- Enhanced Thermal Conductivity of Epoxy Composites Filled with Cu Foam and Functionalized with MWCNT 3D Network
School of Chemical Engineering & Materials Science, Chung-Ang University, Seoul 06971, Korea
- Epoxy/Cu폼/Cu 나노입자가 부착된 MWCNT 고분자 복합체 제조 및 열전도 특성 연구
중앙대학교 공과대학 화학신소재공학부
The thermal conductivity of an epoxy composite with Cu foam as the primary filler and functionalized with multi-walled carbon nanotubes (MWCNTs) is shown to reach 0.62 W/mK, which is 313% higher compared to the untreated epoxy. This enhancement is attributed to the increased interfacial affinity due to the coating with 3-mercaptopropyl trimethoxysilane (MPTMS) and the subsequent construction of a more intricate thermal conduction pathway due to the uniform dispersion of MWCNTs. In detail, the Cu nanoparticles (CuNPs) are attached to the thiol groups of MPTMS to provide more interfacial affinity between the Cu foam and the MWCNTs. The morphology of the functionalized filler inside the epoxy matrix is characterized by field emission scanning electron microscopy (FE-SEM) of the fractured composite surface, and the successful surface treatment is confirmed by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The enhanced thermal property of the composite fabricated in this study makes it a promising thermal interface material for electronic devices.
고방열 특성의 고분자 복합소재를 위하여 Cu폼과 Cu 나노입자가 부착된 MWCNT가 함께 첨가된 에폭시 복합체를 제작하였다. 고분자 내에서의 MWCNT의 분산성 향상을 위해 3-mercaptopropyl trimethoxysilane(MPTMS)를 이용하여 표면처리를 진행하였다. 또한 Cu폼과의 계면친화성을 위해 Cu 나노입자를 MWCNT의 표면에 부착하여 기존 에폭시와 비교하여 높은 열전도도를 확인하였다. 이러한 열전도도 증가는 MPTMS의 코팅으로 인한 MWCNT의 분산성 향상과 보다 복잡한 열전도 통로의 형성 때문이며 이러한 현상은 주사전자현미경과 FTIR, XRD, XPS 등을 통해 확인되었다.
An epoxy composite with Cu foam functionalized with multiwall carbon nanotube was fabricated which exhibited enhanced thermal conductivity compared to untreated epoxy. Increased interfacial affinity between Cu foam and MWCNT was achieved due to 3-mercaptopropyl trimethoxysilane used as a coating agent.
Keywords: multi-walled carbon nanotube, surface modification, thermal conductivity, 3D structure, epoxy
This research was supported by the Chung-Ang University Research Scholarship Grants in 2019 and also supported by the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2020-2020-0- 01655) supervised by the IITP (Institute of Information & Communications Technology Planning & Evaluation.
With development of increasingly smaller and multifunctional electronic devices, the heat dissipation problem has become a critical challenge.1 To deal with this problem, many attempts have been made to develop thermal interface materials (TIMs). Polymers are widely used in TIMs due to their light weight, easy processability, and low price. However, due to the low intrinsic thermal conductivity of polymer materials, conductive fillers such as carbon,2 metal oxides,3 and ceramics4 must be incorporated into the polymer matrix. Although an increasing amount of filler increases the thermal conductivity, a loss of polymeric properties is inevitable. Hence, an approach to increasing the thermal conductivity with a smaller amount of filler is a key challenge in the further development of TIMs.
The construction of 3-dimensional (3D) filler networks is presently gaining attention for the effective enhancement of thermal conductivity with small amounts of filler.5 For example, Wu’s group reported a 3D vertically aligned boron nitride nanosheet network within an epoxy matrix,6 and Ding’s group fabricated 3D carbon fiber and graphene networks within a polyamide-imide matrix. In each case, increased thermal conductivity was achieved by constructing heat conduction pathways.
As a low cost and highly conductive material, Copper (Cu) is widely used as filler in composites.7 In particular, the skeleton structure of Cu foam can be exploited to construct a 3D thermal pathway. Moreover, the high stiffness and conducting properties of metal foams can provide enhanced thermal and mechanical properties when employed as fillers in polymer composites. For example, a high thermal conductivity of 3.49W/mK has been reported when using carbon nanotubes (CNTs) and Cu foam within a paraffin matrix.8 Multi-wall CNTs (MWCNTs) with superior thermal conductivities of ~3000 W/mK9 are widely used as conductive fillers due to their outstanding thermal stability and easy functionalization.10,11 However, their high surface energy leads to the aggregation of particles inside the polymer matrix, thus resulting in a decreased aspect ratio, a low dispersion state, and an increased interparticle distance. Many attempts have been made to solve the aggregation problem by using surface treatment and silane coating.
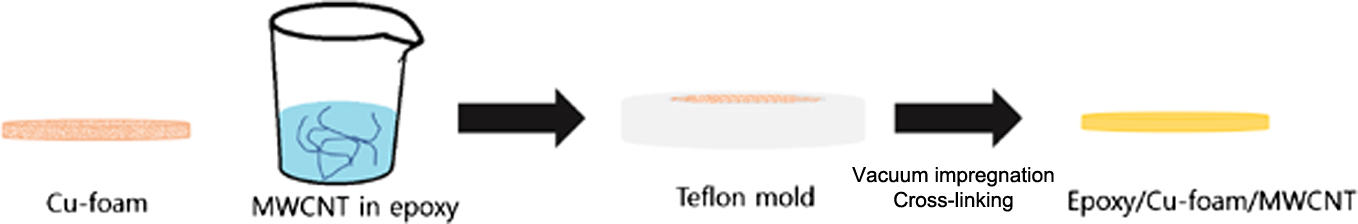
In the present study, Cu foam is used as the primary filler to construct interconnected 3D heat transfer pathways. Epoxy is used as the polymer matrix and MWCNTs are used as the secondary filler. To solve the problem of MWCNT aggregation, a 3-mercaptopropyl trimethoxysilane (MPTMS) coating is applied after surface oxidation by acid treatment. Due to the enhanced interfacial affinity between the MWCNTs and the epoxy, the dispersion state is found to be more uniform and the MWCNT interparticle distance is reduced. The MPTMS is then functionalized by the attachment of ionized Cu nanoparticles (CuNPs) to the thiol groups to obtain a uniform dispersion with enhanced affinity between the MWCNTs and the Cu foam, thus resulting in improved thermal conductivity of the composite with epoxy infiltration.
Materials. The MWCNTs (>90%) and the curing agent 4’-diaminodiphenylmethane (DDM, 97.0%) were supplied by Sigma-Aldrich (St. Louise, MO, USA). The diglycidyl ether of bisphenol-A (DGEBA; epoxy equivalent weight (E.E.W.) = 186.8g/eq) was purchased from Kukdo Chemical (Seoul, Korea). The CuNPs (³99.5%) were obtained from Sigma-Aldrich (St. Louise, MO, USA). The MPTMS (95%) was purchased from Alfa aesar (Haverhill, MA, USA). The Cu foam (110 PPI, ³95% porosity) with a thickness of 2.0 mm was obtained from MTI Korea (Seoul, Korea).
Synthesis of Cu-MPTMS-MWCNT. The MWCNTs (5 g) were dispersed in a 3:1 solution of H2SO4/HNO3 (400mL) in 500mL round flask, and the mixture was refluxed for 20 h at 50°C, then washed with deionized (DI) water until the pH reached 5. The resultant oxidized MWCNTs (150 mg) were dispersed in ethanol (EtOH) by bath sonication for 30 min, then refluxed with 0.3mL of MPTMS at 65°C for 3h. Resultant functionalized MWCNT (50mg) was dispersed in EtOH. Same amount of CuNPs was also dispersed in EtOH. These two solutions were charged in one beaker and followed by sonication for 1h. Cu-MPTMS-MWCNT was obtained after washing process with EtOH.
Fabrication of Cu-MPTMS-MWCNT/Epoxy Composites. The CuNPs-MPTMS-MWCNTs (0.033g) were initially dispersed in epoxy (3g) for 30 min by ultrasonication with power of 80W. The dispersion was then poured onto the Cu foam which had been cut into 25.4 mm discs and placed in a Teflon mold. This was then placed in a vacuum oven at 40°C for 3 min to remove any bubbles from inside the mixture. The mixture was then placed in a box furnace at 180 °C for 30 min for thermal curing.
Characterization. The thermal diffusivity of the samples was measured at room temperature using laser flash analysis (LFA; NanoFlash LFA 467, Netzsch Instruments Co., Selb, Germany). The morphology was analyzed by field-emission scanning electron microscopy (FE-SEM, Carl Zeiss, Sigma, Oberkochen, Germany). The X-ray diffraction (XRD, Bruker-AXS, D8-Advance, Billerica, MA, USA) patterns were collected at a scan rate of 0.2°s-1 over a 2q range of 10-80° with Cu Kα radiation (0.154056 nm). The surface functionalization of the MWCNTs was characterized by X-ray photoelectron spectroscopy (XPS, K-Alpha+, Thermo Fisher Scientific, Waltham, MA, USA) and Fourier transform infrared (FTIR) spectroscopy (Spectrum One, Perkin-Elmer, Waltham, MA, USA).
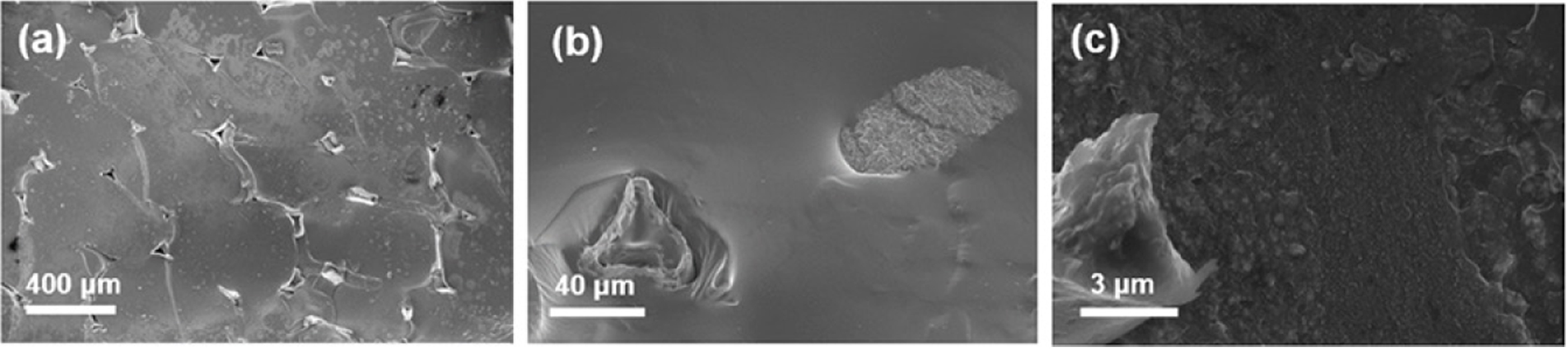
The morphology of the fractured composite surfaces is shown in Figure 1. Here, the Cu foam/epoxy composite is seen to exhibit good packing (Figure 1(a)). The Cu foam acts as the primary conductive filler by providing an uninterrupted thermal pathway throughout the matrix. The MWCNTs (0.5wt%) were added to the Cu foam/epoxy composite to provide a secondary thermally conductive filler. However, aggregation of the MWCNTs inside the polymer matrix is evident in Figure 1(b). This could be ascribed to the van der Waals forces between the MWCNTs leading to the formation of particle bundles and reduced surface area.12,13 In some cases, aggregation of the filler is beneficial for increasing the thermal conductivities of polymer composites due to the reduced phonon scattering at the interface between the filler and matrix. Moreover, enhanced aggregation also results in less interface between the filler and matrix where phonon scattering can occur. However, this case is only for maximum packing of filler inside the polymer. At lower filler fractions, aggregation increases the filler-filler interparticle distances and each aggregated clump can act as a thermal reservoir, thus lowering the heat transport efficiency. As shown in Figure 1(c), this problem is solved by attaching the CuNPs to the surface of the MWCNTs via surface coating with MPTMS to obtain a uniform dispersion of filler. Thus, the increased affinity between the Cu foam and the structurally similar CuNPs leads to an increased dispersion state compared to that obtained with the untreated fillers. Thus, the surface treatment enables a linear dispersion of MWCNTs throughout the epoxy matrix to connect up the Cu foam.
As detailed in the Experimental section, the MWCNTs were functionalized with MPTMS after oxidation by acid treatment.
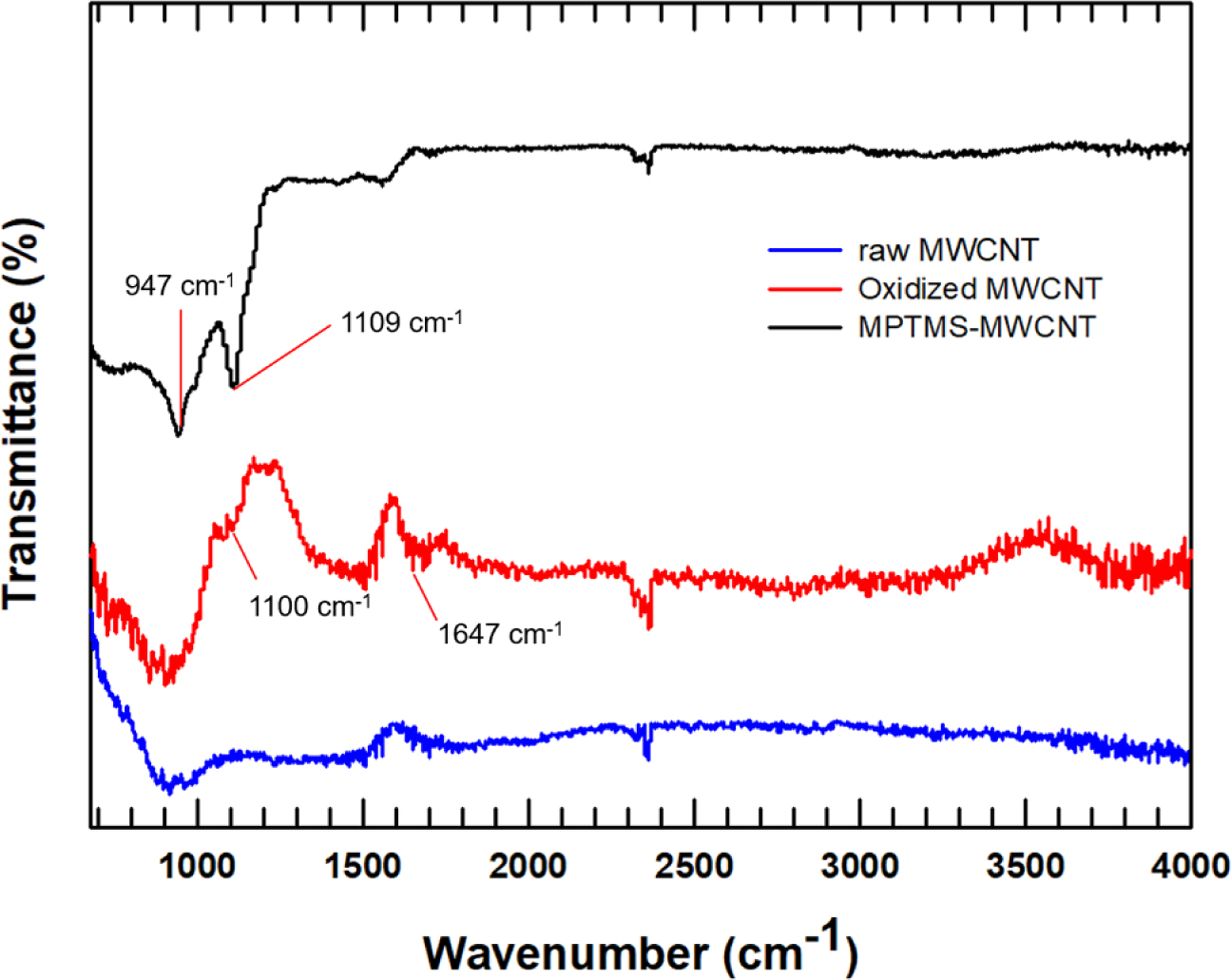
The functionalized MWCNTs were then analyzedby FTIR spectroscopy, as shown in Figure 2. Thus, the FTIR spectrum of the oxidized MWCNTs displays a broad absorption peak at 1100cm-1 and a weak absorption peak at 1647cm-1 corresponding to C=O of the carbonyl group, which is absent from the spectrum of the MWCNTs prior to oxidation.14 After the surface treatment of MWCNTs with MPTMS, an absorption peak at 947 cm-1 is observed, corresponding to Si-OH.14 Moreover, a peak at 1109 cm-1 is attributed to the Si-O-Si stretching vibration and indicates the presence of hydrolyzed MPTMS.15
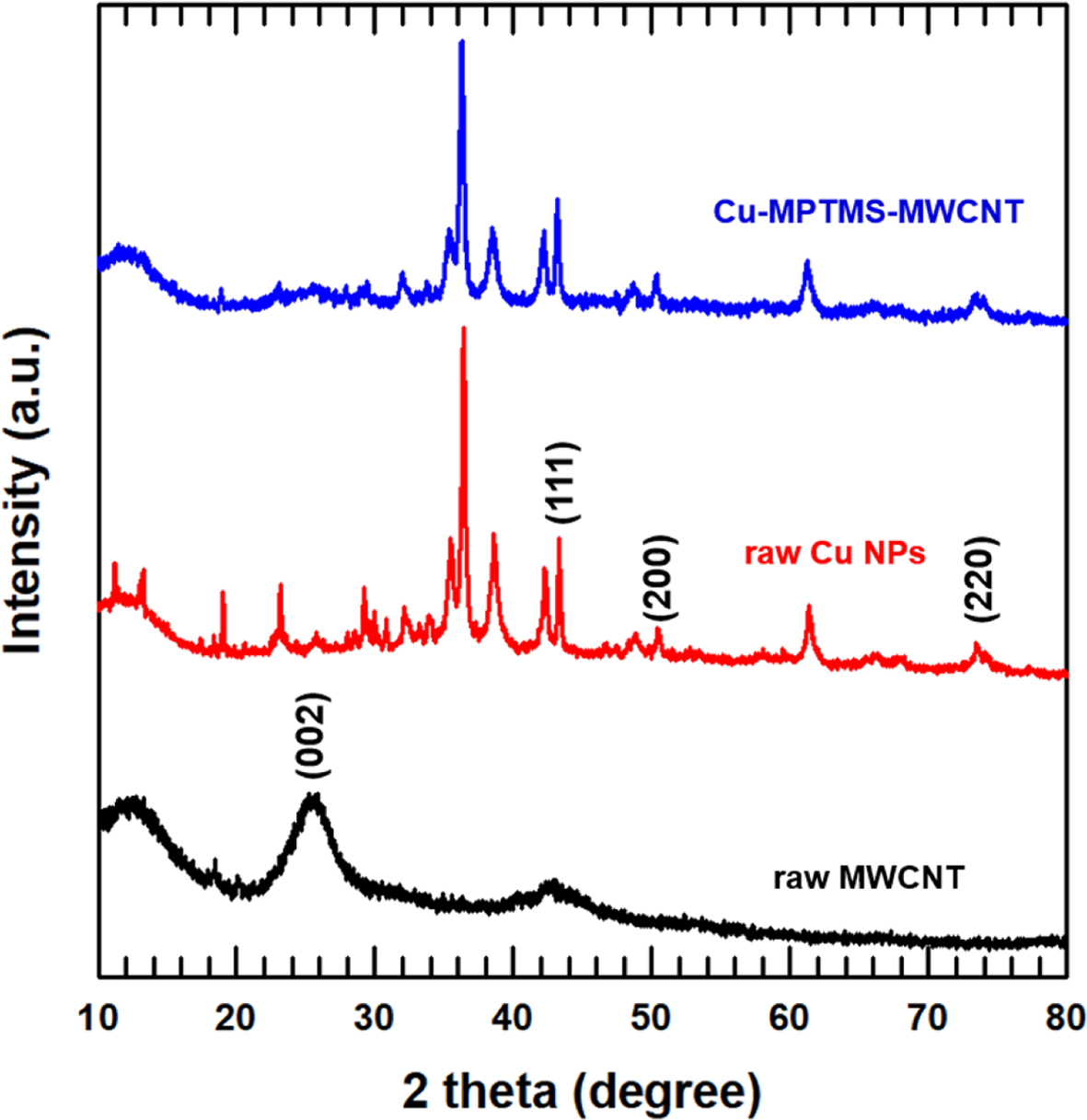
To further confirm the surface functionalization and attachment of CuNPs, the XRD patterns of the untreated and treated MWCNTs are presented in Figure 3. Here, the MWCNT pattern displays a diffraction peak at 25.58°, which is attributed to layered structure,16 while the Cu-MPTMS-MWCNT pattern displays peaks at 2θ ≈ 43.31°, 50.48°, 73.49° corresponding to the (111), (200), and (220) planes of the CuNPs with a d-spacing of 0.21, 0.18, and 0.13nm, respectively,16,17 thus confirming good attachment of the CuNPs to the MWCNTs after the surface treatment with MPTMS.
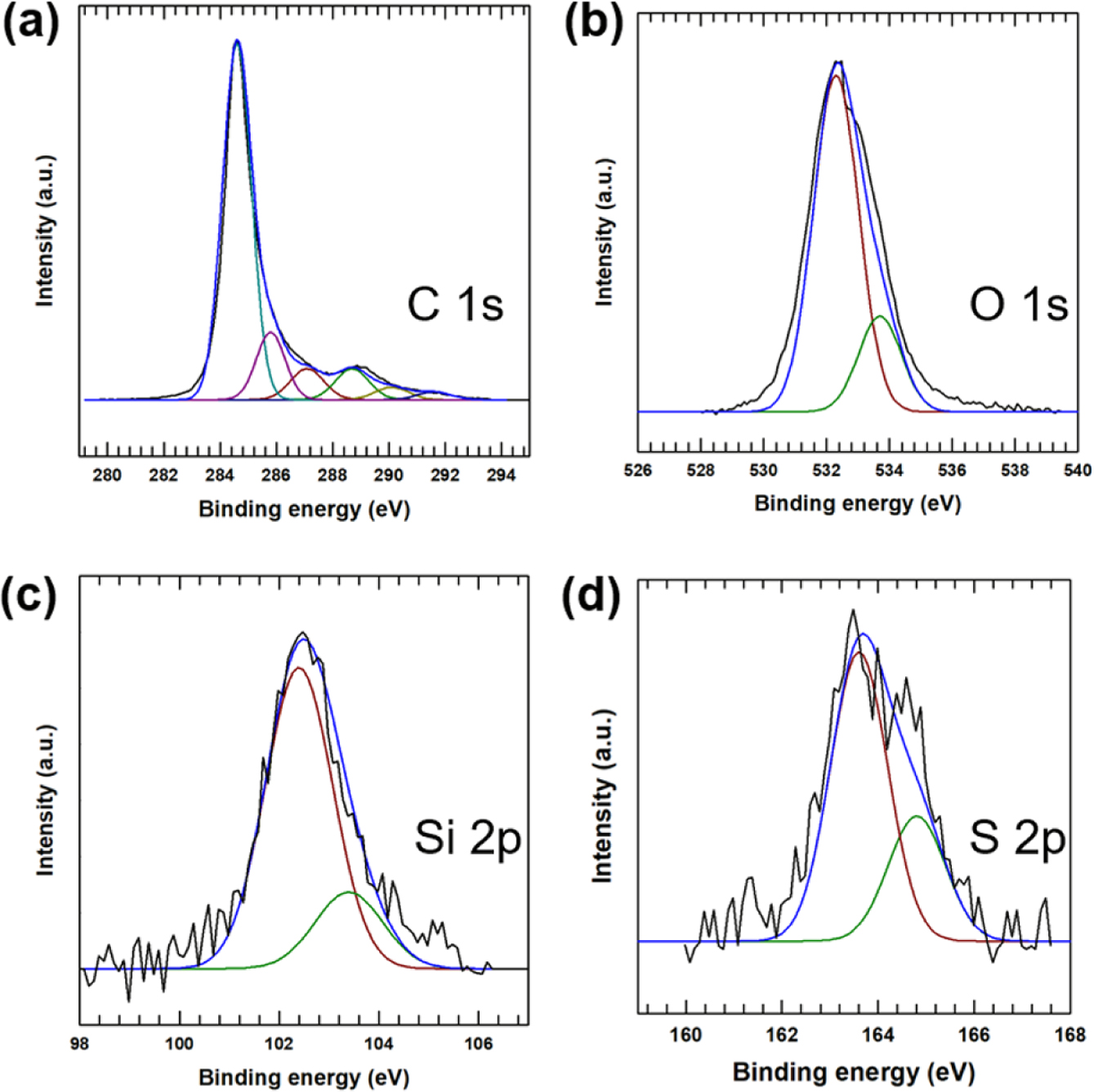
The oxidation of the MWCNTs and the surface treatment with MPTMS are confirmed by the deconvoluted C1s XPS spectrum in Figure 4(a), which reveals six peaks at 284.6, 285.8, 287.1, 288.6, 290.0, and 291.5 eV, due to the graphitic structure of the MWCNTs, the C-C defects on the MWCNTs, the C-O, the C=O ketone, the C=O-O carboxylic group, and the π-π sp2-hybridized carbon atoms, respectively. In particular, the peaks at 285.8 and 290.0 eV indicate oxidation of the MWCNTs prior to surface coating, while the XPS O1s, Si2p, S2p spectra in Figure 4(b, c, and d) further indicate the presence of MPTMS on the surface of the MWCNTs.18
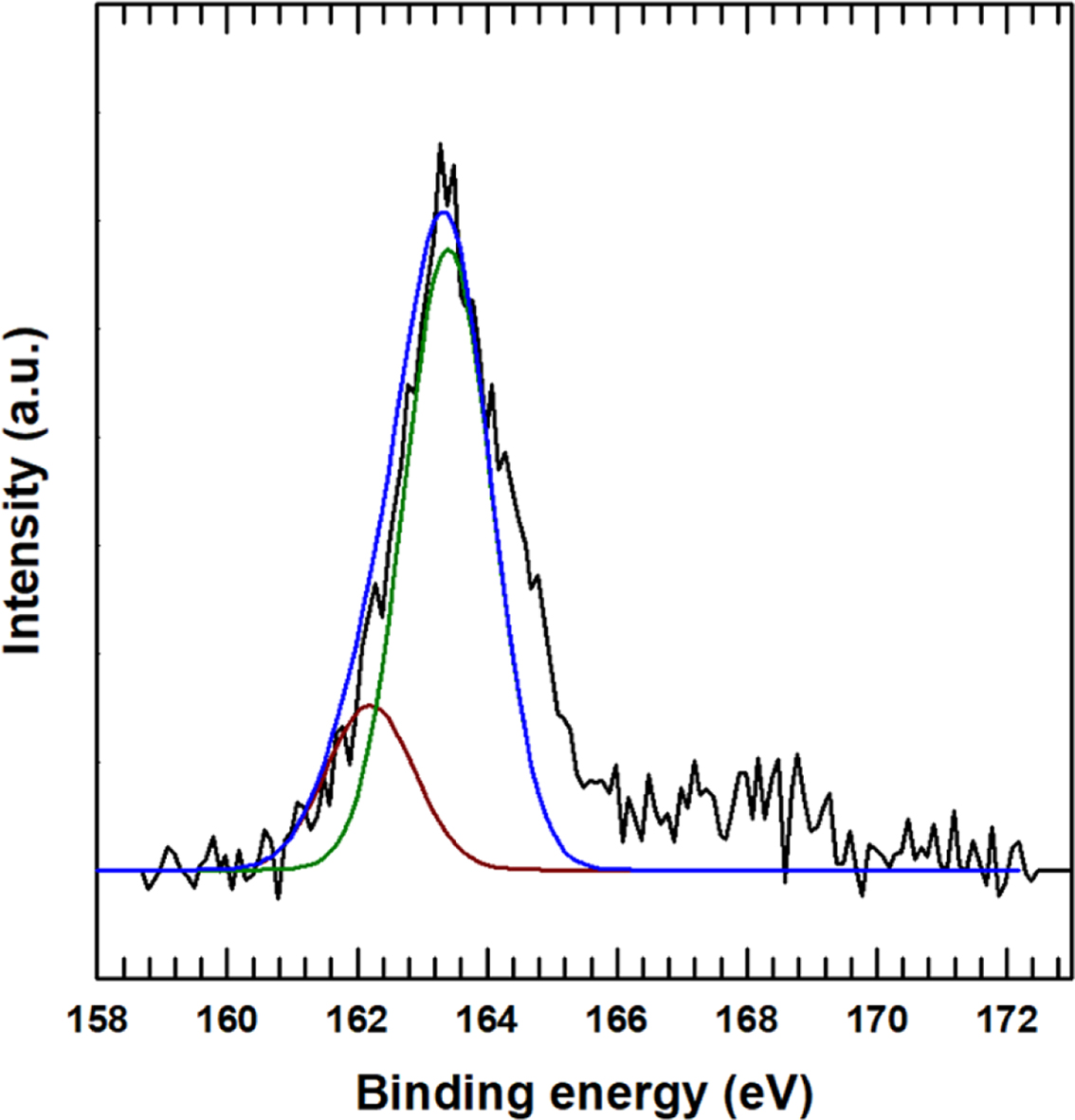
After attaching the CuNPs onto the surface-treated MWCNTs, the XPS spectra in Figure 5 were obtained. Here, the XPS S2p peak is deconvoluted into two peaks with binding energies of 163.6 and 162.2eV attributed to the S-C and Cu-S bonding,18,19 thus indicating that the CuNPs were attached onto the sulfur of the MPTMS after ionization. The XPS Cu2p peak was also deconvoluted into four peaks with binding energies of 933.6, 932.6, 932.5, and 932.2 eV, due to the Cu-O, Cu-SO4, Cu-Cu, and Cu-S bonding, respectively. It is also noted that a satellite peak can be observed in the Cu2p peak.
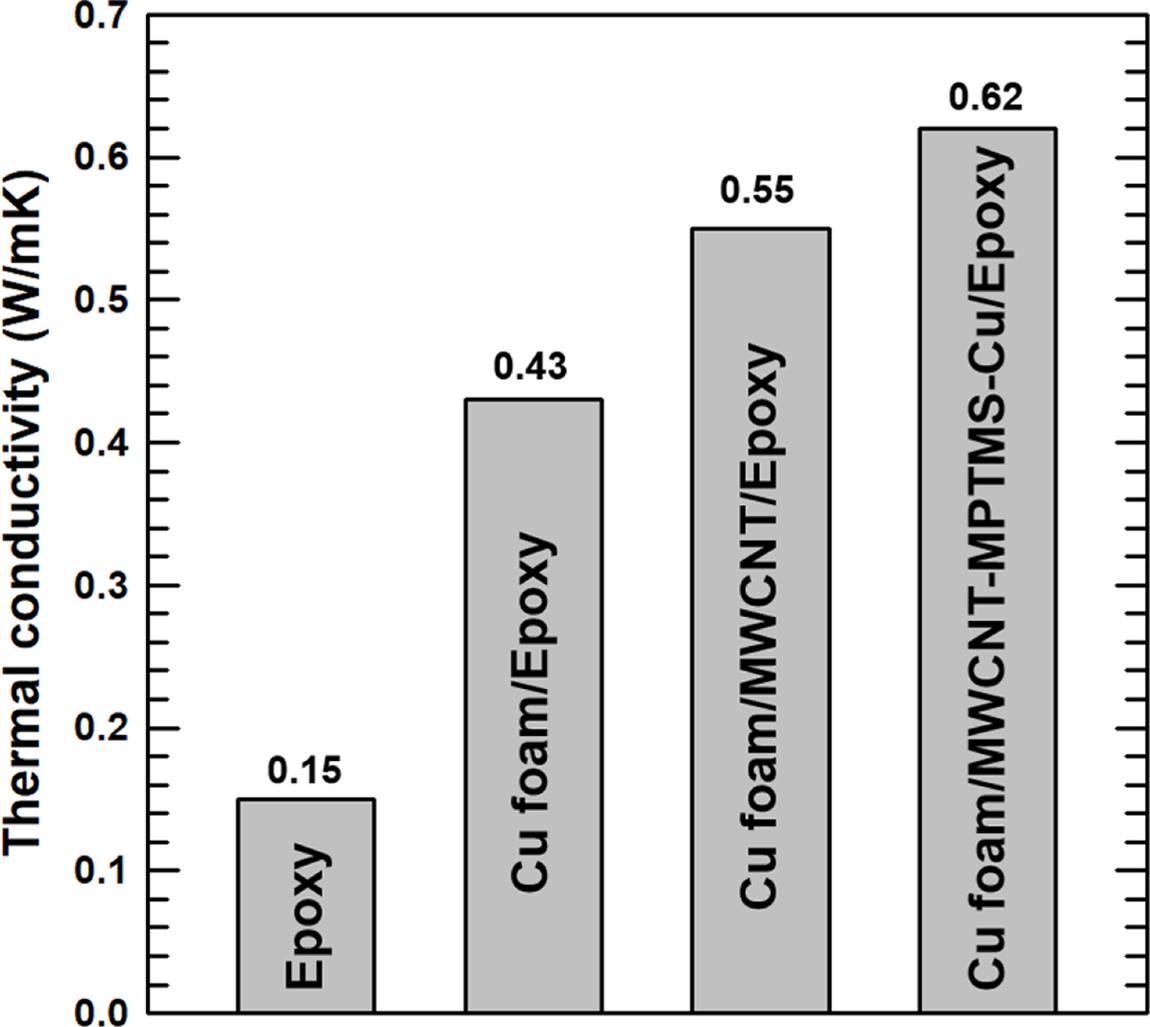
The through plane thermal conductivities of the composites with various fillers are presented in Figure 6. In the untreated epoxy, the amorphous phase of the matrix impedes phonon transfer, thus causing ineffective thermal dissipation resulting in a thermal conductivity of 0.15W/mK. By contrast, the incorporation of Cu foam (0.3g) into the epoxy (3g) is seen to increase the thermal conductivity to 0.43W/mK, which is an 186% improvement compared to the untreated epoxy. This is attributed to the construction of a continuous 3D thermal pathway by the primary filler (i.e. the Cu foam), thus enabling the phonons to be easily transferred.
For further improvement of the thermal conductivity, MWCNTs (0.5wt%) were added into the composite as a secondary filler designed to connect the Cu foam and create a more intricate thermal path. However, as indicated by the FE-SEM image of the fractured surface, aggregation of the MWCNTs has occurred, thus leading to an impeded phonon transfer so that only a slight enhancement in the thermal conductivity (28%) is achieved. This problem was addressed by MPTMS coating followed by attachment of the CuNPs. The silane coating increases the interfacial affinity with the epoxy matrix, thus enhancing the dispersion state of the MWCNTs inside the matrix. Moreover, the increased interfacial affinity reduces the amount of air space between the filler and the matrix, thus enhancing the thermal transport by boosting phonon transfer. In addition, the reaction of mercapto groups with the epoxy matrix enables the formation of a 3D network, thus providing an enhanced thermal pathway,20,21 since thermal conduction is a more effective form of heat transfer than thermal diffusion. Moreover, the CuNPs attached to the surface of the MWCNTs help to construct a thermal pathway connecting the Cu foam due to the structural similarity. For these two reasons, the composite displayed an enhanced thermal conductivity of 0.62 W/mK, which is an 44.2% improvement compared to that of the epoxy composite with only Cu foam, while that of the composite without surface coating was only 28%. This increased thermal conductivity of the fabricated composite indicates a potential for thermal management application in electronic devices.
|
Figure 1 Morphology of the fractured composite surfaces: (a) Cu foam/epoxy; (b) Cu foam/MWCNT/epoxy; (c) Cu foam/MWCNT-MPTMSCu/epoxy. |
|
Figure 2 FTIR spectra of MWCNT and surface modified MWCNTs. |
|
Figure 3 XRD analysis for MWCNT and surface modified MWCNTs. |
|
Figure 4 XPS analysis of MPTMS functionalized MWCNT: (a) C1s spectra; (b) O1s spectra; (c) Si2p spectra; (d) S2p spectra. |
|
Figure 5 S2p XPS analysis of Cu-MPTMS-MWCNT |
|
Figure 6 Thermal conductivity of epoxy composites with different fillers. |
In conclusion, the 3D interconnected thermal pathway within the epoxy matrix was generated by using Cu foam as the primary filler. To make a more intricate thermal pathway, MWCNTs were employed as the secondary filler. The MWCNT aggregation problem was overcome by surface treatment with MPTMS, after which CuNPs were attached to the MWCNTs to enhance their affinity for the Cu foam. The morphology of the filler distribution was observed by FE-SEM. The functionalization of the MWCNTs was further confirmed by XRD, XPS, and FTIR analysis. The thermal conductivity of the Cu-MPTMS-MWCNT/epoxy composite was shown to reach 0.62 W/mK with 10 wt% of Cu foam and 2 wt% of CuNPs-MPTMS-MWCNT. An 313% increase in the thermal conductivity was obtained compared to untreated epoxy, which was attributed to the increased affinity between the filler and matrix after the surface treatment and to the construction of a more intricate thermal pathway by the primary and secondary fillers.
- 1. Ouyang, Y.; Ding, F.; Bai, L.; Li, X.; Hou, G.; Fan, J.; Yuan, F. Design of Network Al2O3 Spheres for Significantly Enhanced Thermal Conductivity of Polymer Composites. Compos. Part A-Appl. S. 2020, 128, 105673.
-

- 2. Ohayon-Lavi, A.; Buzaglo, M.; Ligati, S.; Peretz-Damari, S.; Shachar, G.; Pinsk, N.; Riskin, M.; Schatzberg, Y.; Genish, I.; Regev, O. Compression-enhanced Thermal Conductivity of Carbon Loaded Polymer Composites. Carbon 2020, 163, 333-340.
-

- 3. Liu, C.; Wu, W.; Drummer, D.; Shen, W.; Wang, Y.; Schneider, K.; Tomiak, F. ZnO Nanowire-decorated Al2O3 Hybrids for Improving the Thermal Conductivity of Polymer Composites. J. Mater. Chem. C 2020, 8, 5380-5388.
-

- 4. Li, X.; Li, C.; Zhang, X.; Jiang, Y.; Xia, L.; Wang, J.; Song, X.; Wu, H.; Guo, S. Simultaneously Enhanced Thermal Conductivity and Mechanical Properties of PP/BN Composites via Constructing Reinforced Segregated Structure with a Trace Amount of BN Wrapped PP Fiber. Chem. Eng. J. 2020, 390, 124563.
-

- 5. Zhan, H.; Nie, Y.; Chen, Y.; Bell, J. M.; Gu, Y. Thermal Transport in 3D Nanostructures. Adv. Funct. Mater. 2020, 30, 1903841.
-

- 6. Wang, X.; Wu, P. 3D Vertically Aligned BNNS Network with Long-Range Continuous Channels for Achieving a Highly Thermally Conductive Composite. ACS Appl. Mater. Interfaces 2019, 11, 28943-28952.
-

- 7. Doan, V. C.; Vu, M. C.; Thieu, N. A. T.; Islam, M. A.; Park, P. J.; Kim, S.-R. Copper flake-coated Cellulose Scaffold to Construct Segregated Network for Enhancing Thermal Conductivity of Epoxy Composites. Compos. Part B: Eng. 2019, 165, 772-778.
-

- 8. Zhu, W.; Hu, N.; Wei, Q.; Zhang, L.; Li, H.; Luo, J.; Lin, C.-T.; Ma, L.; Zhou, K.; Yu, Z. Carbon Nanotube-Cu Foam Hybrid Reinforcements in Composite Phase Change Materials with Enhanced Thermal Conductivity. Mater. Des. 2019, 172, 107709.
-

- 9. Kim, P.; Shi, L.; Majumdar, A.; McEuen, P. L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502..
-

- 10. Soltanimehr, M.; Afrand, M. Thermal Conductivity Enhancement of COOH-functionalized MWCNTs/Ethylene Glycol–water Nanofluid for Application in Heating and Cooling Systems. Appl. Therm. Eng., 2016, 105, 716-723.
-

- 11. Kim, K.; Wie, J.; Kim, J. Synergistic Interaction of P and N co-doping EDTA with Controllable Active EDTA-cobalt Sites as Efficient Electrocatalyst for Oxygen Reduction Reaction. Ind. Eng. Chem. 2020, 83, 252-259.
-

- 12. Hassanzadeh-Aghdam, M. K.; Mahmoodi, M. J.; Ansari, R.; Mehdipour, H. Effects of Adding CNTs on the Thermo-mechanical Characteristics of Hybrid Titanium Nanocomposites. Mech. Mater. 2019, 131, 121-135.
-

- 13. Li, H.; Wei, C.; Zhang, D.; Pan, B. Adsorption of Bisphenol a on Dispersed Carbon Nanotubes: Role of Different Dispersing Agents. Sci. Total Environ. 2019, 655, 807-813.
-

- 14. Bag, D. S.; Dubey, R.; Zhang, N.; Xie, J.; Varadan, V. K.; Lal, D.; Mathur, G. N. Chemical Functionalization of Carbon Nanotubes With 3-Methacryloxypropyltrimethoxysilane (3-MPTS). Smart Mater. Struct. 2004, 13, 1263-1267.
-

- 15. Somera, B. F.; Corazza, M. Z.; Yabe, M. J. S.; Segatelli, M. G.; Galunin, E.; Tarley, C. R. T. 3-Mercaptopropyltrimethoxysilane-Modified Multi-walled Carbon Nanotubes as a New Functional Adsorbent for Flow Injection Extraction of Pb(II) from Water and Sediment Samples. Water Air Soil Pollut. 2012, 223, 6069-6081.
-

- 16. Badawy, S. M.; El Khashab, R. A.; Nayl, A. A. Synthesis, Characterization and Catalytic Activity of Cu/Cu2O Nanoparticles Prepared in Aqueous Medium. Bull. Chem. React. Eng. Catal. 2015, 10, 169-174.
-

- 17. Rittermeier, A.; Miao, S.; Schröter, M. K.; Zhang, X.; van den Berg, M. W. E.; Kundu, S.; Wang, Y.; Schimpf, S.; Löffler, E.; Fisher, R. A.; Muhler, M. The Formation of Colloidal Copper Nanoparticles Stabilized by Zinc Stearate: One-pot Single-step Synthesis and Characterization of the Core–shell Particles. Phys. Chem. Chem. Phys. 2009, 11, 8358-8366.
-

- 18. Han, J. T.; Jang, Y.; Lee, D. Y.; Park, J. H.; Song, S.-H.; Ban, D.-Y.; Cho, K. Fabrication of a Bionic Superhydrophobic Metal Surface by Sulfur-induced Morphological Development. J. Mater. Chem. 2005, 15, 3089-3092.
-

- 19. Yang, W. J.; Neoh, K.-G.; Kang, E.-T.; Teo, S. L.-M.; Rittschof, D. Stainless Steel Surfaces with Thiol-terminated Hyperbranched Polymers for Functionalization via Thiol-based Chemistry. Polym. Chem., 2013, 4, 3105-3115.
-

- 20. Yang, D.; Ni, Y.; Kong, X.; Wang, Y.; Zhang, L. A Mussel-like Inspired Modification of BaTiO3 Nanopartciles Using Catechol/polyamine Co-deposition and Silane Grafting for High-performance Dielectric Elastomer Composites. Compos. Part B-Eng. 2019, 172, 621-627.
-

- 21. Li, J.; Zhao, X.; Zhang, Z.; Xian, Y.; Lin, Y.; Ji, X.; Lu, Y.; Zhang, L. Construction of Interconnected Al2O3 Doped rGO Network in Natural Rubber Nanocomposites to Achieve Significant Thermal Conductivity and Mechanical Strength Enhancement. Compos. Sci. Technol. 2020, 186, 107930.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 56-61
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.56
- Received on Jul 13, 2020
- Revised on Aug 10, 2020
- Accepted on Aug 26, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jooheon Kim
-
School of Chemical Engineering & Materials Science, Chung-Ang University, Seoul 06971, Korea
- E-mail: jooheonkim@cau.ac.kr
- ORCID:
0000-0002-6644-7791










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.