- Influence of Sulfur/Accelerator Ratio on Tensile Properties and Structural Inhomogeneity of Natural Rubber
Abdulhakim Masa†
 , Siriwat Soontaranon*, and Nabil
Hayeemasae**
, Siriwat Soontaranon*, and Nabil
Hayeemasae**Sino-Thai International Rubber College, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand
*Synchrotron Light Research Institute, Muang District, Nakhon Ratchasima 30000, Thailand
**Department of Rubber Technology and Polymer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani 94000, Thailand- Sulfur/Accelerator 비율이 천연고무 인장특성 및 구조불균형에 미치는 영향 연구
Sulfur and accelerators play
very important roles in curing natural rubber, influencing the properties of
rubber vulcanizates. Such properties are also associated with the
microstructure of vulcanized natural rubber. In this study, the relationships
between tensile properties, strain-induced crystallization behavior, and
structural inhomogeneity were investigated with special attention to the ratio
of sulfur to accelerator (S/Acc). Increasing the S/Acc ratio increased
crosslink density, simply from having more of the crosslinking agent. The
highest tensile strength was obtained at unit ratio (=1), which was associated
with SIC behavior based on wide angle x-ray diffraction measurements.
Reductions in both the tensile strength and crystallinity were noticed when the
crosslink density was relatively high. Structural inhomogeneity of network
structures induced by crosslinking was investigated by means of small angle
x-ray scattering, which showed the increased size and
improved homogeneity of distribution with increased S/Acc ratio.
Effect of sulfur/accelerator ratio on tensile properties
and structural inhomogeneity of natural rubber was specifically highlighted in
this present study. The highest tensile strength and degree of crystallinity at
various strains were obtained at unit ratio equal to 1. The size and
distribution of crosslink network structure were improved with increased S/Acc
ratio.

Keywords: natural rubber, tensile properties, microstructure, strain-induced crystallization
The authors gratefully acknowledged Prince of Songkla
University for financial support (Grant No. RDO6202102S). Research and
Development Office (RDO), Prince of Songkla University and Assoc. Prof. Dr.
Seppo Karrila are also acknowledged for assistance in editing the English
language in this manuscript.
Vulcanization is a well-known action that turns the viscous and elastic
rubber materials into versatile and elastic materials in the presence of
crosslink agent and heat. Among crosslinking agents used in rubber compounding,
sulfur was found to be the foremost extensively utilized in the rubber
industries due to its efficiency, loosely compatible with compounding
ingredients, and permits to predict the ultimate vulcanizate properties.1
In general, sulfur vulcanization systems are classified into three systems,
depending on sulphur/accelerator (S/Acc) ratio. High S/Acc ratio, e.g., 1.67 to
8.75 is called conventional vulcanization (CV). Moderate S/Acc ratio
(0.42-1.42) refers as the semi-efficient vulcanization (Semi-EV), and low S/Acc
ratio (0.08 to 0.4) is labeled as efficient vulcanization (EV).2 Each
system provides its own advantages depending on the designed
formulation of rubber compounders.
Natural rubber (NR) is extensively used in the rubber industries and is
mostly vulcanized in the presence of sulfur. This type of rubber exhibits
excellent and uncommon mechanical properties. These exceptional properties
combine high tensile strength, elongation at break and good crack growth
resistance, and these are partly associated with the ability of rubber to
crystallize under stretching, which is known as strain-induced crystallization
(SIC).3,4 The crystallites generated by SIC are believed to act as
additional filler or as crosslinks,5,6 so the rubber can
self-reinforce without added reinforcing filler. Thus, the SIC behavior is an
important factor contributing to the mechanical properties of NR.
The SIC of NR has been
extensively studied, and the mechanisms of SIC have been elaborated in some
detail.7 It is believed that the shorter chains are the first ones
to be fully stretched, and subsequently act as nucleation sites for
crystallites. Various factors have been reported to affect the mechanical
properties and crystallization behaviour of NR. In unfilled vulcanizate,
crosslink density is a very important parameter dominating in various final
properties of the products.8-10 Zhao et al.9
demonstrated that the mechanical properties, namely hardness, 300% modulus,
tensile strength, and elongation at break strongly depend on crosslink density.
Similar observations were reported by authors.8,10 The effects of
crosslink density on SIC are also discussed in literature.7,11-14
Trabelsi et al.,11 investigated the effects of crosslink
density on properties and crystallization behaviour of NR, by using three
sulfur contents (0.8, 1.2, and 2 phr). They found that the crystallinity and
crystallization rate both decreased with crosslink density. Furthermore, they
also noticed decreased crystallite size with increased crosslink
density. Later on, Chenal et al.,12,13 investigated the effects of
crosslink density in NR on the crystallization rate during stretching. They
reported that the crystallization rate increased when the crosslink density was
below 1.2×10-4 mol.cm-3.
Crosslink densities beyond 1.2×10-4 mol.cm-3 decreased the crystallization
rate. The former characteristic was attributed to the nucleation of
crystallites, while the latter was governed by the growth of the crystallites
in uniaxial deformation. In conflict with this, Tosaka et al.,7
found that both crystallinity and crystallization rate increased with
crosslink density. So, while the influences of crosslink density on SIC
behaviour have been extensively studied, the results do not show a consistent
pattern. Furthermore, the effects of crosslink density on structural
inhomogeneity (size and distribution) of crosslinked network structures has not
been discussed previously. Therefore, it is necessary to further explore the
effects of crosslink density on mechanical properties and on microstructural
changes in crosslinked NR. The NR samples with various crosslink densities were
prepared by using different sulfur and accelerator ratios. The changes in
crosslink density was studied by means of swelling tests. Tensile properties
and corresponding microstructural changes during stretching were studied by
means of tensile-testing and wide angle x-ray diffraction (WAXD), and sizes as
well as distributions of crosslinked network structures were revealed by small
angle x-ray scattering (SAXS) measurements. This study contributes to the
scientific understanding on how sulfur and accelerators influence the
microstructure in a rubber vulcanizate, and provide useful information for
predicting the performance of natural rubber.
Materials. NR grade STR 5L
was purchased from Chalong Concentrated Natural Rubber Latex Industry Co.,
Ltd., Thailand. Stearic acid and zinc oxide (ZnO) were manufactured by Imperial
Chemical Co. Ltd., Pathumthani, Thailand and Global Chemical Co. Ltd., Samut Prakarn, Thailand, respectively, and these were used as
activators. N-cyclohexyl-benzothiazyl-sulphenamide (CBS) used as
accelerator was purchased from Flexsys America L.P., West Virginia, USA, and
sulfur used as crosslinking agent was supplied by Siam Chemical Co., Ltd., Samut
Prakan, Thailand.
Sample
Preparation. NR and other ingredients such as stearic acid, ZnO, CBS
and sulfur were compounded in a laboratory-sized internal mixer (Brabender®
GmbH & Co. KG, Duisburg, Germany). The compound formulations and mixing
steps are displayed in Table 1. Total mixing time was kept constant at 5 min
and total compounding ingredients were fixed at 107 part(s) per hundred parts
of rubber (phr) for all samples. The compounds were compression molded by hot
pressing at 160 °C each for its respective curing time determined with a
rheometer. In this study, the amounts of curative and accelerator were varied,
but the total of curative and its accelerator was held fixed at 3 phr. The
samples with S/Acc ratios of 0.07, 0.2, 0.5, 1 and 2 were labelled as S0.07,
S0.2, S0.5, S1 and S2, respectively.
Characterizations. Curing Characteristics: The curing
characteristics of the NR compounds with varied S/Acc ratios were investigated
at 160 °C using a moving die rheometer (Montech MDR 3000 BASIC, Germany).
The scorch time (ts1), cure time (tc90),
maximum torque (MH) and torque difference (MH-ML)
were determined.
Swelling
Measurement: The swelling test was performed to estimate the crosslink
density (ν) in each vulcanizate sample. The rubber specimens were immersed in
toluene solvent at room temperature for 168 h. The solvent was refreshed every
24 h. The swelling percentage was calculated as follows.
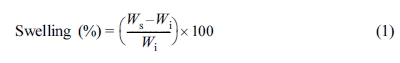
where Wi is the initial weight of sample (g) and Ws
is the weight of swollen sample (g). The results from the swelling test were
used to estimate the crosslink density (ν) in each NR vulcanizate by applying
the Flory-Rehner equation:15
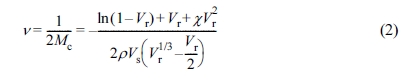
where Vr is the volume fraction of rubber in the
swollen mass, χ is the polymer-solvent interaction parameter (0.39 for
the NR-toluene system),16 ρ is density of the polymer, and Vs
is molar volume of the solvent (106.3 cm3/mol). The Vr
can be calculated from eq. (3):

where W1 is de-swollen weight, W2
is swollen weight, ρr is density of rubber, and ρs
is density of toluene.
Tensile
Measurement: The tensile properties of NR vulcanizates were
investigated by means of a universal tensile testing machine (LLOYD
Instruments, LR5K Plus, UK). Dumbbell shaped test specimens were cut and tested
at room temperature and 500 mm/min crosshead speed according to ISO 37.
Five replicates were done for each type of sample in the tensile test.
WAXD and SAXS Measurement:
Crystallization behaviors under stretching of the NR
vulcanizates were investigated by means of WAXD, while the structural
inhomogeneity, including size and distribution of crosslinked networks were
estimated by means of SAXS measurements. Both WAXD and SAXS were performed at
Beamline 1.3 W, the Siam Photon Laboratory, Synchrotron Light Research
Institute (SLRI), Nakhon-Ratchasima, Thailand. The distance from sample to
detector was 11.5 cm for WAXD, and 4.5 m for SAXS. The WAXD and SAXS data were
collected during continuous stretching with a crosshead speed of 50 mm/min, and
the exposure time for collecting data at a fixed strain was about 30 sec.
All WAXD and SAXS data were normalized and corrected by using SAXSIT data
processing software.
|
Table 1 Compound Formulations and Mixing Schedules for NR with Various S/Acc Ratios |

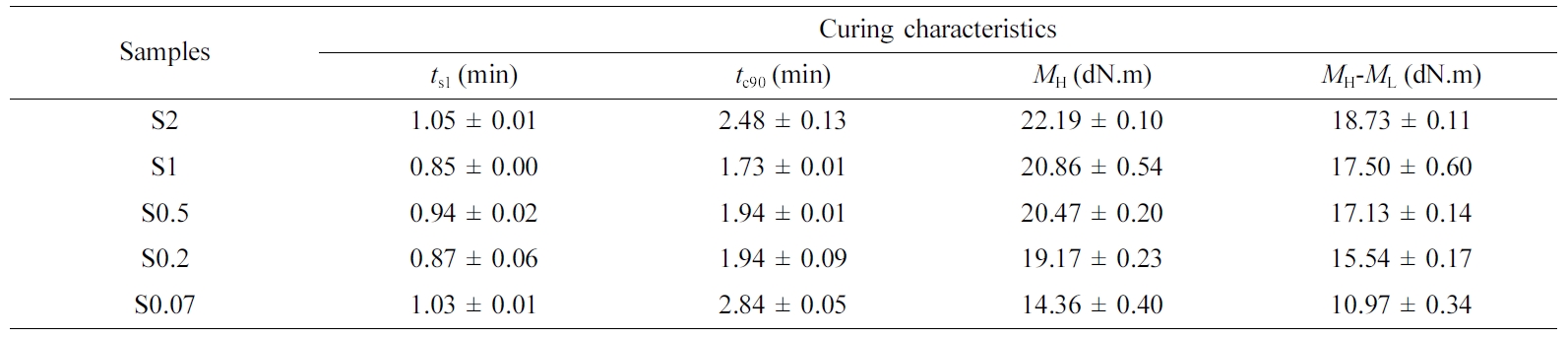
Curing
Characteristics. The curing characteristics in term of ts1,
tc90, MH and MH-ML
at 160 °C of the NR compounds with varied S/Acc ratios is shown in Table
2. The ts1 and tc90 decreased slightly with
increasing S/Acc ratio, shorter ts1 and tc90
indicates faster curing process. This is extremely beneficial as they increase
the production rate. However, an increase of both ts1 and tc90
was again noticed at S/Acc equal to 2, where the accelerator content was too
low due to an insufficient amount of accelerator for the curing reactions. This
is commonly observed as lower content of accelerator was used in the
formulation.8,17,18 It was observed that the MH
and MH-ML increased with increasing
the ratio S/Acc. The MH is a measure of stiffness of the
compounds while the MH-ML indicates the
degree of crosslinking.19 This has suggested that the stiffness and
crosslinking degree of the vulcanizates increased as the ratio of S/Acc
increased. A higher loading of the sulfur crosslinking agent induces a greater
crosslink density due to the higher availability of active sulfurating agents
for crosslinking.
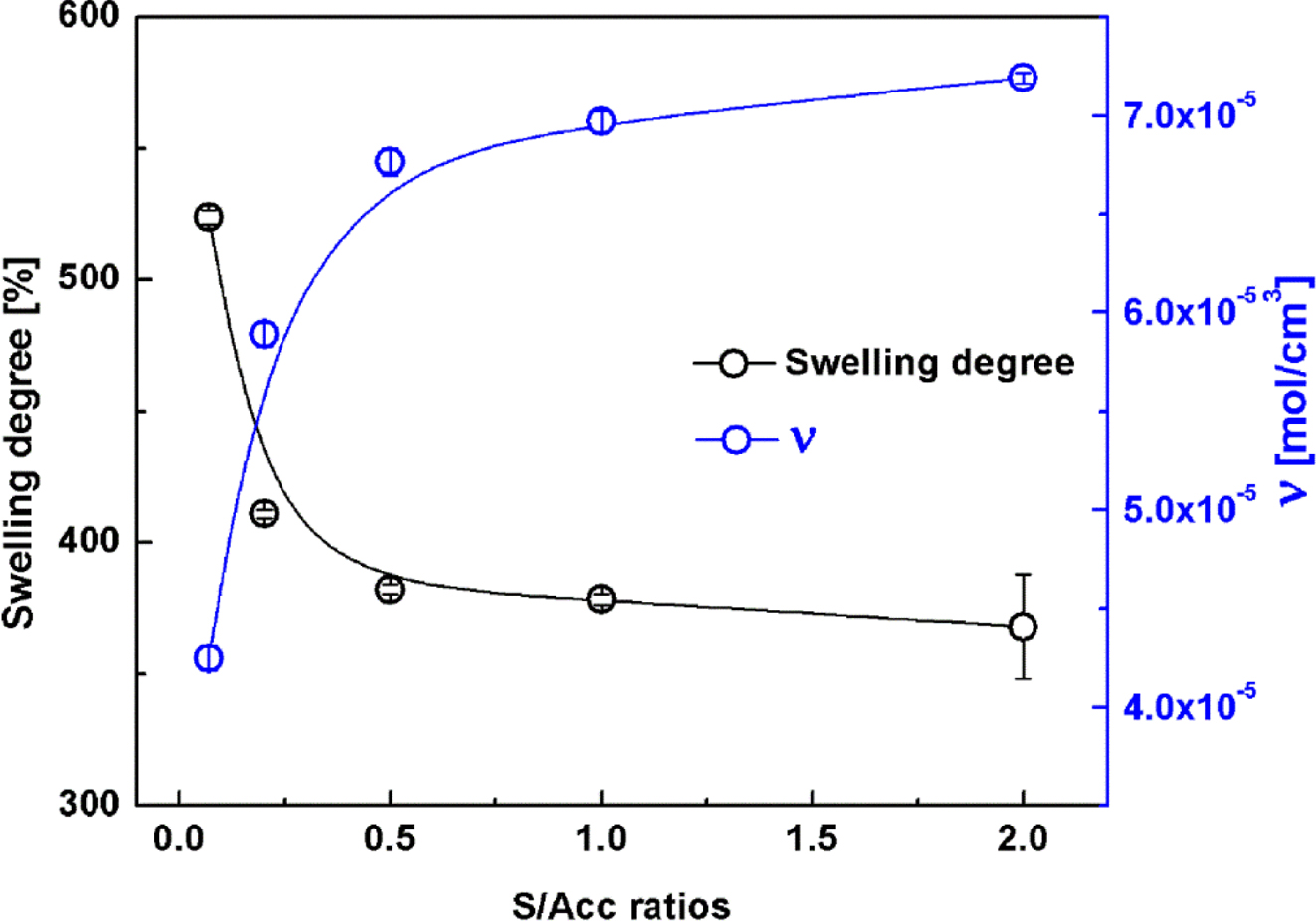
Swelling
Behavior. Figure 1 shows the degree of swelling in NR crosslinked
with the various S/Acc ratios. The swelling of NR vulcanizates decreased with
S/Acc ratio, implying lesser solvent penetration into the samples. It is
well-known that the swelling capacity is inversely related to the total crosslink
density, and a lower swelling degree indicates a higher crosslink density.19
The crosslink density (ν) in the various samples was later estimated by
applying the Flory-Rehner equation,15 and the results are also
included in Figure 1. In accordance with curing characteristics results, the
crosslink density in NR increased with S/Acc ratio. The highest ν was
obtained for the highest concentration of sulfur (S/Acc = 2).
Increase of crosslink density with S/Acc ratio was due to the increased
sulfurating chemicals causing initiation and propagation of the crosslinking
reactions. Thus, crosslink density increased with the S/Acc ratio.
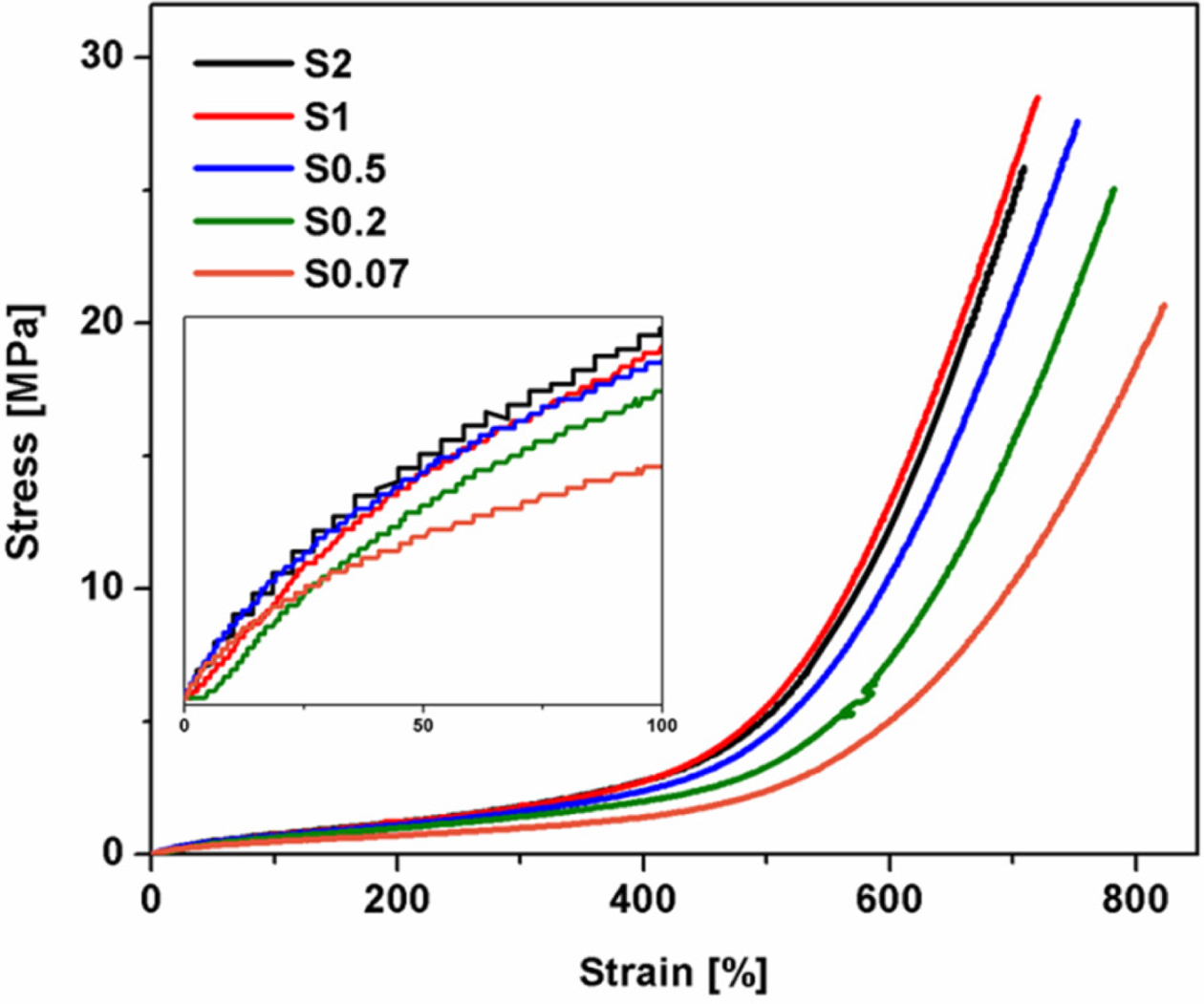
Tensile
Properties. Figure 2 shows stress-strain curves of NR vulcanizates
with the various S/Acc ratios. It is seen that all curves showed a steep
increase in stress at high strains (i.e., over 400% strains) and this pattern
in response was attributed to the SIC behavior.20,21 It is also seen
that the stress at low strain also increased with S/Acc ratio (see the embedded
Figure) due to crosslink density.9
The stress at 100% and 300% strains, tensile strength and elongation at
break are shown in Figures 3 and 4. Figure 3 shows the stresses at 100% and
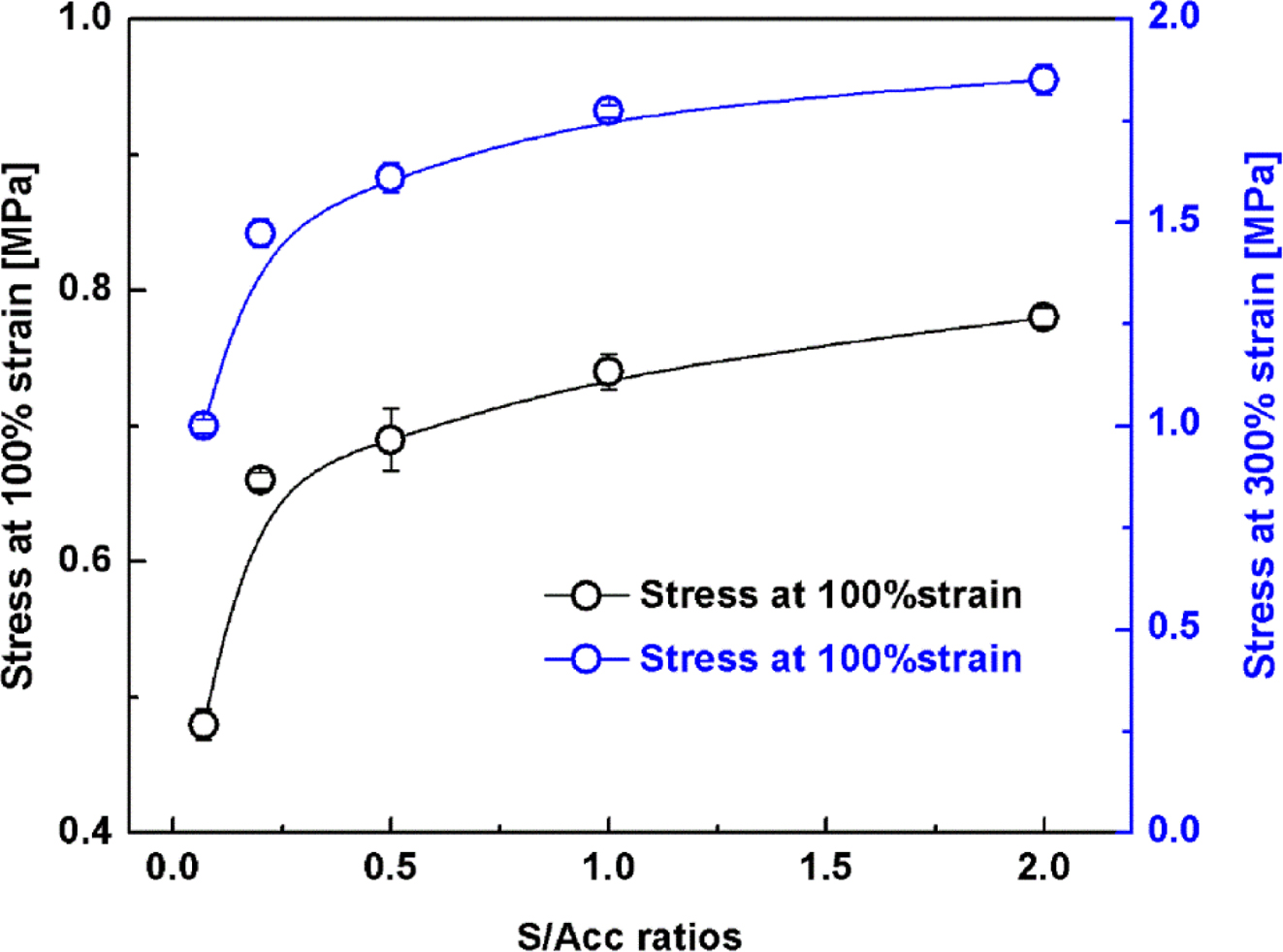
300% strains for the NR vulcanizates with varied S/Acc ratios. Both stresses
increased gradually with S/Acc ratio, which is again attributed to crosslinking
density. A higher crosslink density gives a stronger network and thus enhances
the stress at 100% and 300% strains.
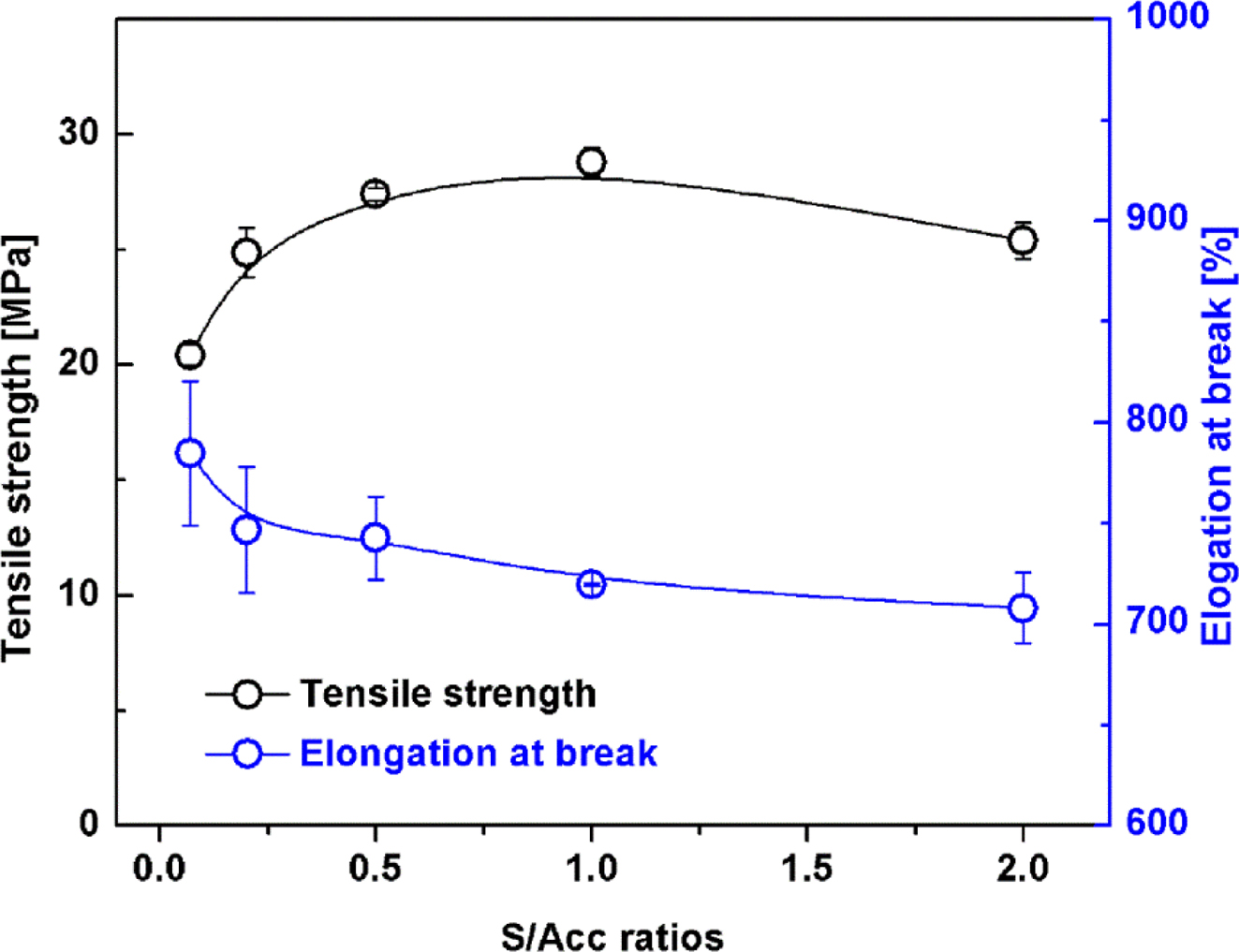
Figure 4 shows tensile strength and elongation at break of the NR
vulcanizates. The tensile strength increased passing through a maximum as S/Acc
ratio increased. The highest tensile strength was obtained at S/Acc equal to 1,
with the crosslink density approximately 7×10-5 mol/cm3.
Reduction of tensile strength after the maximum was attributed to excessive
crosslink density. When the crosslink density is relatively high, the chain
length between two adjacent crosslinking points is short, restricting
orientation of the chains during stretching; thus the tensile strength
decreases.9,22 The elongation at break decreased with S/Acc ratio
because stiffness of the rubber increased with crosslink density, restricting
rubber chain mobility.
Based on the tensile testing, increased crosslink density was responsible
for increasing stress at 100% and 300% strains, while the tensile response from
SIC occurs at higher strains. SIC provides self-reinforcement to vulcanized
rubber.4,23 Therefore, crystallization behavior during stretching
should be given attention. To gain further understanding of the effects of
S/Acc ratio on high strain tensile behaviors, WAXD measurements were conducted
during tensile deformation.
WAXD
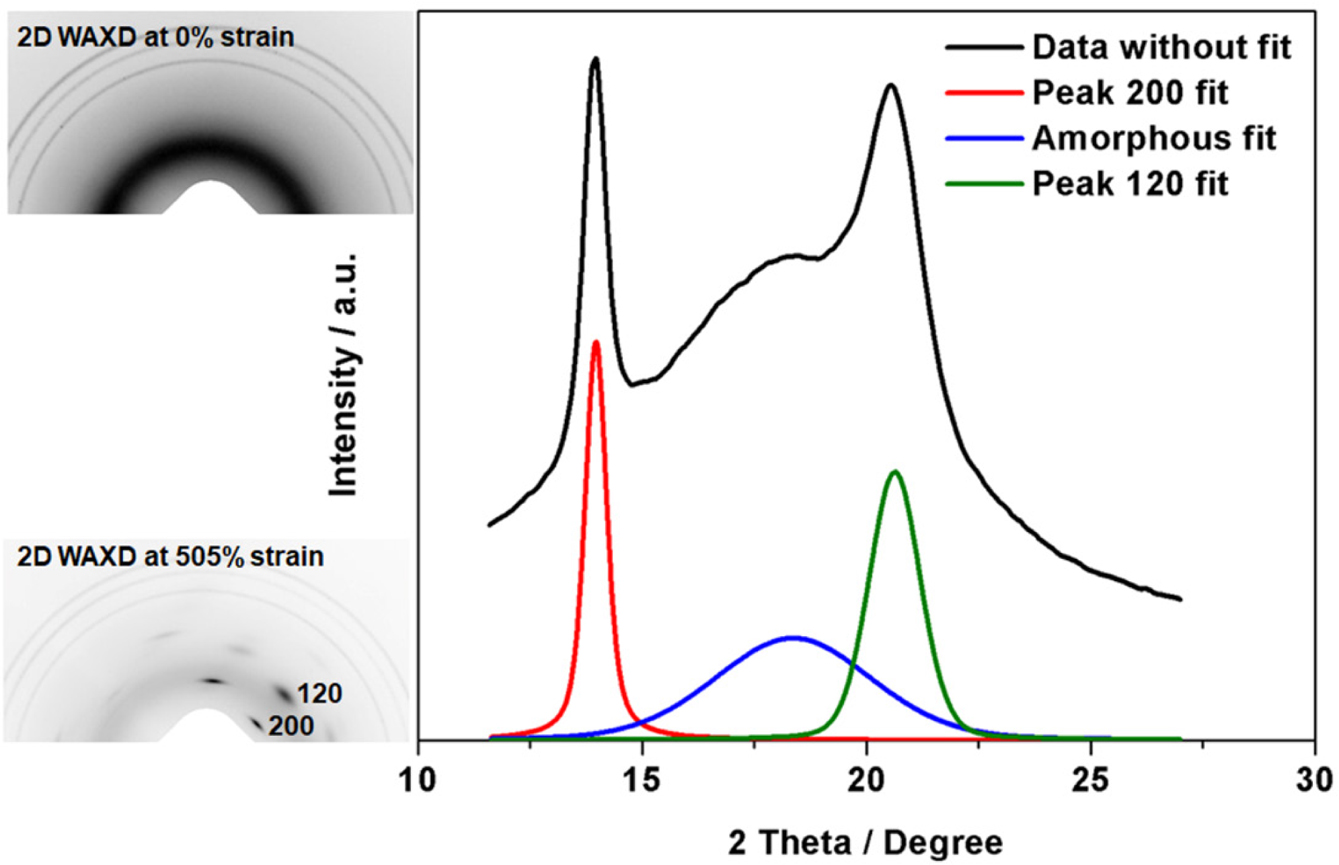
and SAXS. Figure 5 shows representative 2D WAXD images of
unstretched (0% strain) and highly stretched states (505% strain) of vulcanized
samples, and the linear 1D data at highly stretched state are also included.
Without deformation, no reflection spots were observed in the 2D image and the
corresponding 1D pattern appeared as the blue line (amorphous pattern). In a
highly deformed state, reflection spots were detected in the 2D image and a
crystalline peak appeared in the corresponding 1D pattern (black line). After
peak fitting with SAXSIT software, the areas of amorphous and crystalline peaks
were recorded. Among the diffraction spots, the crystallographic planes (200)
and (120) are of particular importance. In this study, variation of the
crystallinity and crystallite size corresponding to (200) plane was assessed
during deformation.
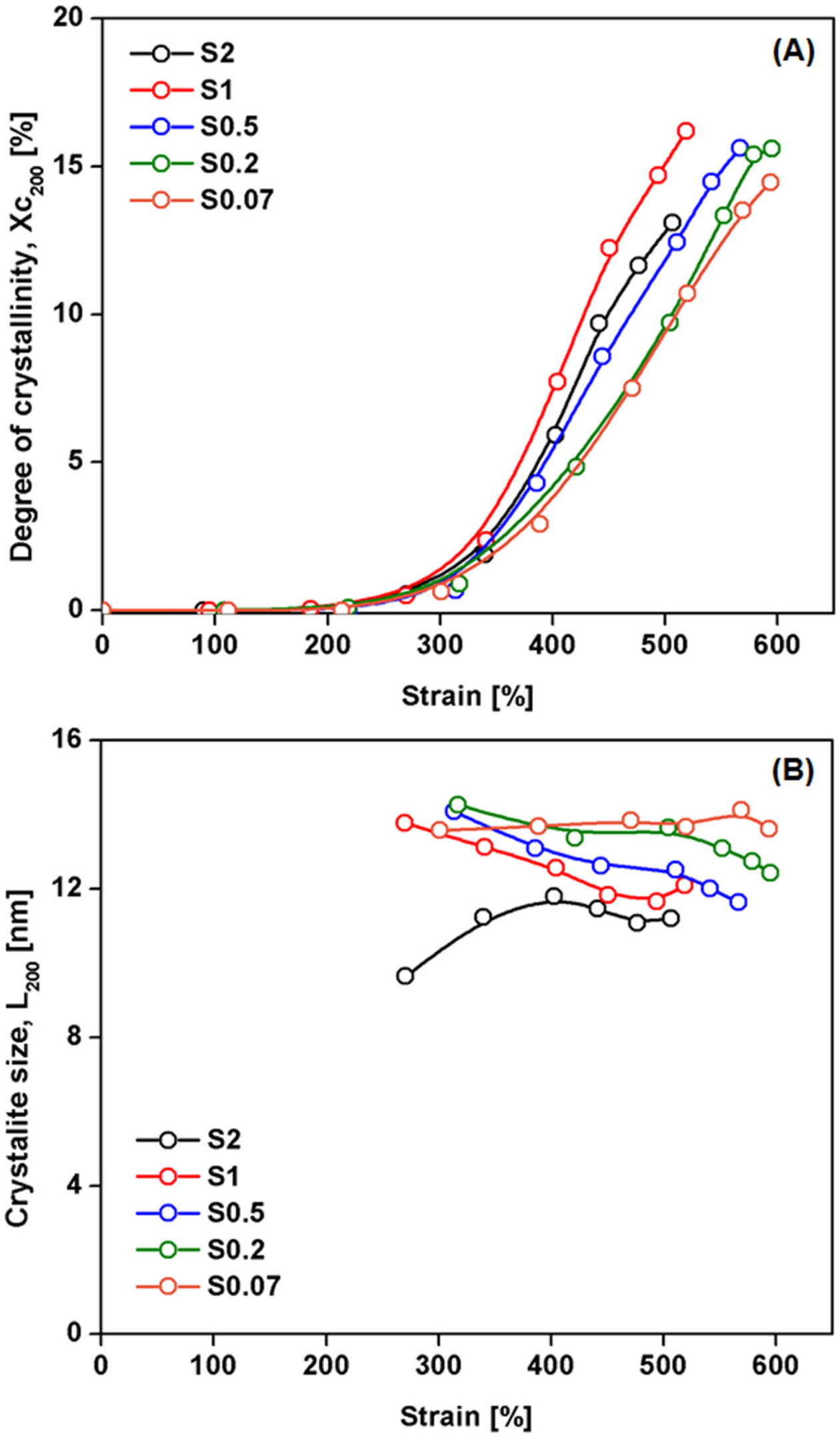
Figure 6 shows a plot of (A) degree of crystallinity (%), and (B) of
lateral crystallite size corresponding to (200) plane, during stretching of the
NR vulcanizates with various S/Acc ratios. The degree of crystallinity (DC) was
calculated as follows;24

Here, Ac is the area of crystalline peaks assigned to
the (200) plane, and Aa is the area of the amorphous halo.
The Scherrer equation was used to estimate the average crystallite size
in the direction perpendicular to the (200) plane (L200) as
follows:25,13

where the K is 0.89, λ is the wavelength, β is the
half-width at half-height, and θ is the Bragg angle.
As can be seen from Figure 6(A), the crystallinity in all cases increased
with strain, because stretching oriented the rubber chains. It is believed that
initially SIC originates from highly stretched short chain segments between
crosslinks that are heterogeneously distributed. The shorter chains are fully
stretched and became nucleation sites for crystallites.24 These
crystallites behave as filler or additional crosslinks,5,6 providing
self-reinforcement to the rubber, and thus stress increases rapidly at high
tensile strains. It is also seen that the crystallinity at any given strain
increased with S/Acc ratio (via the crosslink density), and the highest
crystallinity was found for S/Acc ratio equal to 1. It should be mentioned that
the rate of crystallization seemed to increase with S/Acc ratio, except that
the crystallization rate was decreased at ratio 2. This result suggests that
the best choice of S/Acc is about 1. Further increase in S/Acc reduced the SIC
effects due to excessive crosslinking that hindered crystallization.26
The variation of crystallinity corresponds well to the stress-strain curves
(Figure 2), so the tensile properties can be partly explained by the SIC
behavior.
Figure 6(B) shows two interesting phenomena regarding the crystallite
size during stretching. When the ratio of S/Acc was 2, and the crosslink
density is excessive, the crystal size developed from small to large while
other cases showed opposite behavior. Moreover, the size of crystals was also
smaller than in the other cases at any given strain. These observations can be
explained by two mechanisms: 1) the large amount of short chains serving as
crystallite nucleation is favored in the sample containing high crosslink
density. When the crystal formed in the densely crosslink area, high number of
crystal formation suppressed each other and thus the size of crystal becomes
small; and 2) when the crosslink density was high, the molecular chains between
crosslinks were short. Shortening the free segments of molecular chains
suppresses chain mobility and orientation responses that produce large
crystallites during stretching,11,24 while the crystallite size in
less crosslinked rubber sample was larger due to the longer free chains between
crosslinks.11
To assess the network structures in the samples with various S/Acc
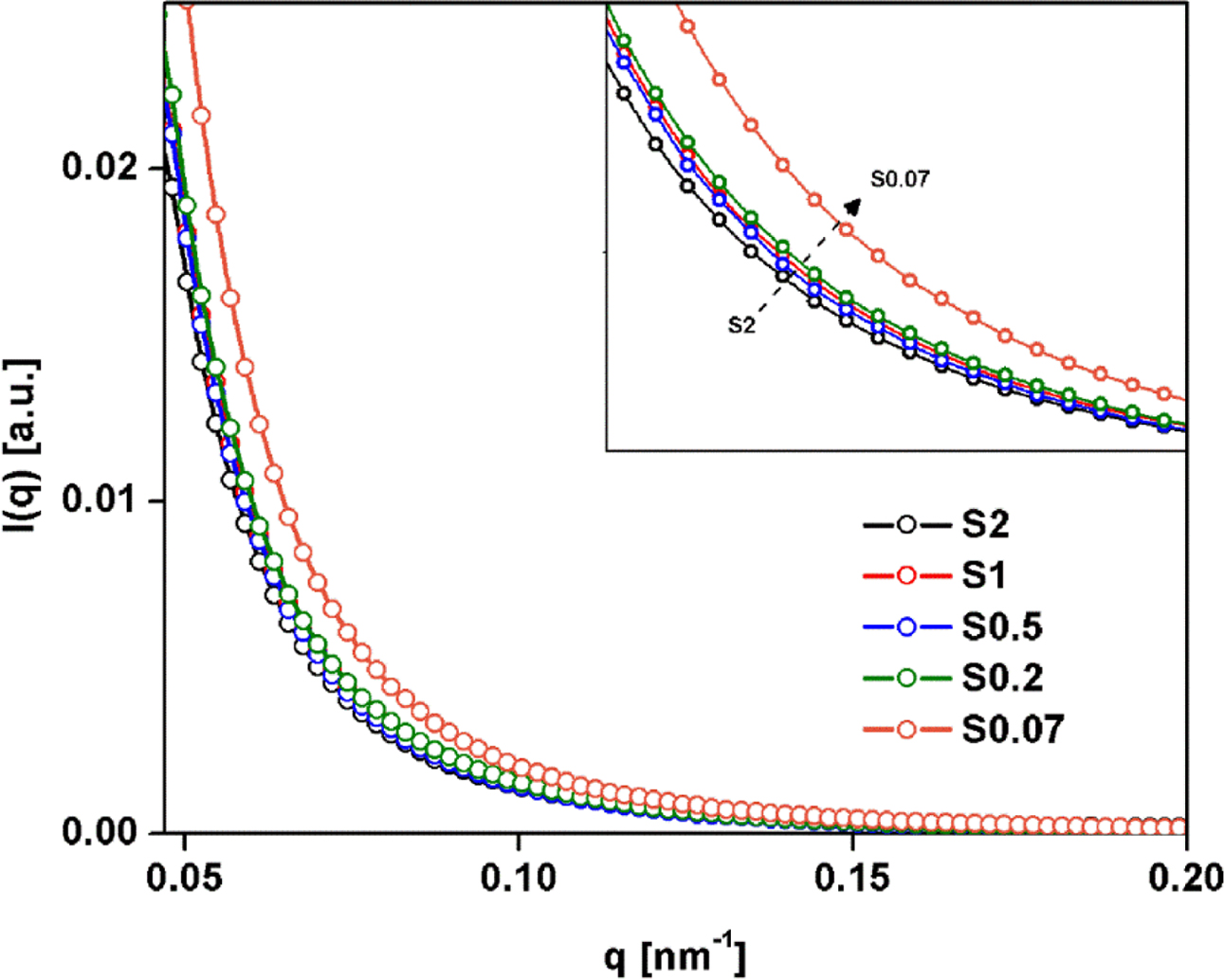
ratios, SAXS measurements were conducted. Figure 7 shows plot of I(q)
as a function of q for the NR samples with various S/Acc ratios. I(q)
is the scattering intensity at q, and q is the scattering vector
(q = (4π/λ) sin q, where l and 2q is the wavelength
and scattering angle). Owing to the vulcanizates contained various elements,
e.g., ZnO, sulfur and other impurities in NR, argument of these elements
affected electron density fluctuations in the SAXS patterns may arise. However,
it has been reported that the ZnO particles do not contribute to the SAXS
patterns, due to the micrometer size range that is not contributing the small q
range, and the SAXS intensity patterns for uncrosslinked rubber compound
containing sulfur displayed lower intensity than that for crosslinked sample.27
Therefore, the effects of ZnO, unreacted sulfur and others components
(including impurities) can be ignored and the pattern of SAXS after
vulcanization should be attributed to crosslinked networks. The crosslinking
reactions changed the local electron density at the crosslink and in its
neighborhood, and can be detected by SAXS.
From Figure 7, it is clearly seen that all cases showed an upturn in
intensity within the low-q region, caused by the heterogeneous network
structures in the vulcanizates created by chemical crosslinking.28,29
Interestingly, the intensity decreased with S/Acc ratio (or with crosslink
density), indicating decreasing heterogeneity.28 Increasing the
crosslink density improved homogeneity of networks in the rubber vulcanizates.
Previously it has been suggested that excessive crosslinking may create
inhomogeneous network structures, although no convincing evidence was provided.30
Within the range of S/Acc ratios in recent studies, the SAXS results suggest
that increasing crosslink density improved homogeneity, so the inhomogeneity of
crosslinks should not be responsible for cracks in highly crosslinked
specimens. The cracks in highly crosslinked sample will be discussed later.
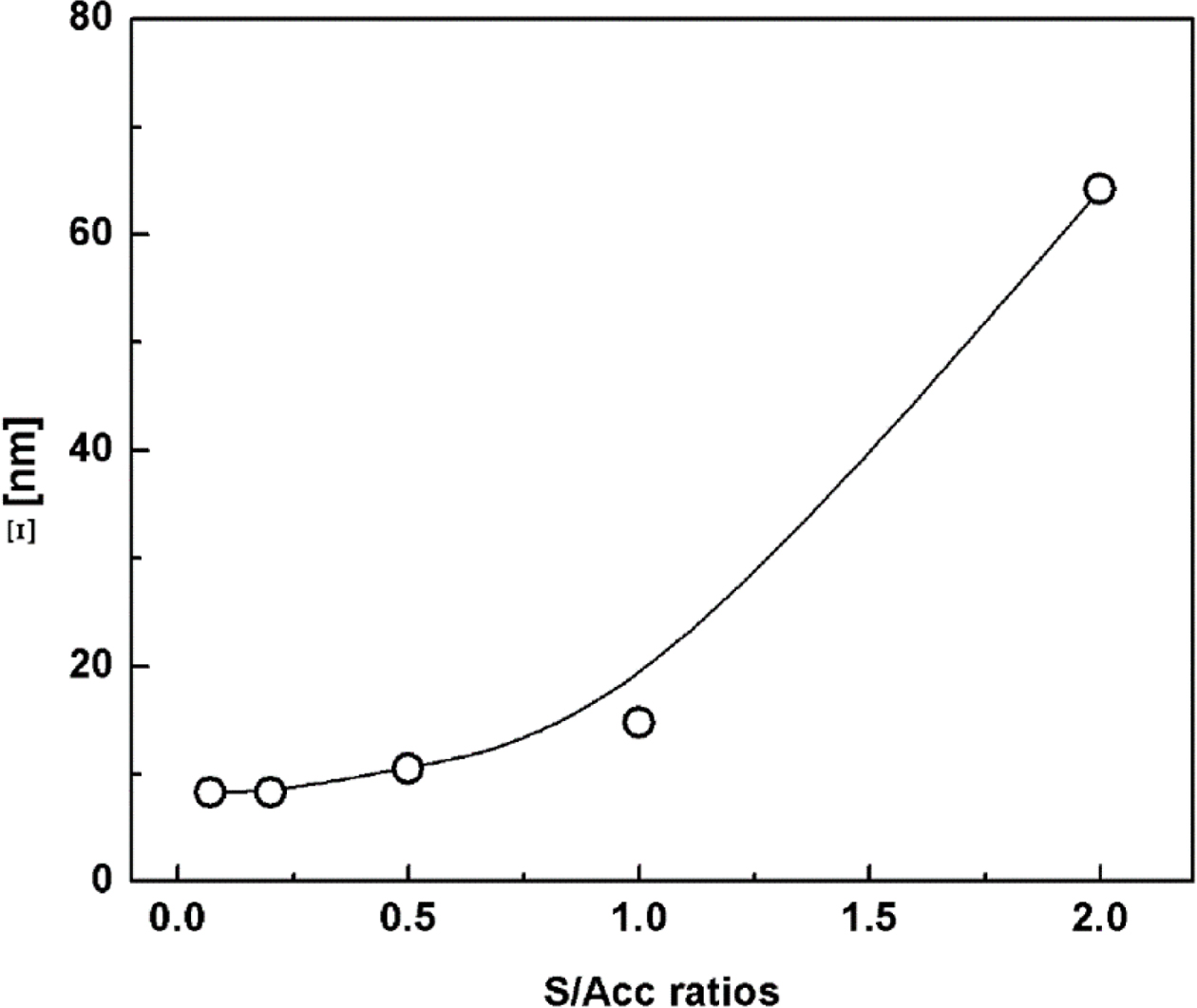
In addition to the distribution of network structures, the characteristic
size of crosslinked structures formed during vulcanization is another factor
contributing to the SAXS profile. The characteristic size of crosslinked
network structures can be estimated by applying the Debye-Bueche equation:31,32

where Ξ is the characteristic size of the heterogeneous network structures.
Figure 8 shows the characteristic size of the crosslinked structures in
the various vulcanizate samples. The characteristic size increased with S/Acc
ratio, suggesting that the size of crosslinked networks became larger as the
crosslink density increased. This might be attributed to the greater number of
crosslink agents readily available for crosslinking reactions. Increase of
characteristic size of crosslinked structures with increment of crosslink agent
was also reported previously, based on small-angle neutron scattering.29
It is interesting that large-sized crosslinked networks contain lots of
crosslinks and thus the number of short free chains would be also high. These
short chains would then limit the extension and orientation of molecular chains
around the crosslink junctions during deformation, and cracking could be
initiated where the short chains are dense. This could be the mechanism of
crack formation with high crosslink density.
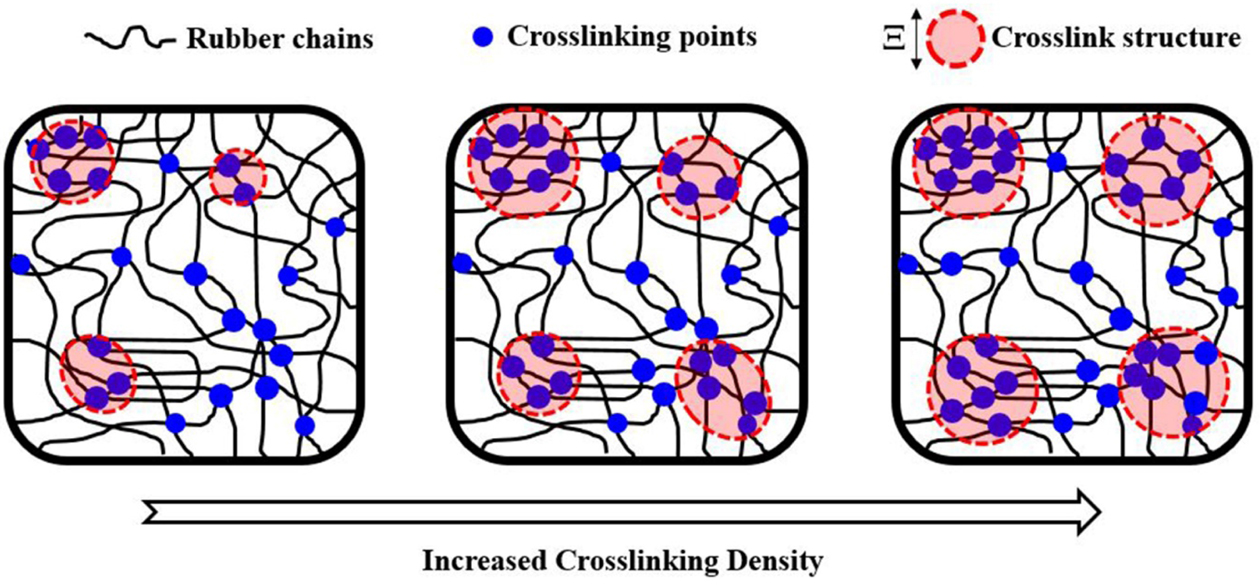
Based on the WAXD and SAXS results, a model illustration of how crosslink
density influences size and homogeneity of the network structures could be
developed, and is shown in Figure 9. When the crosslink density is small,
inhomogeneity of the network sizes would be seen initially, as a result of high
competition for the reactive sites on rubber chains to interact with a small
amount of crosslinking agents. Increasing the amount of crosslinking agents
reduced competition between the active chains for reacting with the sulfur
crosslinking agents, thus increasing the size of crosslinked structures with
less heterogeneity.
Improved homogeneity of the crosslinked network structures contributed to
SIC. However, the size of network structure also increased with the ratio
S/Acc. Large size of the crosslinked network structures may have negative
effects on the crystallization in NR and tensile properties, as suggested by
the highest S/Acc ratio in this current study. Therefore, the mechanical
properties of rubber vulcanizates depend on various factors, including
crosslink density, distribution and size of network structures, and their
ability to crystallize during stretching. Carefully selecting the proper
balance between sulfur and its accelerator could improve the mechanical
properties of vulcanizates.
|
Figure 1 Degree of swelling and crosslink density of NR compounds with various S/Acc ratios. |
|
Figure 2 Stress-strain curves of NR vulcanizates with various S/
Acc ratios. |
|
Figure 3 Stress at 100% and 300% strains of NR vulcanizates with
various S/Acc ratios. |
|
Figure 4 Tensile strength and elongation at break of NR vulcanizates with various S/Acc ratios. |
|
Figure 5 Representative 2D WAXD images and 1D data in
unstretched and highly stretched states (sample S/Acc = 2). |
|
Figure 6 Plot of (A) degree of crystallinity (%); (B) lateral crystallite size corresponding to the (200) plane during stretching of the
NR vulcanizates with various S/Acc ratios. |
|
Figure 7 Linear SAXS patterns of NR vulcanizates with various S/
Acc ratios. |
|
Figure 8 Characteristic sizes (Ξ) of the heterogeneous network
structures in NR vulcanizates with various S/Acc ratios. |
|
Figure 9 Illustration of crosslinked network structure changes with
crosslink density |
|
Table 2 Cure Characteristics at 160 °C of the NR Compounds with Varied S/Acc Ratios |
In this study, effects of various sulfur to accelerator (S/Acc) ratios,
ranging from 0.07 to 2.0, on swelling and mechanical properties, and structure
inhomogeneity of NR vulcanizates were investigated. Increasing the S/Acc ratio
usually increased maximum torque, torque difference and crosslink density,
because there was more crosslinking agent in the compound. Stress at low
strains also increased with the S/Acc ratio, but the highest tensile strength
was obtained when the ratio was 1 and the crosslink density was about 7×10-5
mol/cm3. Deformation induced crystallization was assessed by
means of WAXD and it matched well the observed tensile strengths. It is
interesting to note that the crystallite size developed from small to big in
the sample where the crosslink density was the highest among those tested.
Furthermore, the sizes of crosslinked network structures were found to
increase, with a more homogeneous distribution, when the ratio S/Acc (or the
crosslink density) was increased. Based on SAXS measurements both these
structural parameters played important roles in crystallization and in tensile
strength of the NR vulcanizates.
- 1. P. A. Ciullo and N. Hewitt, The Rubber Formulary, William Andrew, New York, 1999.
- 2. R. N. Datta, Rubber Curing Systems, Smithers Rapra Technology, Shawbury, 2002.
- 3. K. Bruning, K. Schneider, S. V. Roth, and G. Heinrich, Polymer, 54, 6200 (2013).
-

- 4. Y. Fukahori, Polymer, 51, 1621 (2010).
-

- 5. B. Huneau, Rubber Chem. Technol., 84, 425 (2011).
-

- 6. M. Tosaka, K. Senoo, S. Kohjiya, and Y. Ikeda, J. Appl. Phys., 101, 084909 (2007).
-

- 7. M. Tosaka, S. Kohjiya, S. Murakami, S. Poompradub, Y. Ikeda, S. Toki, I. Sics, and B. S. Hsiao, Rubber Chem. Technol., 77, 711 (2004).
-

- 8. L. Gonzalez, A. Rodriguez, J. L. Valentin, A. Marcos-Fernandez, and P. Posadas, Kautschuk und Gummi Kunststoffe, 58, 638 (2005).
- 9. F. Zhao, W. Bi, and S. Zhao, J. Macromol. Sci. B, 50, 1460 (2011).
-

- 10. K. Boonkerd, C. Deeprasertkul, and K. Boonsomwong, Rubber Chem. Technol., 89, 450 (2016).
-

- 11. S. Trabelsi, P. A. Albouy, and J. Rault, Macromolecules, 36, 7624 (2003).
-

- 12. J. M. Chenal, C. Gauthier, L. Chazeau, L. Guy, and Y. Bomal, Polymer, 48, 6893 (2007).
-

- 13. J. M. Chenal, L. Chazeau, L. Guy, Y. Bomal, and C. Gauthier, Polymer, 48, 1042 (2007).
-

- 14. Z.-T. Xie, M.-C. Luo, C. Huang, L.-Y. Wei, Y.-H. Liu, X. Fu, G. Huang, and J. Wu, Polymer, 151, 279 (2018).
-

- 15. P. J. Flory and J. Rehner, Jr., J. Chem. Phys., 11, 512 (1943).
-

- 16. S. Musto, V. Barbera, V. Cipolletti, A. Citterio, and M. Galimberti, Express Polym. Lett., 11, 435 (2017).
-

- 17. B. H. Park, I. G. Jung, and S. S. Park, Polym. Korea, 25, 63 (2001).
- 18. S. Rabiei and A. Shojaei, Eur. Polym. J., 81, 98 (2016).
-

- 19. H. Nabil, H. Ismail, and A. R. Azura, J. Vinyl Addit. Technol., 21, 79 (2015).
-

- 20. A. Masa, R. Saito, H. Saito, T. Sakai, A. Kaesaman, and N. Lopattananon, J. Appl. Polym. Sci., 133, 43214 (2016).
-

- 21. M. Tosaka, Polym. J., 39, 1207 (2007).
-

- 22. G. R. Hamed and N. Rattanasom, Rubber Chem. Technol., 75, 935 (2002).
-

- 23. B. Ozbas, S. Toki, B. S. Hsiao, B. Chu, R. A. Register, I. A. Aksay, R. K. Prudhomme, and D. H. Adamson, J. Polym. Sci., Part B: Polym. Phys., 50, 718 (2012).
-

- 24. M. Tosaka, S. Murakami, S. Poompradub, S. Kohjiya, Y. Ikeda, S. Toki, I. Sics, and B. S. Hsiao, Macromolecules, 37, 3299 (2004).
-

- 25. Y. Ikeda, Y. Yasuda, K. Hijikata, M. Tosaka, and S. Kohjiya, Macromolecules, 41, 5876 (2008).
-

- 26. E. H. Andrews, J. Polym. Sci., Part B: Polym. Phys., 4, 668 (1966).
-

- 27. W. Salgueiro, A. Somoza, I. L. Torriani, and A. J. Marzocca, J. Polym. Sci., Part B: Polym. Phys., 45, 2966 (2007).
-

- 28. N. Osaka, M. Kato, and H. Saito, J. Appl. Polym. Sci., 129, 3396 (2013).
-

- 29. Y. Ikeda, N. Higashitani, K. Hijikata, Y. Kokubo, Y. Morita, M. Shibayama, N. Osaka, T. Suzuki, H. Endo, and S. Kohjiya, Macromolecules, 42, 2741 (2009).
-

- 30. W. Sainumsai, S. Toki, S. Amnuaypornsri, A. Nimpaiboon, J. Sakdapipanich, L. Rong, B. S. Hsiao, and K. Suchiva, Rubber Chem. Technol., 90, 728 (2017).
-

- 31. P. Debye and A. M. Bueche, J. Appl. Phys., 20, 518 (1949).
-

- 32. A. Izumi, Y. Shudo, T. Nakao, and M. Shibayama, Polymer, 103, 152 (2016).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 519-526
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.519
- Received on Mar 14, 2020
- Revised on Apr 16, 2020
- Accepted on Apr 22, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Abdulhakim Masa
-
Sino-Thai International Rubber College, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand
- E-mail: abdulhakim.m@psu.ac.th
- ORCID:
0000-0002-0577-4844









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.