA and PEO have good compatibility. PLGA
can enhance the mobility of PEO molecular chains. So, Tm
shifted to a low temperature with the addition of PLGA. In addition, PEO crystallized
at lower temperatures due to the improved chain mobility. When the content is
higher than 20%, the Tm and Tc begin to
increase. But they are always lower than Tm and Tc
of neat PEO. It shows that PLGA and PEO appear phase separation. Tm
starts back to original Tm of neat PEO.
Weight
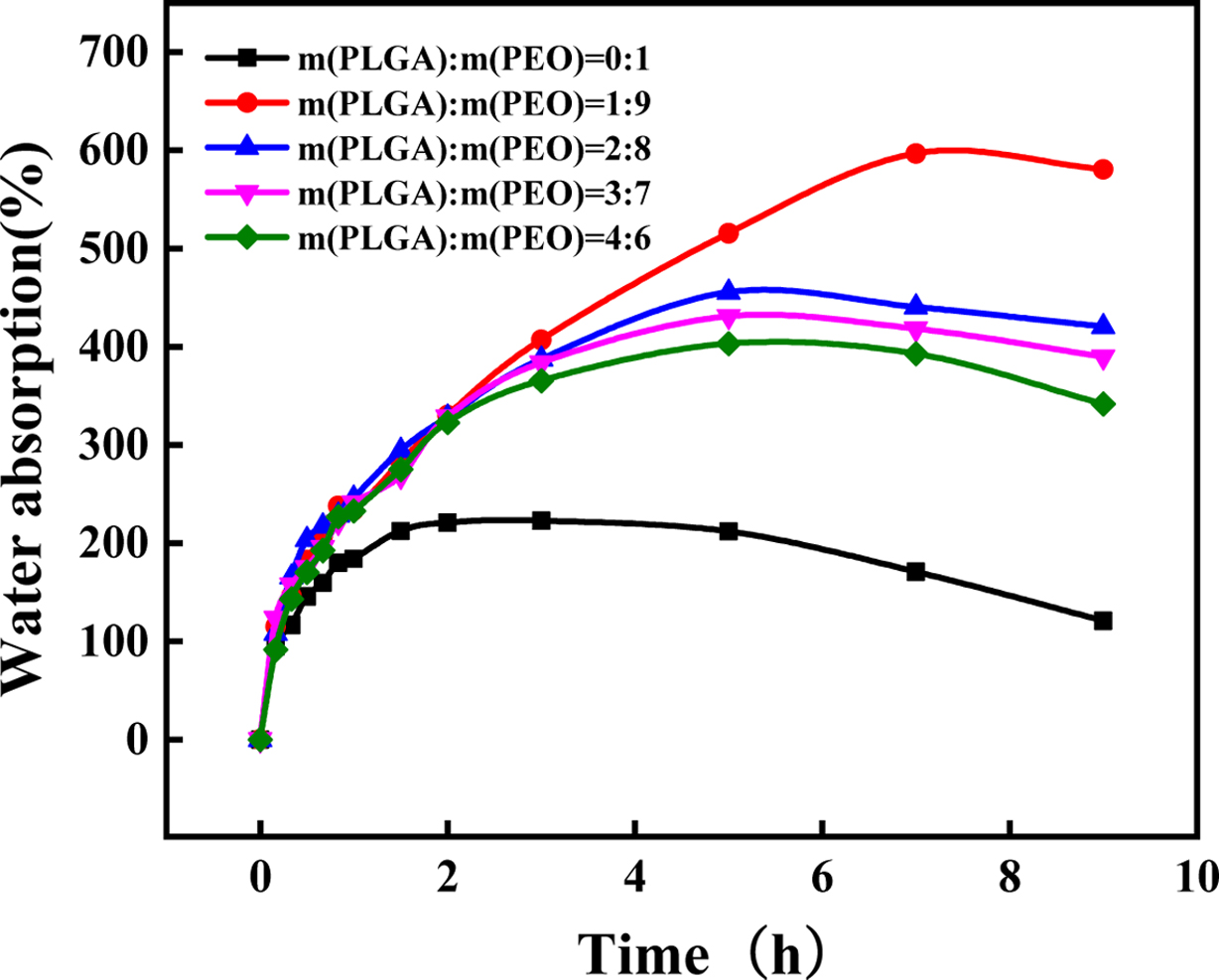
Change in Water. The solubility of polymer materials is a slow process.
First, they must absorb water to swell, and then dissolve. When the rate of
water absorption is fast than the rate of solubility, the weight will increase.
Otherwise it will decrease. PLGA is an insoluble substance, and PLGA/PEO films
will not completely dissolve. When the PLGA content is low, PEO is the matrix
and the dispersed phase is PLGA. When PEO is completely dissolved, PLGA will
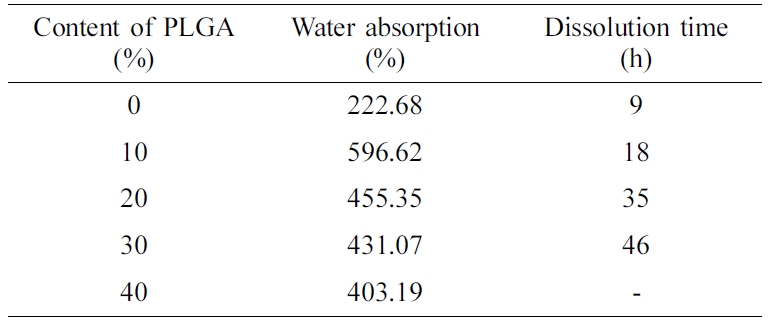
disperse and become particles dispersed in water. It can be known from Table 2
that as the PLGA content increases, dissolution time also increases. When the
PLGA content reaches 40%, the PLGA has been transformed from a dispersed phase
to a continuous phase and will not disperse when placed in water. Besides, it
can be seen from Figure 6 that the addition of PLGA can significantly improve
the water absorption of the material. Because end groups in PLGA and ether bond
in PEO produce hydrogen bonding to form a cyclic structure,
making it difficult for PEO to dissolve out of the material, but water is
locked in the cyclic structure. It enhances the water retention of the film and
increases the water absorption of the material. But when the PLGA content is
more than 10%, water absorption begins to decrease. Because as the
PLGA content increases, hydrogen bonding between PLGA and PEO increase. It
makes PEO hard to absorb water and swell.
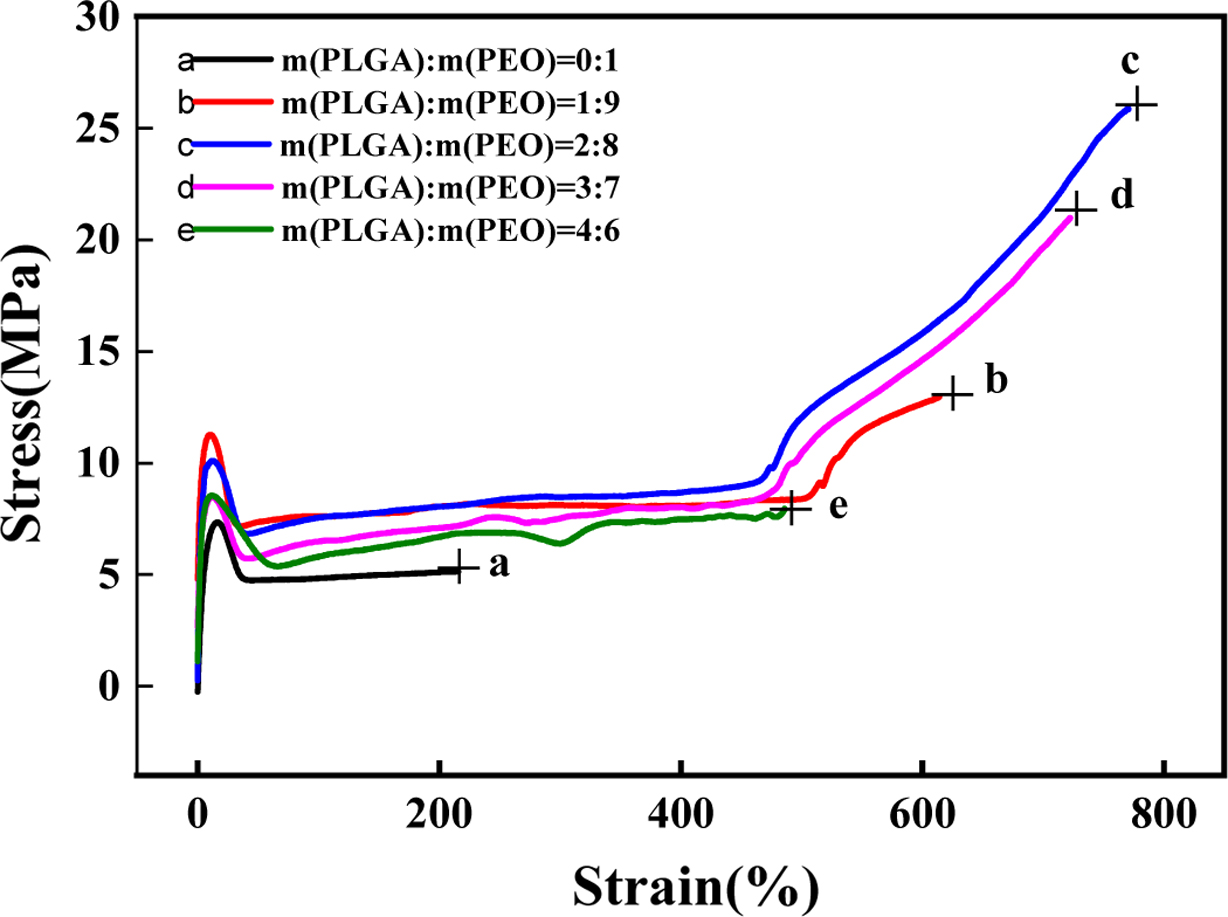
Mechanical Properties. As shown in Figure
7, the mechanical strength of neat PEO is poor, because the molecular
weight of PEO is as high as three million. Although the larger the molecular
weight, the mechanical strength will be increased, after reaching the limit,
the strength of the material will decrease as the molecular weight increases.
Because as the molecular weight of the polymer increases, the intermolecular
forces also increase, which increases the high-temperature flow viscosity of
the polymer, resulting in molecular agglomeration, and the product contains a
lot of bubbles and voids. The molecular weight of PLGA is not high. The insertion
of PLGA can well reduce the force between PEO molecules, thereby reducing the
viscosity of the polymer and improving the toughness of the material. It can be
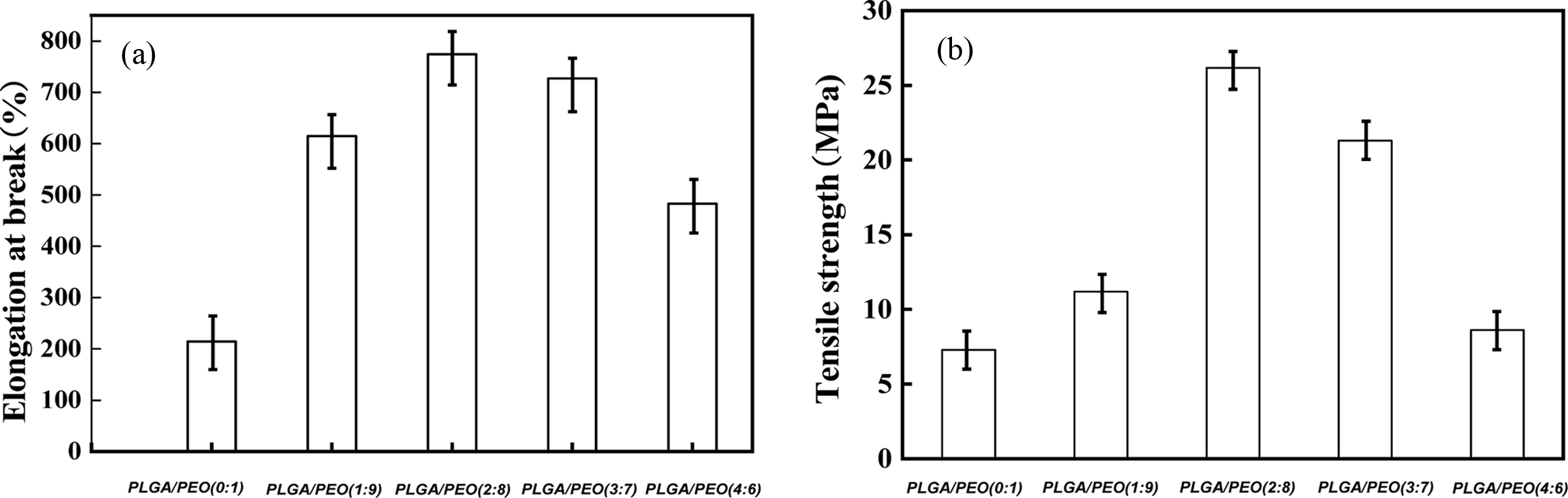
seen from Figure 8, when the content of PLGA is 20%, the tensile strength
reaches 26.1 MPa and the elongation at break reaches 771.2%. The hydrogen
bonding between PLGA and PEO can improve the mechanical strength of the film.
However, when the content of PLGA exceeds 20%, the mechanical properties of the
film gradually decrease. Because PLGA and PEO appear phase separation. As shown
in Figure 7, as the degree of orientation increases, the intermolecular forces
increase. When the deformation of the film reaches about 500%, the tensile
strength increases sharply. The longer the molecular chain, the easier the
molecule is oriented to harden. However, because the molecular chain of PLGA is
not long, the orientation is difficult. So, the elongation at break and tensile
strength decrease.
|
Figure 1 FTIR spectra of PLGA. |
|
Figure 2 1H NMR spectrogram of PLGA. |
|
Figure 3 Distribution of molecular weight of PLGA. |
|
Figure 4 FTIR spectra of PLGA/PEO blends with different mass ratios. |
|
Figure 5 Second heating curves (a); cooling curves (b) of PLGA/PEO blends with different mass ratios. |
|
Figure 6 Weight change of PLGA/PEO blends with different mass ratios in water. |
|
Figure 7 Stress-strain curve of PLGA/PEO blends with different mass ratios. |
|
Figure 8 Tensile properties of PLGA/PEO blends with different mass ratios: (a) elongation at break; (b) tensile strength. |
In this paper, PLGA was prepared by melt
polymerization. Then PLGA and PEO were blended in different proportions. It is
found that there is a hydrogen bonding force between PLGA and PEO, and this
force can improve the mechanical strength and water absorption of the film.
When the PLGA content reached 20 wt%, the tensile strength
and the elongation at break achieved maximum 26.1 MPa
and 771.2%. Compared to neat PEO, blend with 10 wt% of
PLGA had considerably higher water absorption capacity. So, it can be used as a good
water-absorbing material. When the PLGA content was 40 wt%, the film would
not disperse in water. So, it would also be a good insoluble biomaterial. There
is no doubt that the application prospect of PLGA/PEO materials will be more
promising and broader.
- 1. R. S. Lanigan and E. Cosmetic, Int. J. Toxicol., 19, 29 (2000).
- 2. B. S. Lee, J. Mol. Liq., 262, 527 (2018).
-

- 3. P. Ezzati, I. Ghasemi, M. Karrabi, H. Azizi, and I. Fortelny, Polym. Korea, 38, 449 (2014).
-

- 4. L. J. Goujon, A. Khaldi, A. Maziz, C. Plesse, G. T. M. Nguyen, P. H. Aubert, F. Vidal, C. Chevrot, and D. Teyssie, Macromolecules, 44, 9683 (2011).
-

- 5. S. A. M. Noor, A. Ahmad, M. Y. A. Rahman, and I. A. Talib, Ionics, 16, 161 (2009).
-

- 6. G. J. Tudryn, M. V. O’Reilly, S. Dou, D. R. King, K. I. Winey, J. Runt, and R. H. Colby, Macromolecules, 45, 3962 (2012).
-

- 7. X. Zuo, X. Zuo, X. M. Liu, F. Cai, H. Yang, X. D. Shen, and G. Liu, J. Mater. Chem., 22, 22265 (2012).
-

- 8. F. Danhier, E. Ansorenaa, J. M. Silvaa, R. Cocoa, A. L. Bretona, and V. Préat, J. Control. Release, 161, 505 (2012).
-

- 9. H. L. Kim, H. Yoo, H. J. Park, Y. S. Kang, and G. Khang. Polym. Korea, 35, 7 (2011).
-

- 10. M. Ajioka, K. Enomoto, K. Suzuki, and A. Yamaguchi, Bull. Chem. Soc. Jpn., 68, 2125 (1995).
-

- 11. C. M. Dong, K. Y. Qiu, Z. W. Gu, and X. D. Feng, J. Polym. Sci., Part A:Polym. Chem., 38, 4179 (2000).
-

- 12. S. I. Moon, K. Deguchi, M. Miyamoto, and Y. Kimura, Polym. Int., 53, 254 (2004).
-

- 13. Z. Y. Wang, Y. M. Zhao, F. Wang, and J. Wang, J. Appl. Polym. Sci., 99, 244 (2006).
-

- 14. M. Vert, J. Mauduit, and S. Li, Biomaterials, 15, 1209 (1994).
-

- 15. J. F. Lutz, J. M. Lehn, E. W. Meijer, and K. Matyjaszewski, Nat. Rev. Mater., 1, 16024 (2016).
-

- 16. G. ten Brinke, J. Ruokolainen, and O. Ikkala, in Hydrogen Bonded Polymers, W. Binder, Editor, Springer, Berlin, Heidelberg, pp 113-177 (2007).
-

- 17. M. Guo, L. M. Pitet, H. M. Wyss, M.Vos, P. Y. W. Dankers, and E. W. Meijer, J. Am. Chem. Soc., 136, 6969 (2014).
-

- 18. Y. Wang, T. Li, S. Li, R. Guo, and J. Sun, ACS Appl. Mater. Inter., 7, 13597 (2015).
-

- 19. Z. l. Wang, J. L Xua, L. J. Wua, X. Chen, S. G. Yang, H. C. Liu, and X. J. Zhou, Chinese J. Polym. Sci., 33, 1334 (2015).
-

- 20. M. J. S.-Ortega, N. Csaba, L. González, D. B.-González, J. L. O.-Vinuesa, and M. J. Alonso, Colloid Polym. Sci., 288, 141 (2010).
-

- 21. S. Ibrahim and M. R. Johan, Int. J. Electrochem. Sci., 7, 2596 (2012).
- 22. S. Wang, J. Ren, W. Lia, R. Sun, and S Liu, Carbohydr. Polym., 103, 94 (2014).
-

- 23. J. Dong, Y. Ozaki, and K. Nakashima, Macromolecules, 30, 1111 (1997).
-

- 24. V. V. Khutoryanskiy, A. V. Dubolazov, Z. S. Nurkeeva, and G. A. Mun, Langmuir, 20, 3785 (2004).
-

- 25. A. I. Petrov, A. A. Antipov, and G. B. Sukhorukov, Macromolecules, 36, 10079 (2003).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 445-450
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.445
- Received on Jan 29, 2020
- Revised on Mar 3, 2020
- Accepted on Mar 18, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Jianhua Yang, Zhean Xia
-
School of Material Science and Engineering, East China University of Science and Technology, Shanghai, 200237, China
- E-mail: 240296890@qq.com, xiazhean@ecust.edu.cn
- ORCID:
0000-0002-8629-2117, 0000-0002-3904-6271










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.