Different polymerization techniques of poly(N-vinyl caprolactam) (PNVCL) have been reported including the photo polymerization via ultraviolet (UV). However, photo polymerization of PNVCL via UV-C (wavelength of 254 nm) has not been reported. In this study, N-vinyl caprolactam was polymerized via UV-C irradiation at different time periods. Fouirer transform infrared spectroscopy (FTIR) analysis showed that the PNVCL has been successfully synthesized via the photo polymerization method. The optimum conversion time was found as about 70 min and the conversion was found as 71.2%. Gels displayed different swelling behavior at temperatures above and below lower critical solution temperature (LCST), and swelling analysis, dynamic light scattering (DLS) and differential scanning calorimetry (DSC) were performed to investigate the phase change behavior. It was observed that LCST of the synthesized gels occurs between 31 and 35 oC determined by DSC analysis, which is also consistent with DLS and swelling analysis results. In conclusion, a facile and short time period polymerization technique is reported via use of UV-C photo polymerization technique.
A facile and short time period polymerization technique is reported via use of UV-C photo polymerization technique. The water absorption is low due to the hydrophobic nature of the polymer at the temperatures above the lower critical solution temperature. The presence of cross-linker significantly increased the mechanical properties of the gels.

Keywords: poly(N-vinyl caprolactam), UV-C irradiation, polymerization, temperature responsive polymer, lower critical solution temperature
The production and development of polymeric systems that can be tuned according to environmental requirements have been of interest to materials science researchers in recent years. It is an area that has emerged in recent years and has a supra-disciplinary position. Polymers, which are called “smart” or stimuli-responsive”, can respond to small changes in their environment by making a significant change in their microstructure properties.1 Stimuli-responsive polymers are becoming increasingly interesting as scientists learn about the chemistry and triggers that cause conformational changes in material structures and develop ways to exploit and control them.2 The new smart materials can chemically formulate that detect certain environmental changes and adjust predictably, making them useful tools.3 The stimuli of smart polymers are classified as; a) physical, i.e. temperature, electric or magnetic fields, mechanical stress; and ii) chemical, i.e. pH, ionic factors, chemicals, biological agents.4 Currently, temperature and pH-sensitive/responsive systems are attracting research focus because of varying purposes for example a disease could be perceptible using temperature and pH response.5
Stimuli-responsive polymers are frequently used owing to their unique properties and their structure that can be tuned by environmental conditions and it has many other application areas where biotechnology,6 sensors,7 chemical valves,8 enzymes9 and cell immobilization10 are some of the focusing areas.11,12 Smart polymers could also be used as drug capsules when they exhibit biodegradation and biocompatibility.13 Poly(N-isopropylacrylamide) (PNIPAM) and poly(N,N-diethylacrylamide) (PDEAM) are known to be the most studied thermo-responsive polymers14 and poly(N-vinyl caprolactam) (PNVCL) is a well-known thermo-responsive polymer.15 As mentioned before, unique features of PNVCL that are nonionic and nontoxic nature, solubility in water and organics, thermal responsiveness and biocompatibility for biomedical applications16-20 makes it research focused polymer.
N-vinyl caprolactam (NVCL) monomer has amphiphilicity due to hydrophilic carboxylic and amide groups (lactam ring) with also hydrophobic carbon-carbon backbone chains. The lactam ring is very precious when it is the repeating group of PNVCL due to only then the thermoresponsive mechanism could have occurred. When the polymerization occurred with repeating lactam ring, the aqueous solution of PNVCL has a lower critical solution temperature (LCST) within the temperature range of 32-34°C. Due to these properties, it is used in many applications such as drug delivery systems, muscle implantation, and biosynthetic organ development.16-20
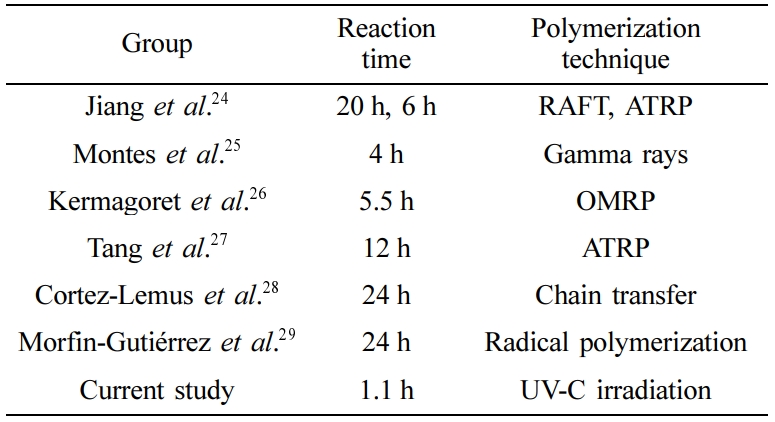
NVCL could be polymerized to form PNVCL via many techniques such as gamma irradiation,18-21 photopolymerization,20 and radical mechanism.5,16,22 Photopolymerization method is used in many applications such as curing adhesives, printing inks, photoresists, and coatings on various materials23 and has advantages compared to other types of polymerization techniques. Gamma irradiation and free radical polymerization of PVNCL have been carried out within previous studies of the former research group. Ultraviolet, which (UV) is a type of electromagnetic radiation with wavelength from 10 nm and UV-C, which is a type of ultraviolet radiation (100–280nm) has not been reported in the literature.16-21 In the literature, several polymerization types have prepared with former studies but their reaction times are very high and this could lead to different economic and production cost problems.24-29
In this study, UV-C irradiation was used to polymerize the NVCL. It is worth to mention, although there are studies available in the literature that reports UV polymerization of the NVCL,20 the photopolymerization via UV-C has not been carried out to our best knowledge. In addition, with usage of photopolymerization, the use of chemicals for the polymerization could be minimized and this would end up with an environmentally friendly approach. Also, during the study, the effect of the crosslinking agent was evaluated, and mechanical tests were done to confirm the change in the polymeric structure due to the presence of the crosslinking agent. Thermal characterization tests were used to determine the thermal properties of the polymerized gels and homopolymers. Moreover, two modes (namely time scan mode and temperature scan mode) of differential scanning calorimetry (DSC) tests were conducted to have a detailed understanding of polymerization characterization. During time scan mode, the polymerization carried out in the DSC pan while determining the thermal parameters of polymerization reactions. Also, the dynamic light scattering technique was employed to determine both particle size distribution and thermal response that causes a change in the particle size of the polymer.
Materials. N-vinyl caprolactam (NVCL), 1-hydroxycyclohexylphenylketone (Irgacure® 184) and ethylene glycol dimethacrylate (EGDMA) was obtained from Sigma Aldrich (US), and these chemicals were used without any further treatment.
Synthesis and Characterization of Homopolymers and Gels by Photo Polymerization Method. Initially, NVCL monomer was kept in a water bath for approximately 60 min at 70°C before its use. The PNVCL polymer was obtained in two ways that are (a) with and (b) without the presence of EGDMA crosslinking agent thus two types of polymers were synthesized (a) homopolymer that has no crosslinking agent, and (b) gel that has a crosslinking agent. The recipe used for the polymerization process is given in Table 1. The components of the recipe transferred into a 50 mL beaker with the monomer in aqueous form. The content of the beaker was stirred using a magnetic stirrer at 50 °C for 75 min until the solution is homogeneous. 1mL solution was put into the cylindrical-shaped polydimethylsiloxane (PDMS, silicone) moulds of 23.85 mm in diameter and 1.90 mm of thickness. Photopolymerization was carried out in a UVP 1000CL UV cabinet with UV-C light source having a 254 nm wavelength. The irradiation flux was 0.7W.m-2.nm-1 according to the producer instructions. The irradiations were carried out for the periods of 15, 30, 50, 70, 90 and 120min. At the end of each period of irradiation, the polymer synthesized (either homopolymer or gel) were removed from the PDMS moulds with no shape distortion during removal. The gels obtained were kept in diethyl ether solution for 24h and purified from the residual non-reacted monomers. Finally, the gels were dried in an oven for 24h until reaching a constant weight. The synthesized polymers (homopolymers and gels) were subjected to characterization and the other tests.
Percentage of gelation for the gels synthesized was found using eq. (1) where WD is dried gel weight (g) and WI is initial monomer weight in grams.
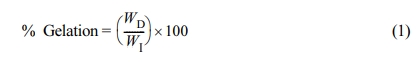
Water absorption of PNVCL was determined for different periods of 15, 30, 45, 60, 90, 120, 180, 240, 300 and 360 min at room temperature and also at different temperatures of 5, 10, 15, 20, 30, 40, 50, 60, 70, and 75°C for a constant 15 min period in water. Water absorption tests of crosslinked PNVCL polymers were performed in a water bath containing 5L of distilled water.
The percent water absorption was found using eq. (2)17,30 where WSE is swelled gel weight (g) and WD is dried gel weight in grams.
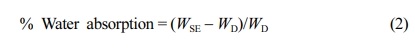
Cloud point of homopolymer was determined. For that purpose, homopolymer (non-crosslinked polymer) was dissolved in 25 mL of distilled water and mixture was transferred to test tube. The tube was placed in the water bath with a starting temperature of 23°C. Colour changes of the solution at temperatures of 25, 28, 36 and 41°C were observed.
Fourier transform infrared spectrometer (FTIR) analysis of gels was carried out using a Perkin Elmer mark FTIR between the frequency of 450 to 4000 cm-1. The particle size distribution of the homopolymer PNVCL was determined via Malvern mark Zetasizer Nano ZS model equipment. PNVCL was dissolved in distilled water to form 1% by weight PNVCL solution and the tests were carried out. Particle size distribution for the gels was not carried out since the gels have, by the theory, infinite molecular weight. Morphological characteristics of the polymers (homopolymer and gel) were determined via Zeiss mark Supra 55 model SEM equipment with a voltage of 10-15 kV and the samples were coated with Pt before the analysis. Perkin Elmer mark DSC 4000 model DSC equipment was used for the DSC tests to investigate the thermal properties of monomer, polymer and the gelation process. Two modes of scanning were used namely; time scan and temperature scan modes. For time scan mode, DSC thermograms were taken at a constant temperature of 70°C. The purpose of this analysis was to investigate the gelation process that was carried out in the DSC pan at a constant temperature of 70°C. Also, the phase transition temperature of the NVCL monomer was determined with the DSC equipment at heat rates of 0.5°C/min and 1°C/min. Dynamic mechanical analysis (DMA) tests were carried out for the polymers using a Perkin Elmer mark Pyris Diamond Model DMA equipment and tests were carried out at 1 Hz frequency in tension mode with 5°C/min heat rate between 20°C and 200°C. Mechanical tests were carried out using a Shimadzu mark AG-X model equipment according to ASTM D638 standard. Mechanical properties of the synthesized polymers were obtained.
|
Table 1 Composition of PNVCL Containing 1-Hydroxycyclohexylphenylketone and EGDMA |
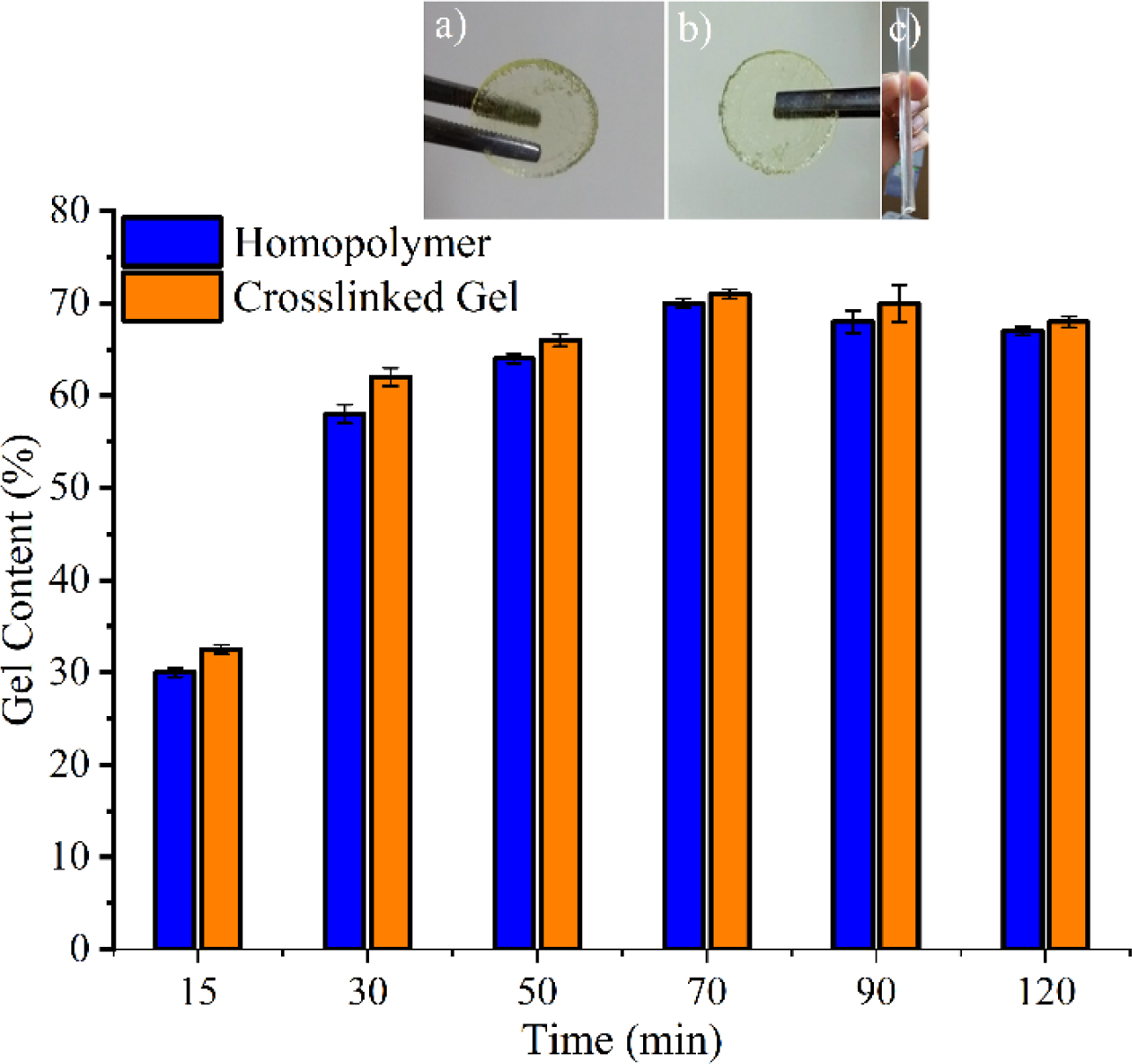
Gel Content Determination. Figure 1 shows that the gel content and the appearance of the prepared polymers. The maximum percentage of gelation for the gels obtained by UV-C polymerization was 71.2% and this conversion was obtained at 70min. Low standard derivation values indicate that high repeatability could be achieved. With this polymerization technique, the polymerization times are very few compared to other PNVCL studies. Different polymerization of PNVCL studies is compared in Table 2. Close results between homopolymer and crosslinked gel showed that the crosslinking reaction did not affect the gel content of the final material. UV irradiation is advantageous than the other polymerization methods because gels/polymers were obtained without any solvent content and a high conversion rate was obtained in a relatively short period.
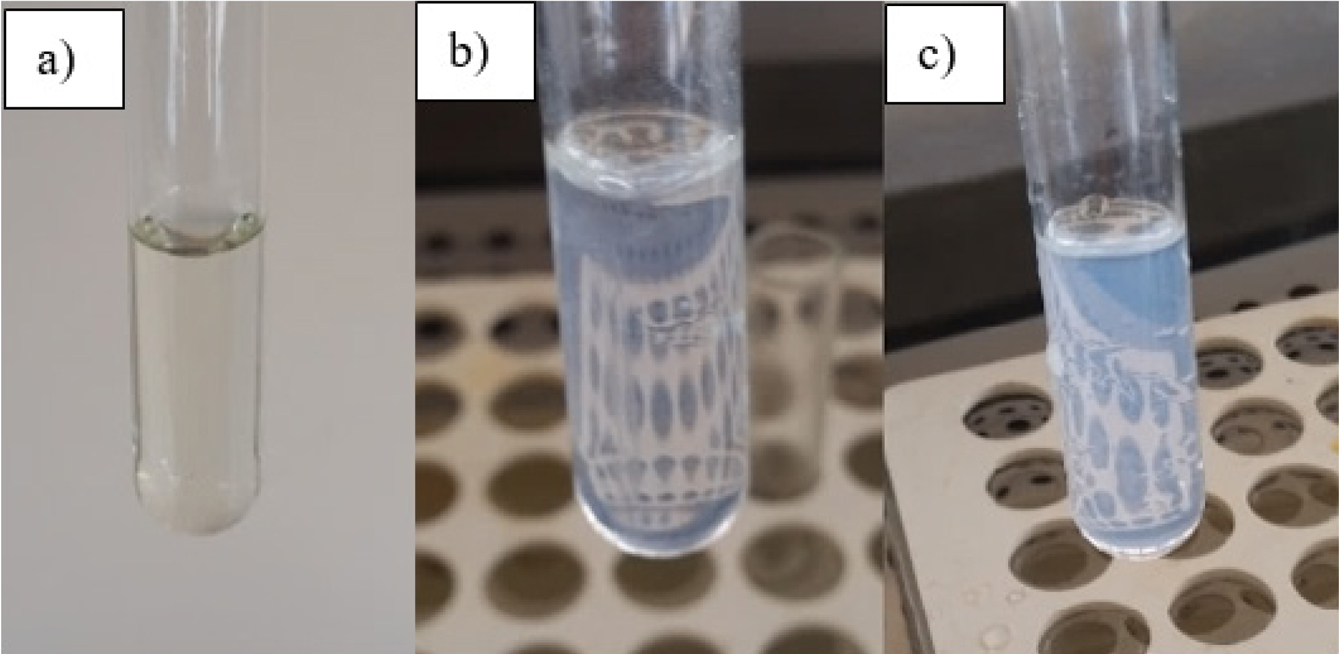
Cloud Point Determination. Cloud point analysis was used to observe the color change at temperatures above and below LCST of the polymer. Homopolymer was dissolved in a 25mL of distilled water and mixture was transferred into a glass tube. The tube was placed in a water bath with a starting temperature of 23°C. Color changes at temperatures of 25, 28, 36 and 41°C were observed. The appearance of PNVCL solution below and above LCST is given in Figure 2.
It is well known that some polymers have a phase separation when the temperature exceeds a definite value, causing shrinkage. This temperature is defined as the LCST.13 The gels exhibit a shrink behavior via heating due to the hydrophobic-hydrophilic balance within the polymer chains. Due to an increase in temperature, the entropy of tensile force moving between hydrophobic groups increases, thus these hydrophobic interactions become dominant at the temperatures higher than the critical solution temperature resulting in a shrinkage of the polymer chain.31 While there is no change in the appearance of the gels at 25 and 28°C that is below the lower critical solution temperature, there was a blurred appearance that is gradually increasing at temperatures between 36 to 41°C. In other words, the temperature is above the LCST temperature at this point.
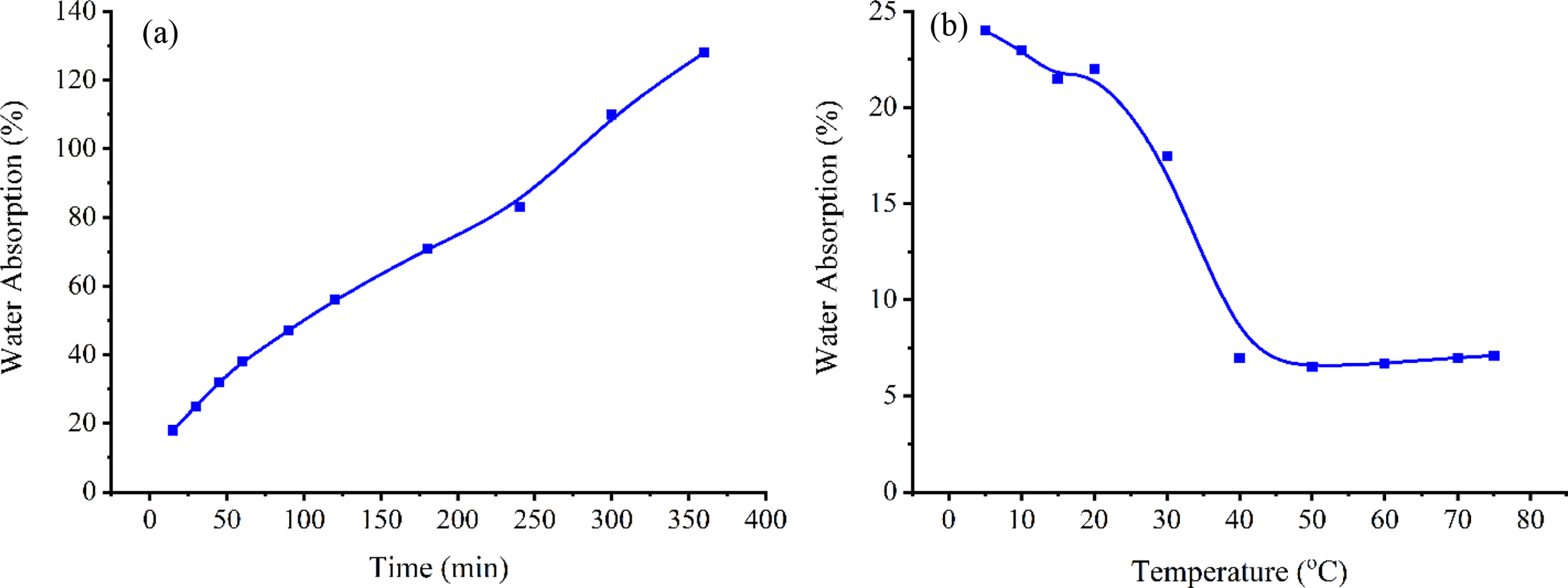
Water Absorption of Gels. Figure 3 shows the water absorption of the gels for different periods and different temperatures within the range of 5 to 75 °C. The percentage of water absorption after keeping the samples in distilled water for 15 min at each temperature was calculated. The increased water absorption after prolonged periods shows that the decrement in swelling ratio could be bigger and it can lead to sharp LCST values. Crosslinked gels exhibited different properties at the temperatures below and above the LCST regarding water absorption. Figure 3 shows a gradual decrease in swelling ratio due to the predominance of the hydrophobic property of the polymer at the temperatures above the LCST. It has been found that the swelling ratio is higher at temperatures below the LCST since the hydrophilic properties of the polymer are dominant.
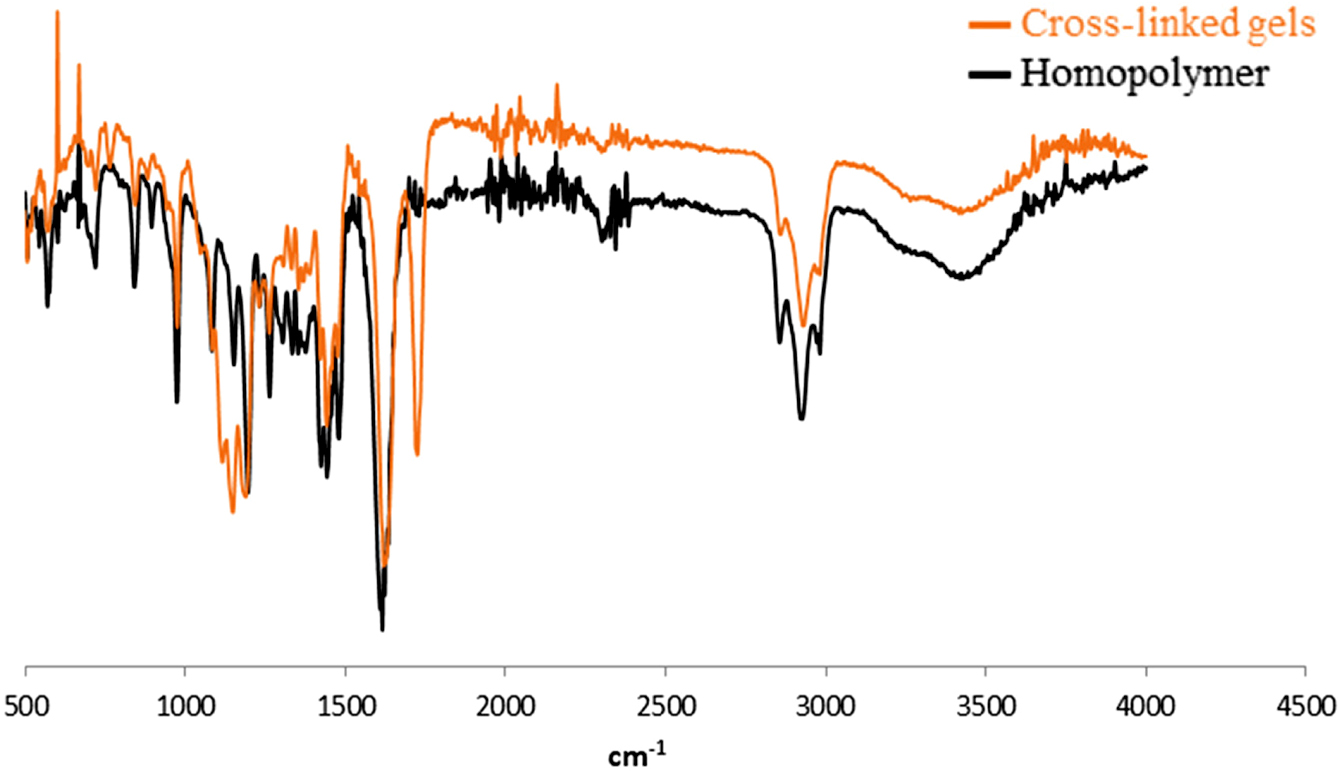
FTIR Analysis of Gels. FTIR characterization was performed to determine the changes in the structure of crosslinked polymers and homopolymers. FTIR spectra of the homopolymer and crosslinked gels are given in Figure 4. Besides FTIR analysis has been carried out on whether the PNVCL synthesis can be achieved successfully by examining the functional groups in the structure of the material.
The peaks observed approximately at 1650 and 1620cm-1 for the spectrum of the NVCL monomer show characteristic C=O (amide I) and C=C (vinyl band) stretching vibrations, respectively. The peaks assigned to -CH= and CH2= are observed at 3000 cm-1.14-16 The presence of a single peak of C=O at 1610 cm-1 in the gel and the absence of C=C bond indicate that the polymerization occurs via the vinyl group. The peak at 1770cm-1 is the because of the crosslinking between polymer chains. C=O stretching band belongs to EGDMA in crosslinked gels observed at 1728 cm-1. The bands at about 1419, 1440, and 1482 cm-1 (triplet) are due to the characteristic vibrations of the lactam ring.14 The peak intensity increase in this range shows that the crosslinking reactions occurred in the polymer chain successfully.
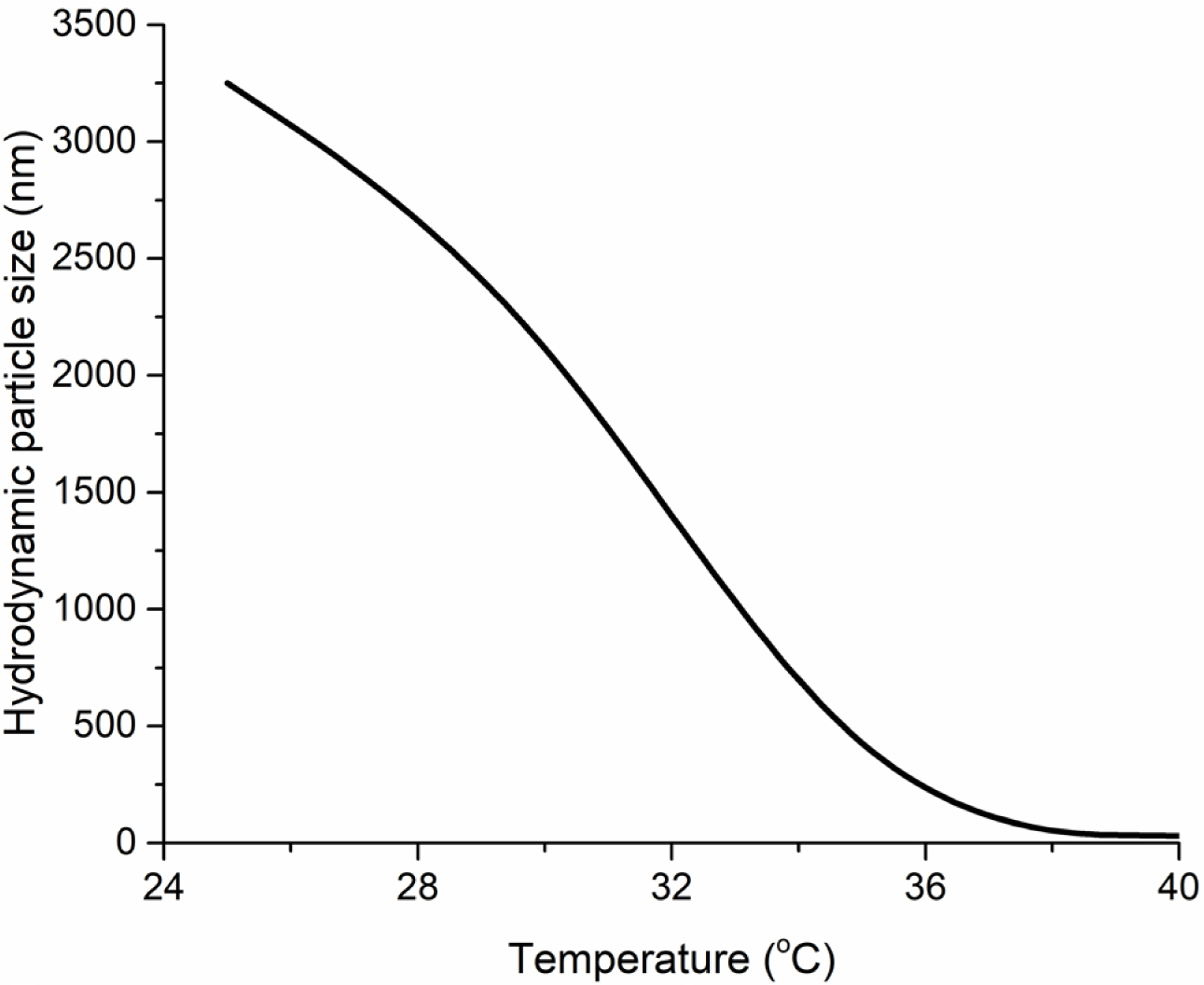
Temperature Effect on the Particle Size of Gels. Dynamic light scattering equipment (DLS) was used to determine the LCST value of synthesized homopolymer. The homopolymer was completely soluble in water while the crosslinked gel was completely insoluble, and it was not convenient to determine the LCST value for the gel synthesized due to the theoretical knowledge that the gels have infinite chain length. In DLS analysis, water was used as a solvent to form a solution. In this regard, 1% (w/w) of the polymer was added to 99% (w/w) distilled water, and the solution was centrifuged at 2000 rpm for 2 h. The effect of the medium temperature on the particle size of the colloidal homopolymer in the prepared solution is shown in Figure 5. As can be seen from Figure 5, the medium temperature considerably effects the hydrodynamic diameter of the homopolymer. Essentially, this is a result because the homopolymer’s swelling/de-swelling as the medium temperature changes due to the interactions between hydrophilic (carboxylic and amide groups) and hydrophobic groups (carbon-carbon backbone) in the NVCL structure. DLS analysis results demonstrated that the hydrodynamic diameter of the homopolymer decreased with the increment of a medium temperature. Moreover, it was significantly decreased within the range of 30-35oC, which was an expected result due to the LCST value of PNVCL.
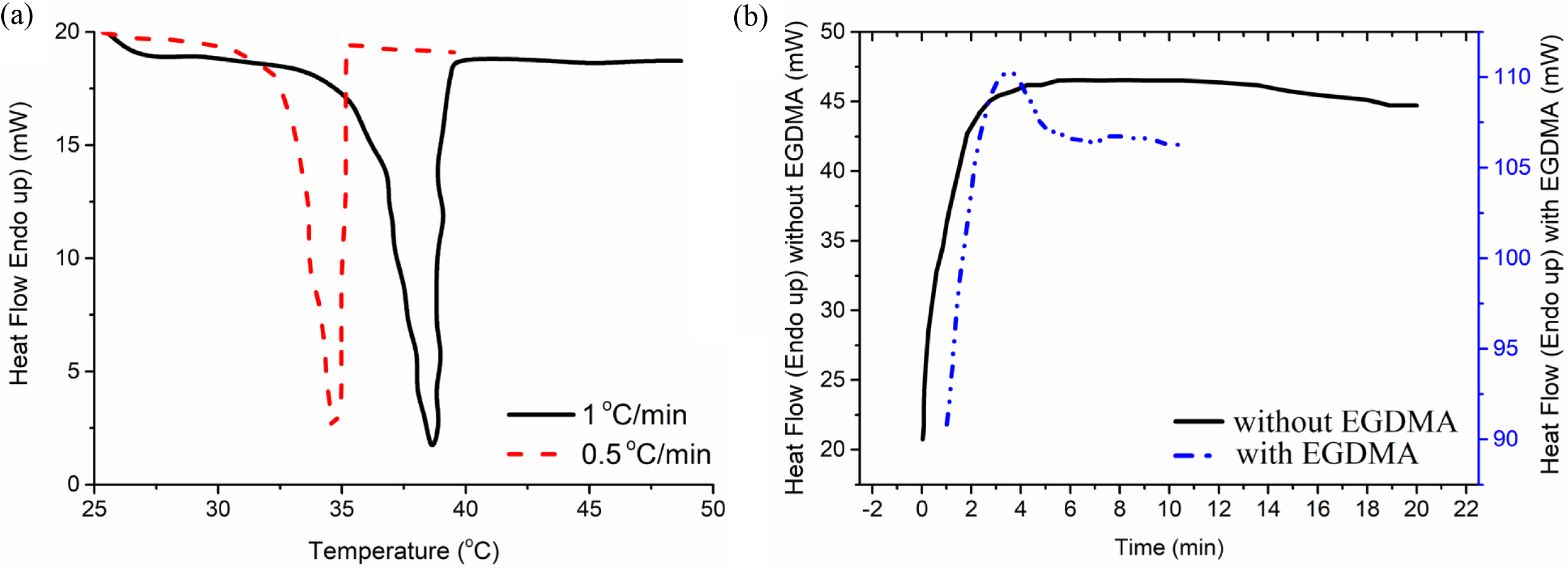
DSC Test Results. Phase transition temperature (LCST) of NVCL was determined via DSC tests and the resulting thermograms are given in Figure 6(a) where an exothermic peak is observed ascribed to the phase transition temperature of the NVCL. It is worth stating that the left side of the peak given in Figure 6(a) is different from the right side of the peak which was rather a straight line. This was probably because of the thermal responsiveness of the monomer molecules that occur at the same temperature, while the critical solution state reached is affected by the transient (rather non-homogeneous) heat transfer to the DSC pan that results with a time delay to reach a homogeneous temperature within the material matrix.
The breakage of hydrogen bond, polymer chain movement, and conformational restructuration as well as the rearrangement of water molecules around the polymeric chains occurs as an endothermic process, while hydrogen bond formation and the collapse of hydrophobic groups proceed in an exothermic manner.32 At the peaks in Figure 6(a) correspond to the collapse of hydrophobic groups that cause a phase separation in the NVCL. The exothermic heat flow corresponds to entropy-driven phase separation for the NVCL monomer. The exothermic phase transition shows that the entropy increases, and the system becomes less ordered that results with the exothermic heat release by the system at the specified temperature range. Thus, the entropy change (ΔS) and enthalpy change (ΔH) is negative for the phase separation of the NVCL.
In addition, the heat rate during the DSC tests was an important parameter for determining the phase transition temperature. The peak has the range of 31-35oC for the case of heat rate of 0.5oC/min whereas the peak has the range of 35-39oC for the case of heat rate of 1oC/min. These observed peaks correspond to LCST of the monomer NVCL. The slower heat transfer rate (0.5oC/min) results with a rather homogeneous temperature distribution in the DSC pan that results with more realistic data for the LCST range. The LCST found from cloud point analysis and DSC tests were compatible with each other.
The isothermal (time scan) mode of DSC was carried out and the polymerization was carried out in the DSC pan for two cases (i) without, and (ii) with the presence of a crosslinking agent. The resulting thermograms are given in Figure 6(b). An exothermic peak was observed for the case of polymerization with the presence of a crosslinking agent (gel synthesis) within a short period of time while this was not the situation for the case of polymerization reaction without a crosslinking agent (homopolymer synthesis).

The time scan (isothermal) mode of DSC has shown an exothermic peak that means the crosslinking reaction occurred in the DSC pan and the peak gives the value of energy released during the crosslinking reaction of the NVCL. Since the enthalpy change (ΔH) and the entropy change (ΔS) are both negative and thermodynamically favorability of the crosslinking reaction depends certainly on the temperature of the system. The change in Gibbs free energy (ΔG) is given in eq. (3) should be negative in order to have a spontaneous reaction. The polymerization reaction is enthalpy-driven because the exothermic reaction is dominant over the decrease in entropy as given in eq. (4). That is the exothermic heat flow corresponds to enthalpy driven polymerization of the NVCL monomer. The exothermic peak is due to a decrease in the entropy resulting from a more ordered system that ends up with an exothermic reaction by the system during the polymerization and crosslinking of the NVCL to synthesize the PNVCL gels.
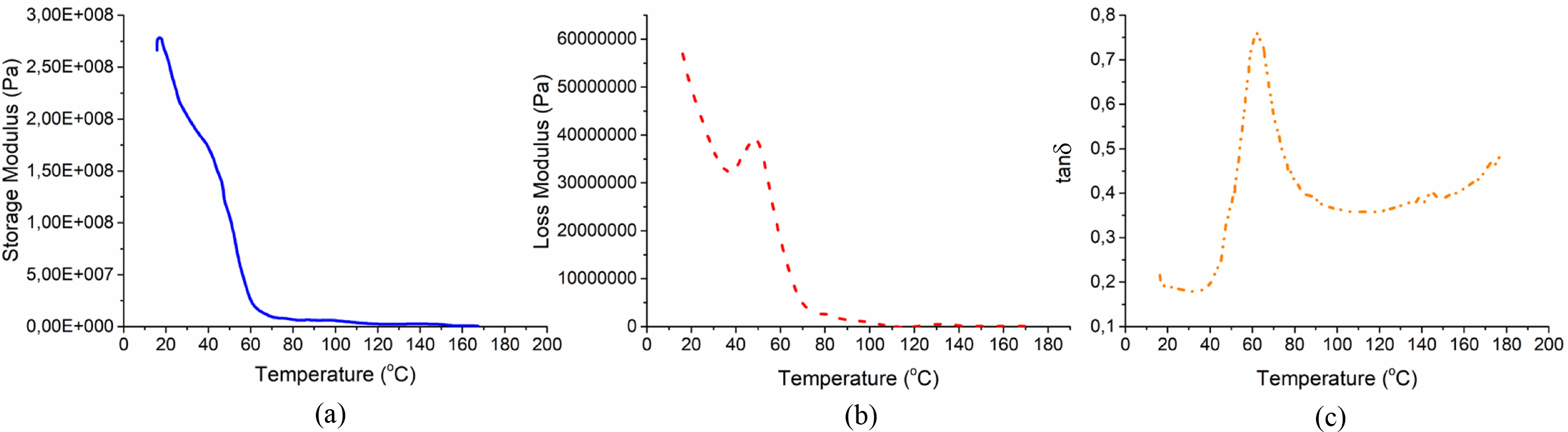
DMA Test Results. DMA is an effective way to investigate how the viscoelastic behavior of materials changes as a function of a medium temperature. In DMA analysis, storage modulus (E'), loss modulus (E''), and tand parameters are examined. E' gives information about how much energy stored in the polymer matrix and the rigidity of the polymer. As can be seen in Figure 7, the E' was found as 270 MPa at about 16.5 °C. On the other hand, the E″ refers to the capability of polymers to dissipate its energy. The E″ was determined as about 55.7 MPa at around 16.5°C. The alpha relaxation peak, which is the first peak seen on the tand curve, represents the glass transition temperature (Tg). Tg is known as the temperature limit at which the polymers lose its glassy properties and begins to acquire viscous properties and is a unique feature for polymers. From the DMA test results, the Tg peak was observed at 62.6°C that means the Tg is higher than the human physiological temperature. It is well known that the higher the Tg means the higher the mechanical properties of the polymer. Tg could be tailorable by means of the content of the crosslinking agent, the increase in the crosslinking agent content would increase the Tg to a certain extent.
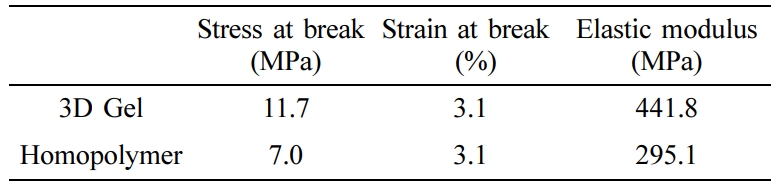
Mechanical Test Results. The characterization of the mechanical properties of the responsive polymers allows an idea of the potential application areas of the polymers. In particular, the mechanical strength of the responsive polymers to be used in drug delivery systems is desired to be stable throughout the release process. In this regard, mechanical analyses of both the homopolymer and the crosslinked gels were performed. Samples were prepared according to ASTM D638, type IV standard, and were tested at a crosshead displacement rate of 0.5mm/min. The obtained mechanical properties of samples are summarized in Table 3. The tensile tests showed that the crosslinked gels have higher mechanical properties compared to non-crosslinked PNVCL. Mechanical properties have shown the increase with the presence of a cross-linking agent.
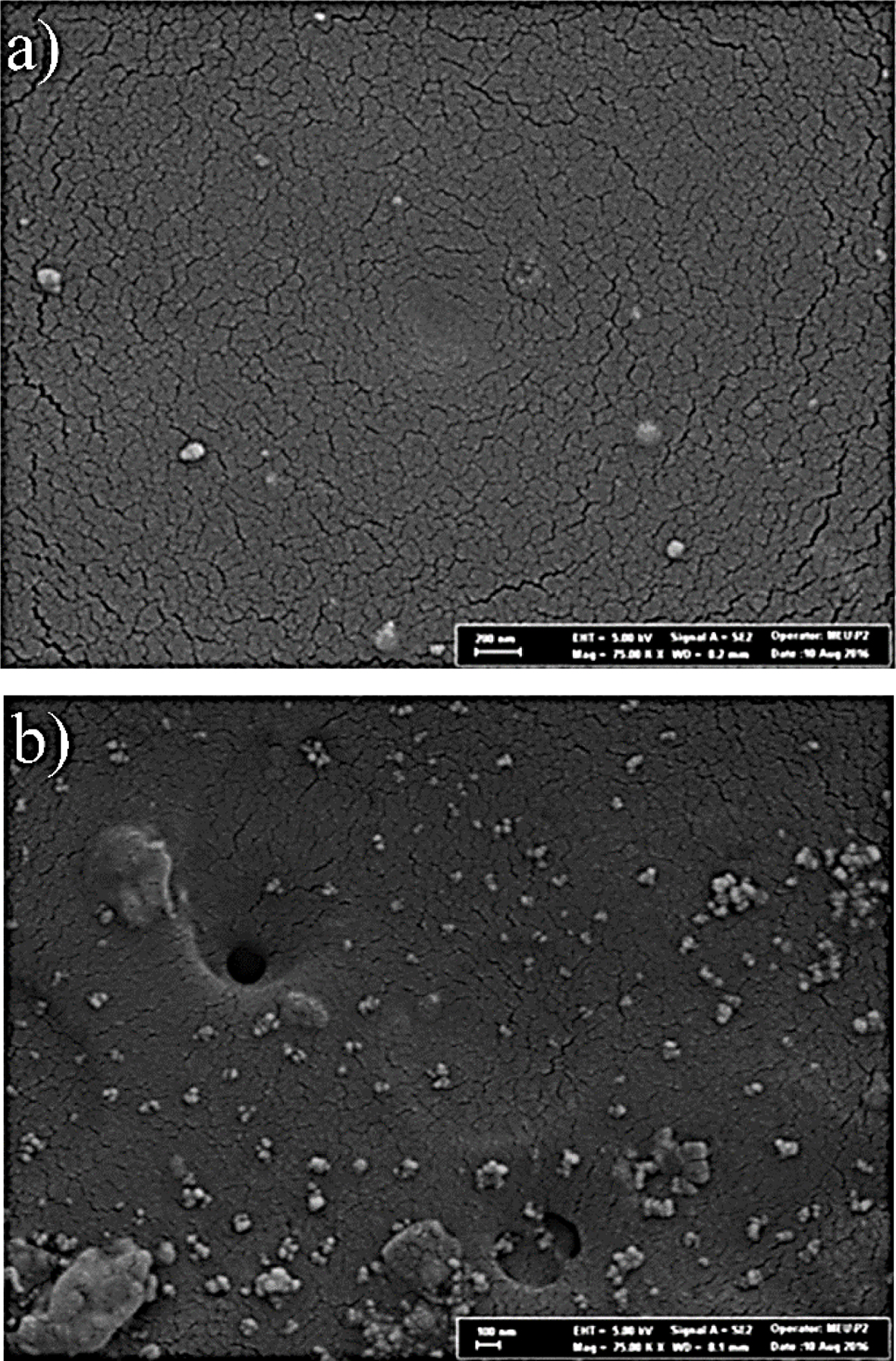
Morphology of Gels. SEM imaging was performed to investigate the surface morphology of homopolymer and crosslinked gels obtained by photopolymerization. Figure 8 shows SEM images of homopolymer and crosslinked gels with magnifications of 7.5×104 times. SEM scans shown that the small particles were present on the surface of the gels. Moreover, it was observed that the homopolymer has a smooth surface compared to the crosslinked gels, which was an expected outcome. During cross-linking reactions, undesirable side reactions may occur or non-crosslinking moiety may accumulate on the surface of the material.
|
Figure 1 Percentage gelation depending on period of UV-C irradiation and appearance of gels synthesised (a) and (b) coin shaped; (c) cylindirical shaped. |
|
Figure 2 PNVCL solution below and above LCST (a) solution at 25 °C; (b) solution at 36 °C; (c) solution at 41 °C. |
|
Figure 3 Change in the percentage of water absorption of crosslinked gels at (a) room temperature; (b) different temperatures. |
|
Figure 4 FTIR spectrums of the synthesized gel and homopolymer. |
|
Figure 5 Effect of the medium temperature on the particle size of the colloidal homopolymer. |
|
Figure 6 DSC thermograms of the monomer NVCL (a) for the purpose of LCST determination with temperature scan mode; (b) for investigation of crosslinking reaction within the DSC pan with time scan mode at 70 ℃ constant temperature. |
|
Figure 7 DMA test results of (a) storage modulus; (b) loss modulus; (c) tanδ. |
|
Figure 8 SEM images of (a) homopolymer; (b) crosslinked gels with magnifications of 75. |
|
Table 2 Comprasion of Reaction Times for Polymerization of PNVCL according to Different Studies |
Photopolymerization via UV-C is an effective way of synthesizing both homopolymers and gels. The maximum percentage of gelation for the gels obtained by UV-C polymerization was 71.2% at 70min. It is understood from the FTIR spectra of the gels synthesized under optimum conditions, that PNVCL synthesis has been successful. Phase changes in gels with the change of ambient temperature were investigated by cloud point, water absorption, DLS, and DSC analysis. It has been observed that the water absorption is low due to the hydrophobic nature of the polymer at the temperatures above the LCST. As the temperature is above the LCST, water molecules are scaled down and out by the hydrophobic segments in the polymer chains. This has caused the polymer to collapse and to appear in a globular form. When the effect of temperature change on the particle size of gels is examined, it can be concluded from DLS analysis that the gel starts to shrink in 30-35oC range and the particle size decreases. The phase transition temperature of NVCL was determined from the DSC analysis. The effect of the crosslinking agent was seen from the DSC thermograms. In addition, it was determined that the glass transition temperature (Tg) of the crosslinked gels was approximately 62.6°C obtained from the DMA test. Mechanical tests showed that the final flexural strength was 5.7 MPa and the elastic modulus was 34.9MPa. It was observed that the presence of cross-linker significantly increased the mechanical properties of the gels. Consequently, it has been concluded that gels that are synthesized quickly and easily using the UV-C photopolymerization technique are highly sensitive to temperature change.
- 1. Hoogenboom, R. Temperature-responsive polymers: properties, synthesis and applications, in Smart Polymers and their Applications; Aguilar, M. R., Román, J. S., Eds.; Woodhead Publishing: U. K., 2014; pp 15-44.
-

- 2. Aguilar, M. R.; San Román, J. Introduction to Smart Polymers and Their Applications. Smart Polym. Their Appl. 2014, 3, 1-11.
-

- 3. Kuckling, D.; Doering, A.; Krahl, F.; Arndt, K.-F. Stimuli-Responsive Polymer Systems. in Polymer Science: A Comprehensive Reference, Elsevier B.V.: 2012; pp 377-413.
-

- 4. Rinaudo, M.; Osada, Y. Stimuli Responsive Polymers. Actual. Chim. 2006, 300, 31-35.
- 5. Medeiros, S. F.; Barboza, J. C. S.; Giudici, R.; Santos, A. M. Thermally-sensitive and Biocompatible Poly(N-vinylcaprolactam): A Kinetic Study of Free Radical Polymerization in Ethanol. J. Macromol. Sci. Part A 2013, 50, 763-773.
-

- 6. Soto-Figueroa, C.; Galicia-García, T.; Rodríguez-Hidalgo, M. del R.; Vicente, L. Theoretical Study of Thermoresponsive Dendritic Polymeric Micelles: Micellar Phase Control and the Extraction of Organic Molecules by Temperature Effects. Eur. Polym. J. 2020, 127, 109596.
-

- 7. Rullyani, C.; Singh, M.; Li, S.-H.; Sung, C.-F.; Lin, H.-C.; Chu, C.-W. Stimuli-responsive Polymer as Gate Dielectric for Organic Transistor Sensors. Org. Electron. 2020, 85, 105818.
-

- 8. Baniasadi, M.; Yarali, E.; Foyouzat, A.; Baghani, M. Crack Self-healing of Thermo-responsive Shape Memory Polymers with Application to Control Valves, Filtration, and Drug Delivery Capsule. Eur. J. Mech. A/Solids 2021, 85, 104093.
-

- 9. Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-Responsive Polymeric Micelles of Cabazitaxel for Prostate Cancer Targeted Therapy. Acta Biomater., 2020, 113, 501-511.
-

- 10. Zhao, L.; Li, L.; Zhu, C.; Ghulam, M.; Qu, F. pH-responsive Polymer Assisted Aptamer Functionalized Magnetic Nanoparticles for Specific Recognition and Adsorption of Proteins. Anal. Chim. Acta 2020, 1097, 161-168.
-

- 11. Liu, X.; Guan, J.; Lai, G.; Xu, Q.; Bai, X.; Wang, Z.; Cui, S. Stimuli-responsive Adsorption Behavior Toward Heavy Metal Ions Based on Comb Polymer Functionalized Magnetic Nanoparticles. J. Clean. Prod. 2020, 253, 119915.
-

- 12. Li, J.; Li, X.; Liu, H.; Ren, T.; Huang, L.; Deng, Z.; Yang, Y.; Zhong, S. GSH and Light Dual Stimuli-responsive Supramolecular Polymer Drug Carriers for Cancer Therapy. Polym. Degrad. Stab. 2019, 168, 108956.
-

- 13. Qiu, Y.; Park, K. Environment-sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2001, 53, 321-339.
-

- 14. Çavuş, S.; Çakal, E. Synthesis and Characterization of Novel Poly(N-vinylcaprolactam-co-itaconic Acid) Gels and Analysis of pH and Temperature Sensitivity. Ind. Eng. Chem. Res. 2012, 51, 1218-1226.
-

- 15. Liu, L.; Bai, S.; Yang, H.; Li, S.; Quan, J.; Zhu, L.; Nie, H. Controlled Release from Thermo-sensitive PNVCL-co-MAA Electrospun Nanofibers: the Effects of Hydrophilicity/Hydrophobicity of a Drug. Mater. Sci. Eng. C 2016, 67, 581-589.
-

- 16. Kozanoǧlu, S.; Özdemir, T.; Usanmaz, A. Polymerization of N-Vinylcaprolactam and Characterization of Poly(N-Vinylcaprolactam). J. Macromol. Sci. Part A 2011, 48, 467-477.
-

- 17. Çakal, E.; Çavuş, S. Novel Poly(N-vinylcaprolactam-co-2-(diethylamino)ethyl methacrylate) Gels: Characterization and Detailed Investigation on Their Stimuli-Sensitive Behaviors and Network Structure. Ind. Eng. Chem. Res. 2010, 49, 11741-11751.
-

- 18. Cheng, S.; Feng, W.; Pashikin, I.; Yuan, L.; Deng, H.; Zhou, Y. Radiation Polymerization of Thermo-sensitive Poly(N-vinylcaprolactam). Radiat. Phys. Chem. 2002, 63, 517-519.
-

- 19. Jun, L.; Bochu, W.; Yazhou, W. Thermo-sensitive Polymers for Controlled-release Drug Delivery Systems. Int. J. Pharmacol. 2006, 2, 513-519.
- 20. Dalton, L. G. M.; Halligan, S.; Killion, J.; Murray, K. A. Smart Thermosensitive Poly(N-vinylcaprolactam) Based Hydrogels for Biomedical Applications. Adv. Environ. Biol. 2014, 8(24), 1-6.
- 21. Usanmaz, A.; Özdemir, T.; Polat, Ö. Solid State Polymerization of N-vinylcaprolactam via Gamma Irradiation and Characterization. J. Macromol. Sci. Part A 2009, 46, 597-606.
-

- 22. Wan, D.; Zhou, Q.; Pu, H.; Yang, G. Controlled Radical Polymerization of N-vinylcaprolactam Mediated by Xanthate or Dithiocarbamate. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3756-3765.
-

- 23. Jiang, X.; Yin, J. Copolymeric Photoinitiators Containing In-chain Thioxanthone and Coinitiator Amine for Photopolymerization. J. Appl. Polym. Sci. 2004, 94, 2395-2400.
-

- 24. Jiang, X.; Li, Y.; Lu, G.; Huang, X. A Novel Poly(N-vinylcaprolactam)-based Well-defined Amphiphilic Graft Copolymer Synthesized by Successive RAFT and ATRP. Polym. Chem. 2013, 4, 1402-1411.
-

- 25. Montes, J. Á.; Ortega, A.; Burillo, G. Dual-stimuli Responsive Copolymers Based on N-vinylcaprolactam/Chitosan. J. Radioanal. Nucl. Chem. 2014, 303, 2143-2150.
-

- 26. Tang, G.; Hu, M.; Ma, Y.; You, D.; Bi, Y. Synthesis and Solution Properties of Novel Thermo- and pH-responsive Poly(N-vinylcaprolactam)-based Linear-dendritic Block Copolymers. RSC Adv. 2016, 6, 42786-42793.
-

- 27. Kermagoret, A.; Mathieu, K.; Thomassin, J.-M.; Fustin, C.-A.; Duchêne, R.; Jérôme, C.; Detrembleur, C.; Debuigne, A. Double Thermoresponsive Di- and Triblock Copolymers based on N-vinylcaprolactam and N-vinylpyrrolidone: Synthesis and Comparative Study of Solution Behaviour. Polym. Chem. 2014, 5, 6534-6544.
-

- 28. Cortez-Lemus, N. A.; Castro-Hernández, A. Intrinsic Viscosity of Poly(N-vinylcaprolactam) with Varying the Architecture. J. Polym. Res. 2020, 27, 225.
-

- 29. Morfin-Gutiérrez, A.; Meléndez-Ortiz, H. I.; Puente-Urbina, B. A.; García-Cerda, L. A. Synthesis of Poly(N-vinylcaprolactam)-Grafted Magnetite Nanocomposites for Magnetic Hyperthermia. J. Nanomater. 2018, 2018, 1-6.
-

- 30. Sanna, R.; Fortunati, E.; Alzari, V.; Nuvoli, D.; Terenzi, A.; Casula, M. F.; Kenny, J. M.; Mariani, A. Poly(N-vinylcaprolactam) Nanocomposites Containing Nanocrystalline Cellulose: a Green Approach to Thermoresponsive Hydrogels. Cellulose 2013, 20, 2393-2402.
-

- 31. Makhaeva, E. E.; Thanh, L. T. M.; Starodoubtsev, S. G.; Khokhlov, A. R. Thermoshrinking Behavior of Poly(Vinylcaprolactam) Gels in Aqueous Solution. Macromol. Chem. Phys. 1996, 197, 1973-1982.
-

- 32. Zhao, J.; Wang, H. Exothermic Nonreversing Process in the Phase Transition of Poly(N-isopropylacrylamide) Studied with Stochastic Temperature-modulated DSC. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1869-1877.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 22-30
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.22
- Received on Jul 3, 2020
- Revised on Sep 16, 2020
- Accepted on Sep 23, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Habil Uzun, Ahmet Güngör, İsmail Kutlugün Akbay
-
Department of Chemical Engineering, Mersin University, Mersin, Turkey
- E-mail: ahmet.gungor@mersin.edu.tr, akbay@mersin.edu.tr
- ORCID:
0000-0002-8319-1652, 0000-0002-0685-8660










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.