- Nanomaterial Enhanced Polyelectrolyte Membranes for Hydrogen-Oxygen Fuel Cells
Abdul Kalam Azad*, Lakshmi Unnikrishnan**,†
 , Smita Mohanty***, and Sanjay Kumar Nayak*, **, ***
, Smita Mohanty***, and Sanjay Kumar Nayak*, **, ****Central Institute of Plastics Engineering & Technology (CIPET), Guindy, Chennai 600032, India
**CIPET, School for Advanced Research in Polymers (SARP)-APDDRL, Hi Tech Defence and Aerospace Park, Bengaluru 562149, India
***CIPET, School for Advanced Research in Polymers (SARP) - LARPM, B/25, CNI Complex, Patia, Bhubaneswar 751 024, India- 수소-산소 연료 전지용 나노물질 첨가 고분자 전해질 막
The solid electrolyte membrane for a hydrogen-oxygen fuel cell was prepared and investigated from the potential of sulfonated poly(ether ether ketone) (SPEEK) embedded together with montmorillonite (MMT). Acid hydrophilized poly(ether ether ketone) (PEEK) and MMT with varying sulfonation levels of 25-70% prepared through solvent casting were investigated for their performance. The nuclear magnetic resonance (1H NMR) spectra at 7.5ppm affirmed the occurrence of sulfonation reaction, and its degree was studied by both 1H NMR and titration method. The reaction time was varied to achieve sulfonation levels from 25 to 70%. The effect of incorporation of pristine and sulfonated MMT into SPEEK was examined. The membranes, prepared using solvent casting technique, were involved for water uptake over the wide range of temperature, thermal stability, and proton conductivity measurements. The cross-sectional surface arrangement of the membrane and clay dispersion was deliberated using SEM. The proton exchange membrane fuel cell (PEMFC) single cell’s tests revealed that sulfonated PEEK membrane demonstrated service performance comparable to that of Nafion, as validated using MATLAB software.
Victrex poly(ether ether ketone) was sulfonated and optimized (68.74%DS) successfully to prepare the series of SPEEK, SPEEK/MMT and SPEEK/SMMT nanocomposite membranes. Maximum power density of 185mW/cm2 was accomplished for SPEEK and 153mW/cm2 for SPEEK/SMMT nanocomposite membranes.

Keywords: poly(ether ether ketone), fuel cell performance, proton conductivity, sulfonated montmorillonite
The authors intend to express their gratitude to Dr. Sangeetha D. and Mr. Muruganantham R, Department of Chemistry, Anna University, Sardar Patel Road, Chennai - 600025, India for their support during characterizing the membranes for (PEMFC) performance at their laboratory.
MATLAB validated data is available for SPEEK, SPEEK/SMMT and NAFION membranes during its fuel cell performance test. The materials are available via the Internet at http://journal.polymer-korea.or.kr.
PK_2020_045_01_101_Supporting_Information_template.pdf (179 kb)
Supplementary Information
It is appreciable for environmental protection that has soared the demand for eco-friendly power generation. This has encouraged and supported the research in different fuel cell technologies, if one takes into account all the parameters considered to choose an energy conversion devices, the one which stands out and fulfill all the parameters such as low pollution and low maintenance cost in the fuel cell.
Any electrochemical technology which basically converts fuel such as hydrogen into electricity can be considered as fuel cell technology. In this technology, there is no recharging as normally done in battery technology. Fuel cell technology is the act of producing heat through electrochemical oxidation of fuel such as hydrogen to generate heat and electricity. Different types of electrolytes are used in fuel cell technology but when it comes to considering electricity for vehicles and buildings, the proton exchange membrane fuel cells (PEMFCs) seem to attract a considerable deal of curiosity.1-3 The mechanism for polymer electrolyte membrane (PEM) is transferring protons from the positively charged electrode (anode) to the negatively charged electrode (cathode), thus allowing only finite mobility of electrons and gases between the electrodes.
PEMs which have good chemical along with mechanical stability are used as electrolytes in PEMFCs. The reason for this is that these electrolytes have high proton conductivity. Nevertheless, their notable drawbacks are viz, exorbitant, high methanol-crossover, low temperature operation and drop of conductivity at elevated temperatures (>80°C) have motivated an exhaustive search for a suitable alternative.4 Several aromatic thermoplastics,5-10 namely poly(aryl ether ketone)s (PAEKs),5,7-12 poly(ether sulfone) (PES),13-15 polysulfone (PSU),16,17 polybenzimidazole (PBI),18,19 etc., have excellent thermo-oxidative stability, better mechanical properties, high chemical resistance and inexpensive. Among them, PEEK with reasonable conductivity characteristics, better thermal stability and magnificent mechanical properties are the suitable candidate for fuel cell membrane application.20-23
The hydrophilic passages in sulfonated PEEK (SPEEK) are more branched and narrower than Nafion as observed by Poppe et al. and Jones et al., respectively.24,25 This caused lower electro-osmotic drag and permeation co-efficient hence making them more suitable for direct methanol fuel cell (DMFC). The post-sulfonation process may weird the mechanical properties and hence impair their long-term stability. Scientists have initiated to focus more on diffusing inorganic fillers in the sulfonated polymer matrix so as to overcome limitations.26-29
Sodium–montmorillonite (MMT) is an inorganic filler which can impact the membrane properties. MMT has the general chemical formula Mx (Al4-x-Mgx) Si8O20(OH)4, which is a silicate with the silica tetrahedral and alumina octahedral sheets, where M is a monovalent cation and x is the degree of isomorphous with a value ranging from 0.5 to 1.3. This nanoclay within the polymer matrix impacts high barrier properties causing improvement in the thermal and mechanical properties. Researchers investigated that the permeability of methanol was reduced noticeably when nanocomposite membranes were used. Other researchers also noted that the SPEEK/MMT nanocomposite membranes are reduced methanol permeability, obstruct swelling at high operational temperatures, increase mechanical strength, thermal stability and maintain optimum hydration levels in the membrane in different cell conditions.30,31
The influence of sulfonation and nanohybridization on the conduct of PEEK was evaluated in this study. 1H NMR as well as conventional titration method was utilized to determine the degree of sulfonation and it is optimized in accordance with the thermal analysis. The effectiveness of SPEEK and SPEEK/MMT nanocomposite membranes was investigated from thermal, morphological and proton conducting properties. A study of the in-service performance of the membranes, single cell performance test was also conducted.
Preparation of Nanocomposite Membranes. Materials: PEEK (Grade 450G, Victrex PLC, England) was utilized as the core polymeric material and conc. sulfuric acid (H2SO4) (98% pure, Merck, Germany) was used in the sulfonation of polymer. Sodium montmorillonite (MMT), (Merck, Germany) was used as a nanomaterial for enhancing the quality of membrane. 3-Aminopropyl triethoxysilane (γ-APS), (Dynasylan, Germany), 4-sulfophthalic acid (50%, Sigma Aldrich, U.S.A.), 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (97-98% pure, Alfa Aesar, England) and ethanol (75%, LOBA Chemie, India) was used in the sulfonation of MMT. Sodium hydroxide (NaOH), (LOBA Chemie, India), phenolphthalein (indicator), (LOBA Chemie, India) was used in the titration process. Laboratory grade dimethyl acetamide (DMAc), (LOBA Chemie, India) was used in membrane preparation and titration as well.
Sulfonation of PEEK: Before sulfonation process, PEEK (10 g) was employed for pre drying action at 100°C for 12h to get rid of moisture and then added into the vigorously stirred 350mL of conc. H2SO4 (98% pure) at approximately 20°C. After requisite reaction time, the sulfonated solution was added into the excess of methanol for recovering SPEEK and the precipitate was washed minutely with distilled water up to the time of neutral pH was ~7. Subsequently, the samples were collected and kept at room temperature for one day, then dried at 100°C in a vacuum oven for 12h in order to eliminate the existence of water completely at the last. In the washing process it was noted that polymers began to swell at higher degree of sulfonation (>60%). This caused it to be difficult to remove the residual acid, this making recovery impossible.
Sulfonation of MMT: A prescribed amount of MMT (4.0 g) was suspended in 1.6 L of ethanol solution (75%) and stirred vigorously for 3h at approximately 20°C prior to drop-wise addition of γ-APS (4.8 mL). The refluxed (80°C for one day) suspension of silane-modified NaMMT (SiMMT) was separated and cleaned repeatedly with deionized water as displayed in step 1, Scheme 1. Subsequently, 4-sulfophthalic acid (1.09×10−2 mole), water conjugating agent (WSC) (1.30×10−2 mole) and SiMMT were dispersed and mixed in deionized water as a medium. The dispersion was again stirred vigorously for about 24h. Finally, sulfonated MMT was separated, cleaned and dried in vacuum oven at 65°C for overnight as displayed in step 2, Scheme 1.

Scheme 1. Sulfonation of MMT.
Preparation of Membranes: The method for the preparation of membrane as outlined in ref 30 was used with slight modification to obtain ~300µm thick SPEEK membrane. The cast viscous solutions were dried in vacuum oven at 100°C for 12h and the acquired membrane was removed cautiously from the Petri dish without any surface defects as the existence of defects would affect the performance of membrane which was observed in the beginning and avoided with the experience. A previously reported method31 was used for preparing SPEEK/MMT and SPEEK/sulfonated NaMMT (SMMT) membranes as clearly depicted in Scheme 2.

Scheme 2. SPEEK/MMT and SPEEK/SMMT nanocomposites.
Measurements. BRUKER 400 ultrashieldTM spectrometer was used to identify the 1H NMR at a reverberation recurrence of 400 MHz. The appropriate parameters were adjusted to a spectral window of 8012 Hz (20 ppm), a pulse angle of 90°, obtaining time of 4 sec and unwinding deferral of 1s.
1H NMR was also used to calculate DS from the proportion between the peak area of the distinct HE signal (AHE ) and the integrated peak area of the signals equivalent to all the other aromatic hydrogens (AHAA|.BB|.CD), using the formula mentioned below as reported in ref 12,
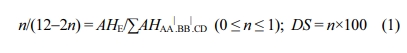
Where, AHE=1; ∑AHAA|.BB|.CD varied with the reaction time and n is the number of HE per repeating unit.
DS measurements were taken using the classical titration method which indicates the % of sulfonated polymeric repeating unit. Ion exchange capacity (IEC), used in the process of indicating the amount of H+ ions in 1g of dried SPEEK, was calculated from DS. The dried SPEEK sample (1.0g) was then dissolved in 20mL of dimethylacetamide (DMAc) and the H+ ions discharged were identified by titration with the standard solution of NaOH utilizing phenolphthalein as an indicator. Lakshmi et al. study was followed in order to calculate the sulfonation degree and IEC.15
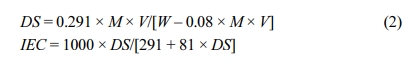
Where,M: Concentration of standard NaOH solution (mole/L),V: Volume of standard NaOH solution used to neutralize (mL),W: Mass of sample (g),291: Molecular mass of PEEK monomer unit, 81: Molecular mass of -SO3H groupBefore water uptake test, membranes of dimension 2×2cm2 were dried in a vacuum oven at 120°C for 24h and weighed; immersed in deionized water at room temperature for 24 h, and then re-weighed. The water uptake in the membrane at higher temperatures viz., 60°C, 80°C and besides 100°C for 2h were noted and calculated by using the subsequent formula:
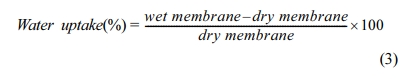
Zeiss EVO MA 15 scanning electron microscope with the speed up voltage of up to 10 kV has been utilized to investigate the cross-sectional morphologies of the prepared SPEEK nanohybrid polymer membranes coated with a fine surface of Au/Pd.
Proton conductivity of extensively hydrated membranes for 24 h at an ambient temperature were recorded by AC impedance spectroscopy method using the Quad Tech 7600 (Model-B) version-3.0 gain phase analyzer in the frequency range of 1×10-5-2MHz with an oscillating voltage range of 20mV-5V and a current range of 250µA-100mA with the usage of two probe for measuring the resistance.
Fabrication of Membrane Electrode Assembly (MEA) and Single Cell Performance. MEA was constructed uniaxially with the dimensions of 5×5cm2 by hot pressing of the cathode and anode onto the membrane under the pressure of 2000kg/cm2 for 2min with a temperature of 100°C as exposed in Figure 1. For PEMFC, the electro catalyst used was 20 wt% Pt on Vulcan XC-72 in teflonized carbon supported cloth for both the cathode (loading 0.0005g/cm2) and anode (loading 0.00025g/cm2) side. The surface of the catalyst was formulated by mixing the catalyst, isopropanol (IPA), distilled water and Nafion solution as binder together with applied on the carbon cloth. Fulfilment of the membrane was recorded by means of PEMFC test stations. The energetic cell area used in the PEMFC experiment was 25cm2 with an externally applied electronic load furthermore influenced manually. Stainless steel test cell has been utilized to deploy the fabricated MEA and all the bolts were compacted with similar torque to execute the test. In this PEMFC, the H2 as well as O2 flow rates were continuously retained at 15 mL/30 sec in the cathode along with anode respectively.
|
Figure 1 Membrane electrode assembly. |
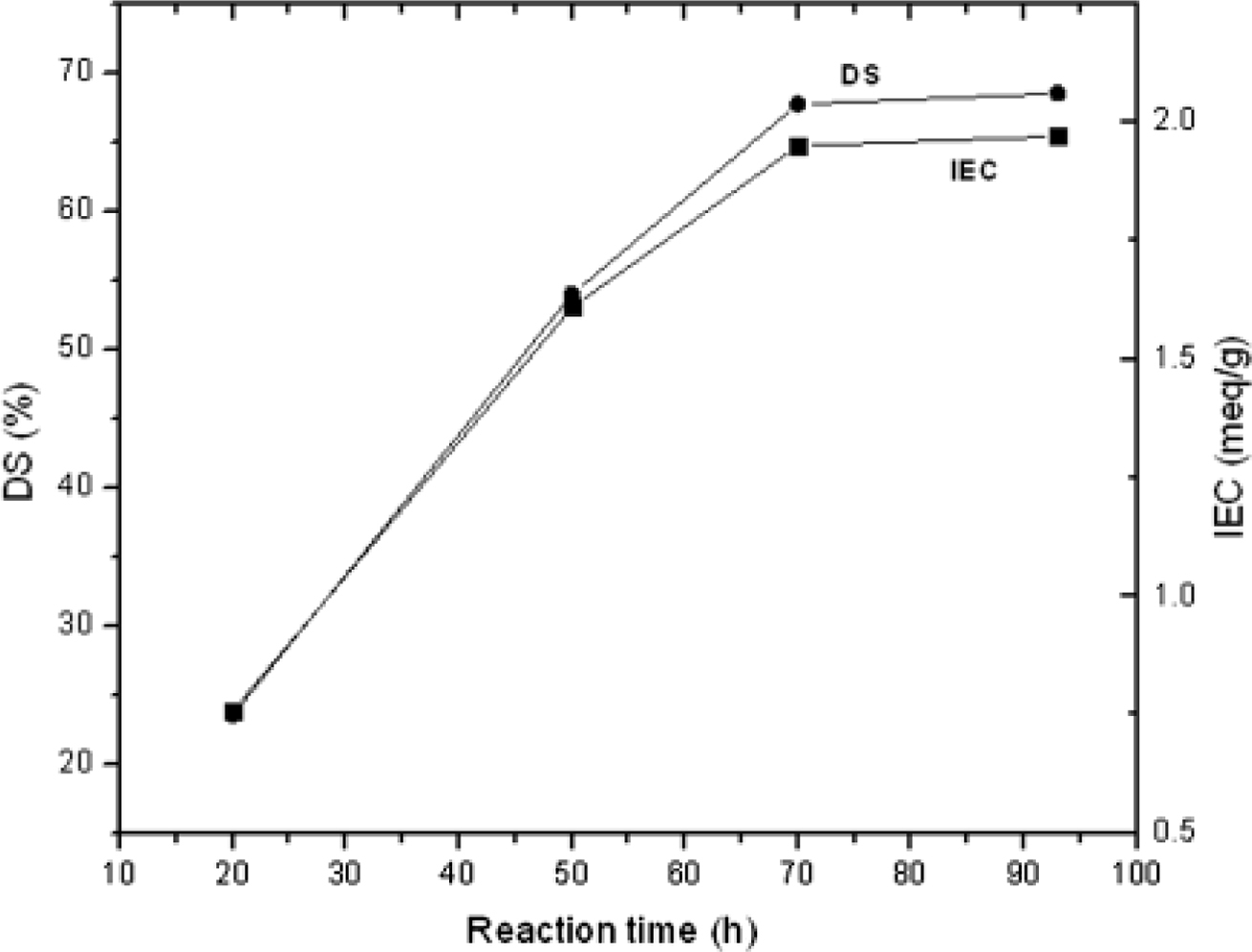
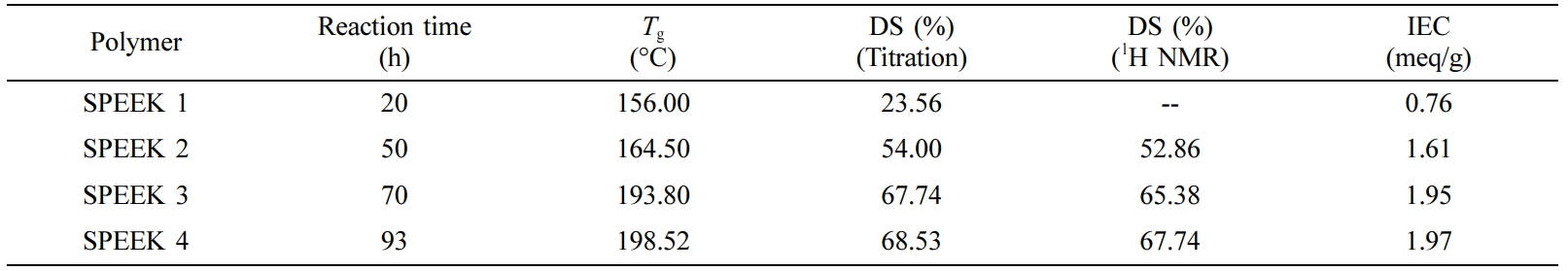
Sulfonation of PEEK. SO3H functionalized PEEK with proposed proton conductivity was prepared through post-sulfonation by differing reaction time with a minimum of 20 to a maximum of 140h. The effectiveness of sulfonation, an electrophilic reaction, relies on the aromatic group of polymer substituents. In the case of PEEK, the quinol unit (in the middle of ether bridges) is easily sulfonated even more under meek conditions towards electrophile reactions.17 As expected, DS and IEC (Table 1), Figure 2 increased with reaction time thereby reducing the crystallinity of PEEK and increasing its solubility.
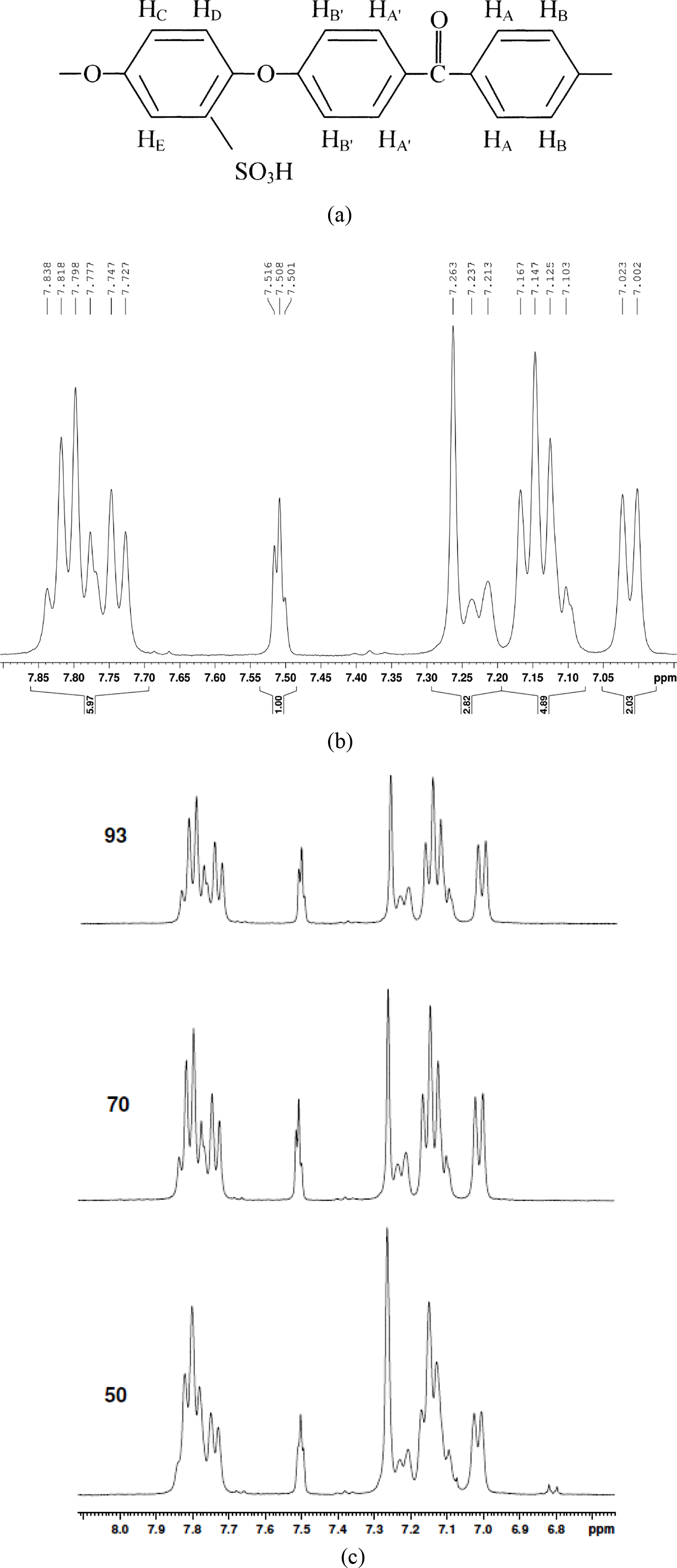
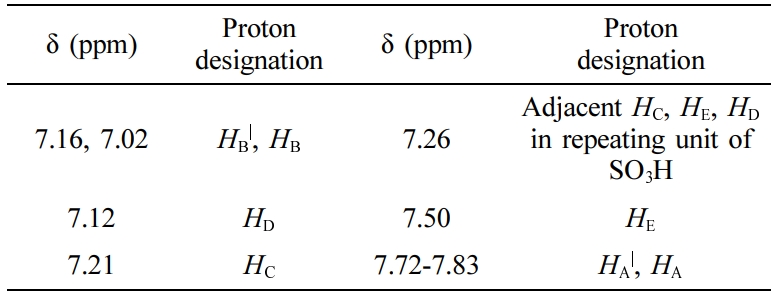
PEEK sulfonation was verified via 1H NMR spectroscopy as shown in Figure 3(b). From the 1H NMR spectra, it was observed that the existence of the sulfonic acid group in the polymer backbone produced a notable downfield of HE hydrogen compared to HC, HD in the quinol compound. The maximum DS is limited to 1.0 and considers only on the four chemically identical site of quinol section.29 The nomenclature of the aromatic protons for the SPEEK repeating unit is given in Figure 3(a) and the chemical shift assignments of 1H NMR spectra are listed in Table 2.
A significant signal for protons at the E location was detected at 7.5 ppm suggesting the presence of -SO3H group. The HE signal intensity makes an estimate of the HE content that is identical with the -SO3H group. Virgin PEEK could not be analyzed as it is insoluble in most of the solvents except strong acids.17 It was carefully observed that, while DS increases, the intensity of HE increases along with singlet HC, HD resonance at 7.26 ppm corresponding to non-sulfonated repeating unit in the polymer chain, decreases. The adjacent doublet peak representing the HC, HD of SO3H attached aromatic ring exhibited increase in intensity at higher DS as shown in Figure 3. The intensities of both HC doublet at 7.21ppm and the two HB| at 7.14 ppm increased proportionally with the intensity of HE. The DS was also determined by 1H NMR from the ratio of the peak area of the independent HE signal (AHE) to the combined peak area of the signals corresponding to all other aromatic hydrogens (AHAA|.BB|.CD), using the below mentioned formula,
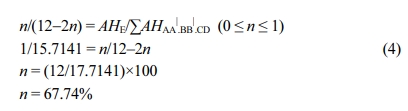
Where, AHE=1
∑AHAA|.BB|.CD=17.7141 and n is the number of HE per repeat unit. An approximation of the degree of sulfonation DS is obtained as: DS=n×100
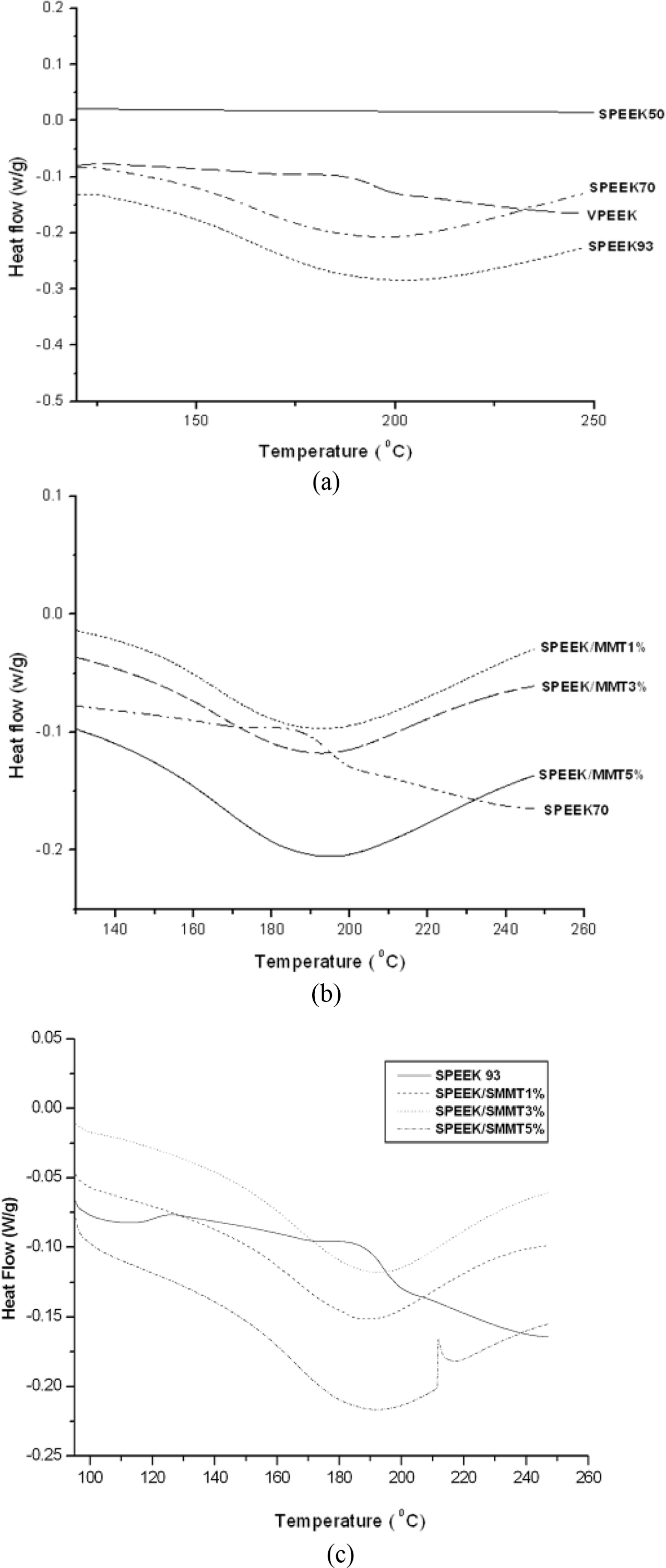
Glass Transition Temperature (Tg). Membrane samples Tg were identified using DSC. In preparation for the execution of proton exchange membranes high temperature, it should possess high Tg owing to the fact that above this Tg, the material loses its firmness which leads to the worsening of its performance characteristics. PEEK is a thermally stable polymer with a Tg of ~152°C as clearly observed in Figure 4(a) and confirmed the study of Xing et al.36
DSC analysis showed that the SPEEK Tg increases to a maximum of 198 °C at 93 h with the increase of the reaction time. In accordance with previous reports,12 the increase in Tg value in ionomeric polymers is normally observed due to the expected strong interplay between sulfonic groups and the introduction of these groups onto the polymer backbone has two outcome on Tg,
· Rise in molecular interplay by side group ions
· Rise in molecular vastness
These two outcome will inhibit the internal rotation of a polymer molecule as a consequence of that increased Tg in sulfonated polymers. As evident from Figure 4(a), the Tg increases with increasing DS. The slight dispersion of Tg values may be attributed to the physical cross-linking effect which could be saturated at certain DS.
Incorporation of unmodified MMT into SPEEK matrix decreased the Tg of nanocomposite, as displayed in Figure 4(b). Different loading of nanoclays were added to SPEEK 90. Addition of 1% MMT decreased the Tg to 177 from 180°C for SPEEK 90. Further, addition of nanoclays to the tune of 3wt%, marginally increased the Tg to 178°C. There was no variation in the Tg of SPEEK/MMT nanocomposite with 5wt% nanoclay loading.
However, addition of SMMT increased the Tg in contrast to that of SPEEK 93 and SPEEK/MMT nanocomposite membranes. The thermograms are shown in Figure 4(c). SPEEK/1wt%SMMT, SPEEK/3wt%SMMT, SPEEK/5wt%SMMT showed Tg of 187, 188 and 186°C accordingly. This rise in Tg values may be attributed to the -SO3H to -SO3H, interplay between the sulfonated polymer and sulfonated nanoclay (SPEEK and SMMT), thus improving their compatibility. Incorporation of various weight% of SMMT had only marginal effects on the thermal stability of the membranes of SPEEK/SMMT nanocomposites. The insertion of SPEEK into the clay structure might occlude the mobility of polymer chain whichever results in chain packaging and enhancing Tg.32
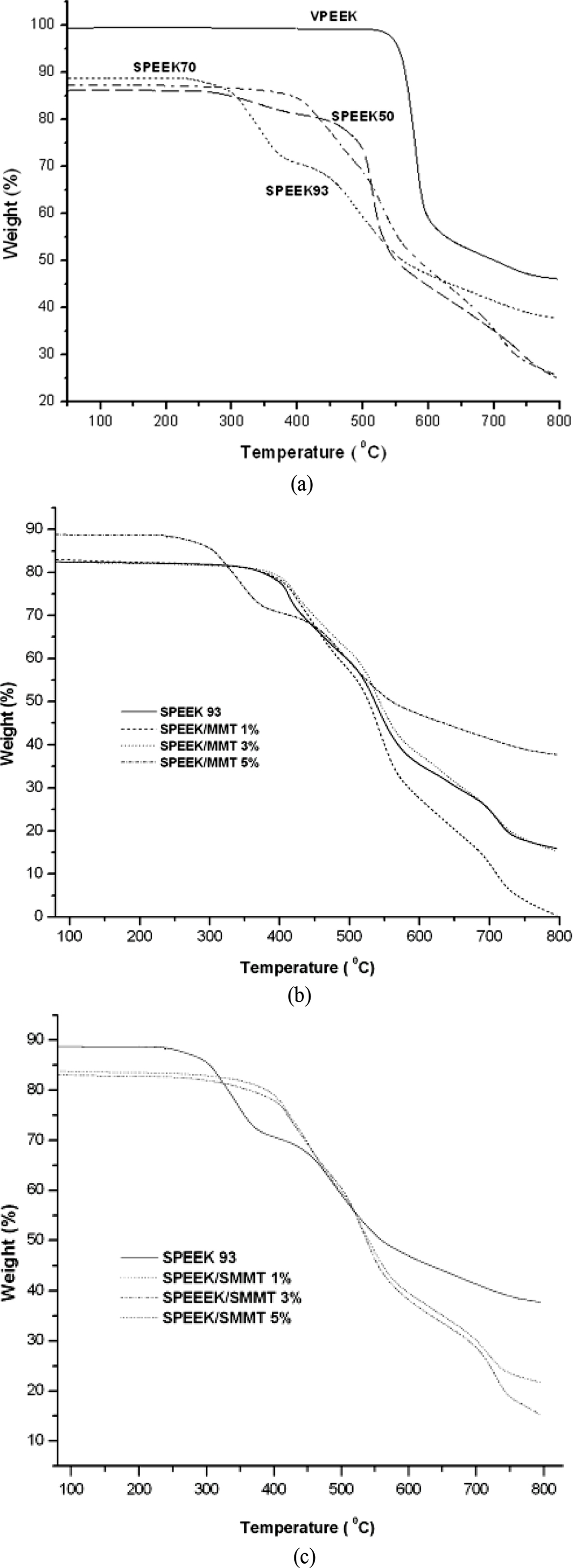
Thermal Decomposition. Figure 5(a), 5(b) and 5(c) reveals the thermal reliability and thermal degradation temperature (Td) of the virgin PEEK, SPEEK, SPEEK/MMT and SPEEK/SMMT nanocomposite membranes throughout a temperature range of 25-850°C studied by TGA.
PEEK, a high temperature resistant polymer, displayed a single-step thermal degradation with initiation of degradation (Td1) at 550°C, whereas SPEEK and nanocomposite membranes showed two-step degradation. SPEEK remained pyrolytically stable up to 280°C. As a result of pyrolytic degradation of PEEK, phenols and benzenes are produced as by-products in the prior chain scission reaction and involve ROR´ linkages rather than RCOR´ linkages.2 The first step is mainly correlated with the elimination of -SO3H groups at 240-300°C and the next step (Td2) which occurs in the range of 400-580°C, may be related to the degradation of PEEK main chain. Due to the insertion of SO3H group into the main polymeric chain, the degradation temperature of SPEEK main chain is lower than that of virgin PEEK, which makes it degrade faster than the virgin PEEK. It was observed from Figure 5(a) that the thermal stability decreased with the sulfonation of PEEK which is agreed with the Robertson et al. study.33 Furthermore, the degradation temperature was decreased with a rise in sulfonation degree intimating the buildup of number of -SO3H groups attached to the backbone of polymer.
MMT and SMMT were integrated to enhance the thermal reliability of sulfonated PEEK membranes and the thermogram results are displayed in Figure 5(b) and 5(c). The addition of unmodified MMT improved the initial degradation temperature as compared to that of SPEEK. This increase in Tdi is conceivably due to the insertion of polymer chains into the surfaces of silicate. However, the maximum degradation temperature (Tdmax) decreased in case of nanocomposite membranes despite increase in nanoclays addition. This phenomenon may be due to the dissociation of -SO3H groups within the nanocomposites.
Further, there was a dramatic increase in the Td1 of SPEEK/SMMT nanocomposites irrespective of the nanoclays loading. As observed from Figure 5(c), the thermal reliability of the nanocomposite membranes initially improved with the inclusion of SMMT. However, the degradation temperature was decreased when the SMMT loading was added from 3 to 5wt%. The aggregation of SMMT layers might have contributed to this decrease as pointed out by Gosalawit et al.34 and Jung et al.,35 respectively.
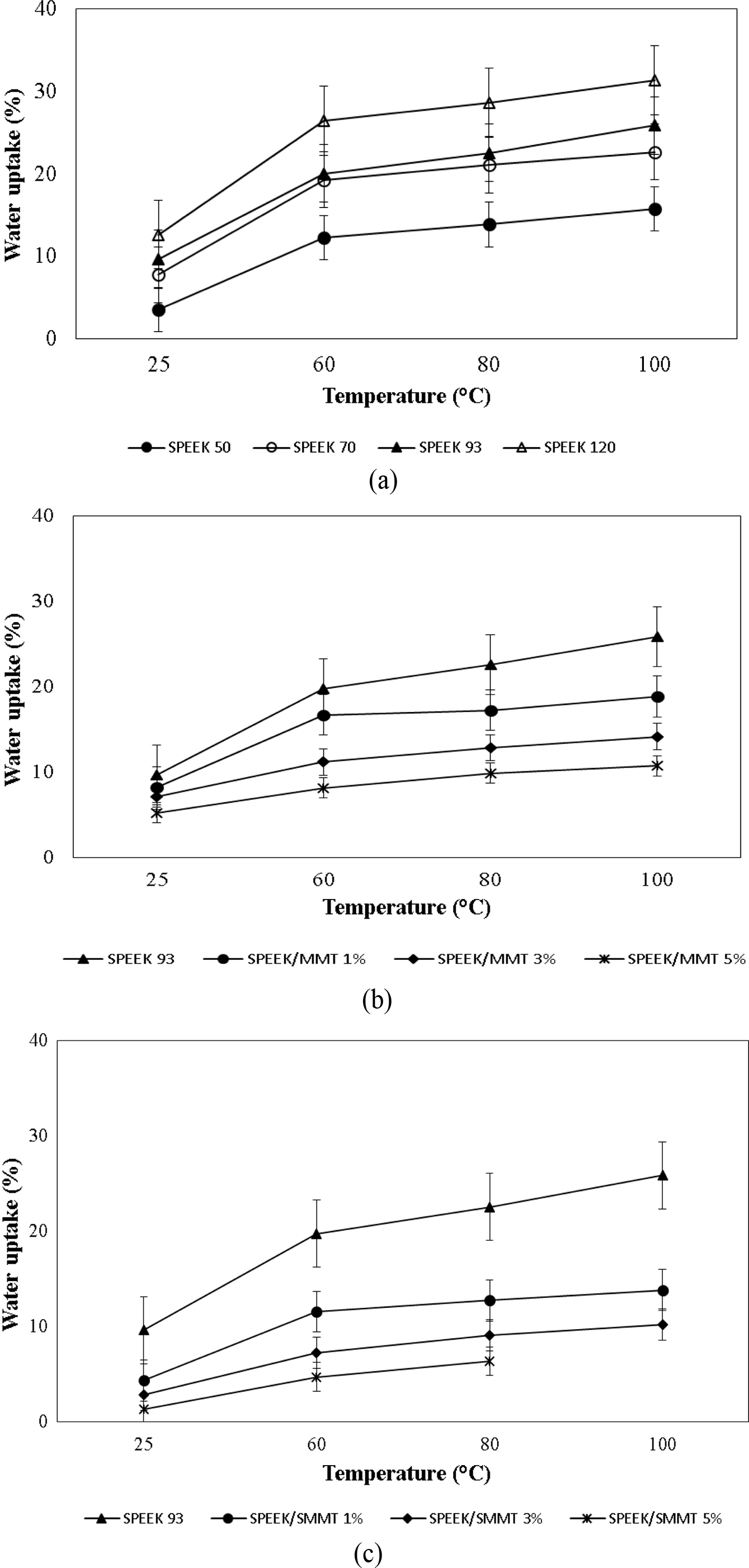
Water Uptake. The membrane stability based on water uptake with a gradual increase of temperatures was studied. The main objective of sulfonating virgin PEEK is to upgrade the state of hydrophobic into hydrophilic polymer in order to attract the water molecules to assist the proton transfer by vehicle mechanism. Generally, the conductivity of protons for ionomeric membranes depends on the existence of usable acid groups and their capacity to distinguish the protons in water. As observed from Figure 6(a), the SPEEK with increased degree of sulfonation shows the attribute of higher water uptake value which is due to the addition of hydrophilic nature of -SO3H group. Furthermore, increase in temperature aggravated the membranes water uptake ability and the study was conducted over the temperature of 25 to 100°C. A maximum uptake of 26% was displayed by SPEEK 93 (DS = 68.74%) at 100°C. However, none of these membranes disintegrate in water even at elevated temperatures. The incorporation of MMT at various loading was observed to decrease the water uptake content. As evident from Figure 6(b), the water uptake decreases further at higher loading levels (3 and 5%) due to the accumulation of clay.
Equivalent tendency was observed in the matter of SPEEK/SMMT nanocomposite membranes (Figure 6(c)). This decrease may be due to the accumulation of silicate-surface with increase in SMMT loading, which may result in weakening of H-bonds between the hydrophilic groups (OH and NH2) and water on the SMMT layers. Moreover, the insertion in MMT layers might occlude the mobility of polymer chain which leads to chain packing.34 However, a steady increase was noted with the increase in temperature from 25-100°C without disintegration of nanocomposite membranes and remained stable even at elevated temperature except SPEEK membrane loaded with 5% SMMT.
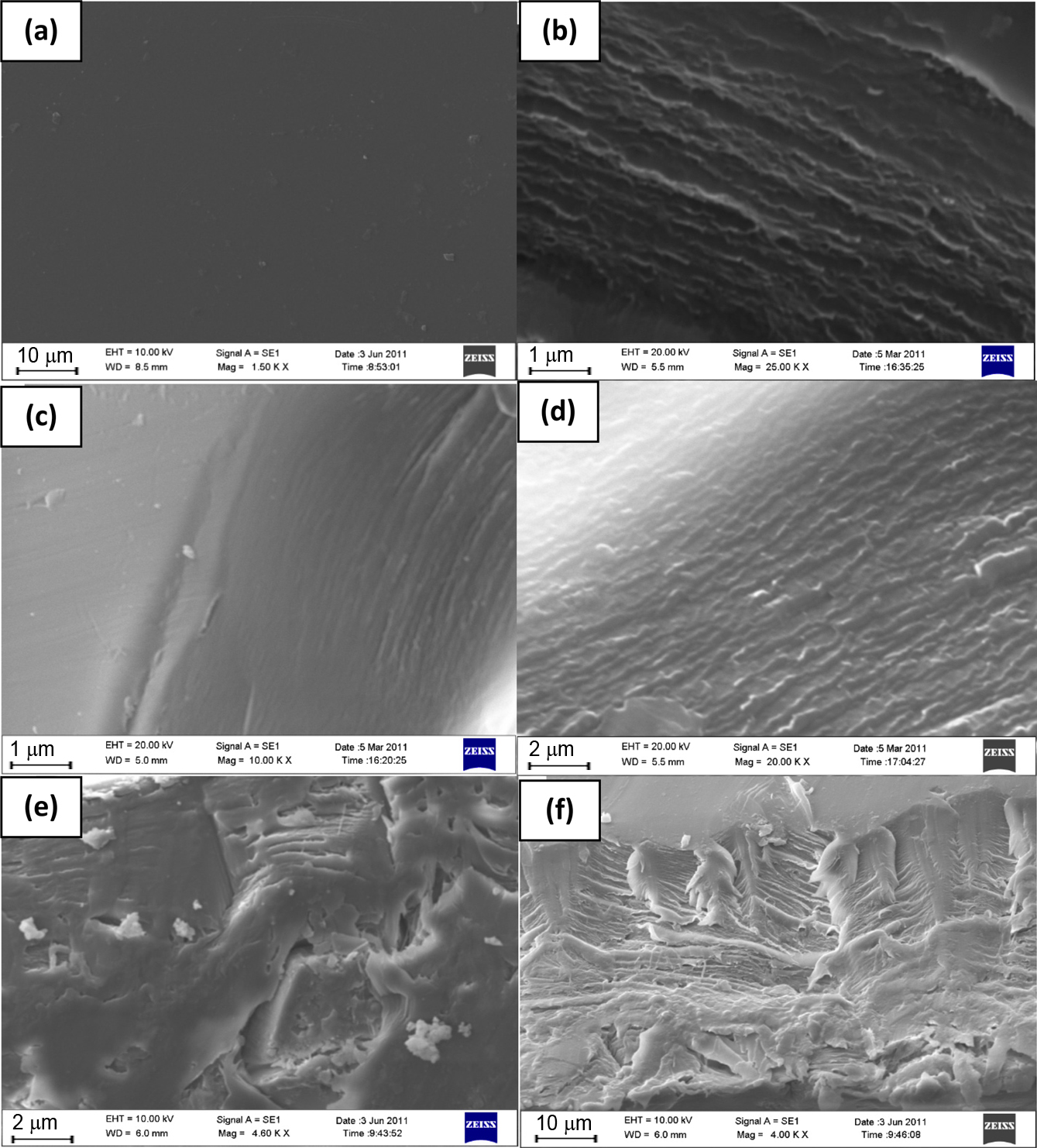
Morphology. The cross-sectional morphology of virgin PEEK, SPEEK and its nanohybrid membranes were analysed by SEM. The micrographs are represented in Figure 7(a-f). The prepared membranes were dense as evident from surface morphology of virgin and sulfonated PEEK (Figure 7(a) and 7(b)).
The smooth morphology of virgin PEEK was replaced by a rough morphology after sulfonation. This is anticipated due to the incorporation of -SO3H groups on the polymer back bone. The variation in degree of sulfonation had negligible effect on the cross-sectional morphology of the sulfonated polymer membranes (Figure 7(c) and 7(d)). Further, the addition of unmodified MMT within SPEEK matrix resulted in formation of clay agglomerates, as displayed in Figure 7(e) and 7(f). The cross-sectional morphology of SPEEK/SMMT nanocomposite membranes revealed uniformly dispersed nanoclays within the SPEEK matrix which could be ascribed to the SO3H – SO3H compromise between SPEEK and SMMT, thus resulted in the proper dispersion and improved compatibility.
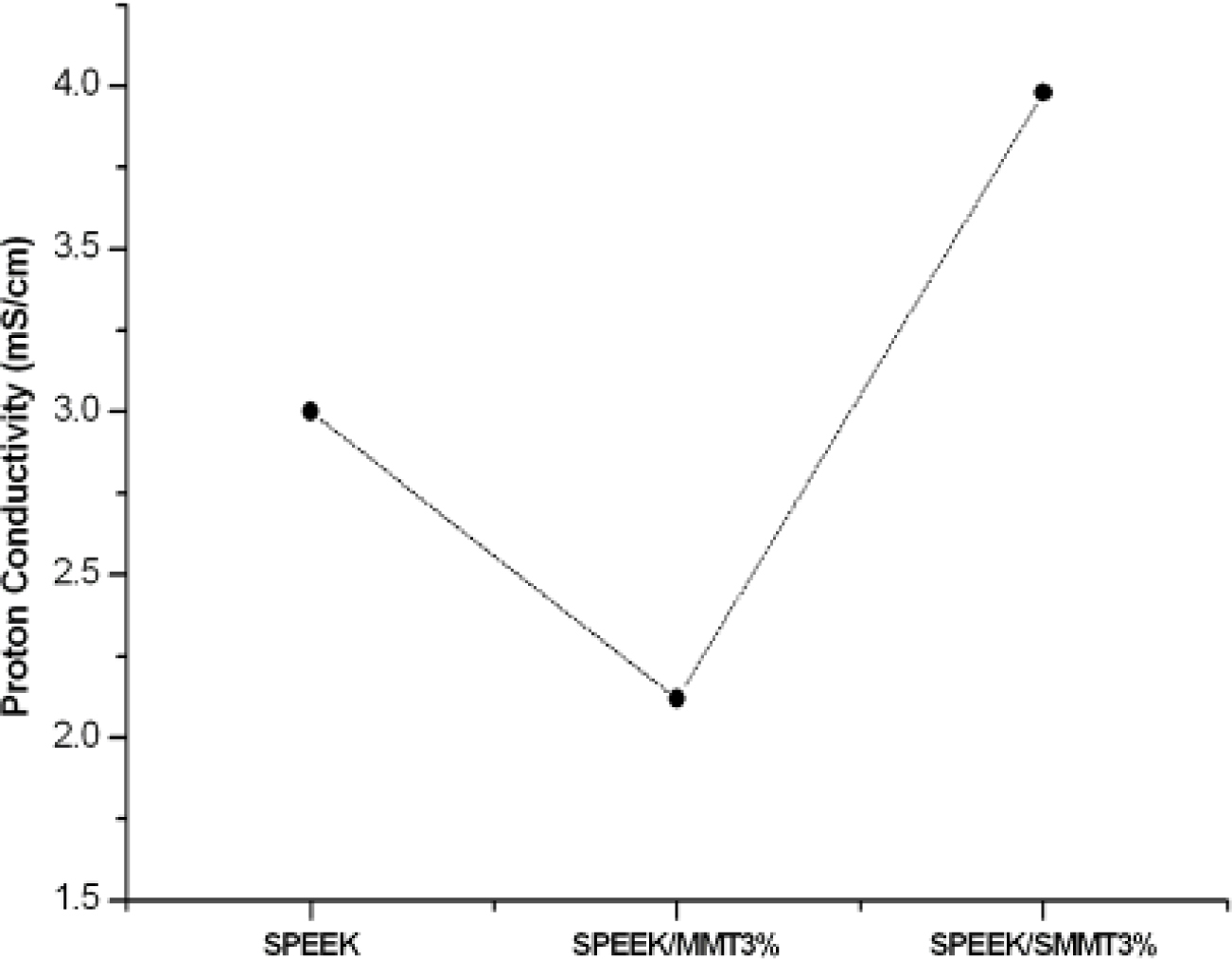
Proton Conductivity Characteristics. Figure 8 displayed the conductivity of protons at ambient temperature for SPEEK and its modified form of nanocomposite membranes. The proton conductivity had very slight variation with phase shift. It was observed that SPEEK membranes showed conductivity within the range of 3 mS/cm. This may be due to the acidic nature and high proton concentration as a result of sulfonic acid group.36,37 The proton conductivity was decreased with the inclusion of unmodified nanoclay. This prominent behaviour draws attention on the outcome of nanoclay such as layered silicates in splitting the molecules of polymer chain from one another. As a consequence, the proton conductivity decreased.34 It may also be explained as a decrease in SO3- groups in polymer chain molecules per unit volume. However, the incorporation of sulfonated MMT further increased the conductivity characteristics of SPEEK to 4mS/cm under room temperature and 100% hydration level. This increase in proton conductivity in SPEEK/SMMT nanocomposites might be due to the preferable potential to preserve water molecules (proton transporter) as a result of H-bond with the hydrophilic groups (OH, NH2, SO3H) of the nanohybrid membranes.
Nevertheless, the conductivity values of SPEEK based membranes with two probe method, which are in the range of 0.06×10-6 S/cm, are quite lower than that of commercially available membrane (Nafion 115). Such activity may be due to the higher membrane thickness which would have increased the route for proton transfer thus decreasing the conductivity of proton.
The conductivity performance was determined for 1% loading of unmodified and modified nanoclays. At higher loading, as evident from SEM, the silicate layers tend to form aggregates which might hinder proton transfer in the membrane.38,39
PEM Single Cell Performance Test. MEA was fabricated and utilized to assess the single cell performance of SPEEK and its nanohybrid membranes as follows, the gas diffusion layers (uncatalyzed carbon papers) and catalyst layers were directly applied on the membranes. 50% Ru black/50% Pt was used as anode catalyst and 40wt% Pt/C as cathode catalyst, respectively. Nafion solution was used to bind the catalyst and also to serve as an electron transporter in MEA geometry. In addition, the presence of Nafion bridges the active catalyst sites in the middle of the interfaces catalysts and membranes. The amount of catalyst deposited were 4mg/cm2 for anode as well as cathode. The single layer MEA was acquired by hot pressing gas diffusion surfaces on each side of the membrane at 100°C.
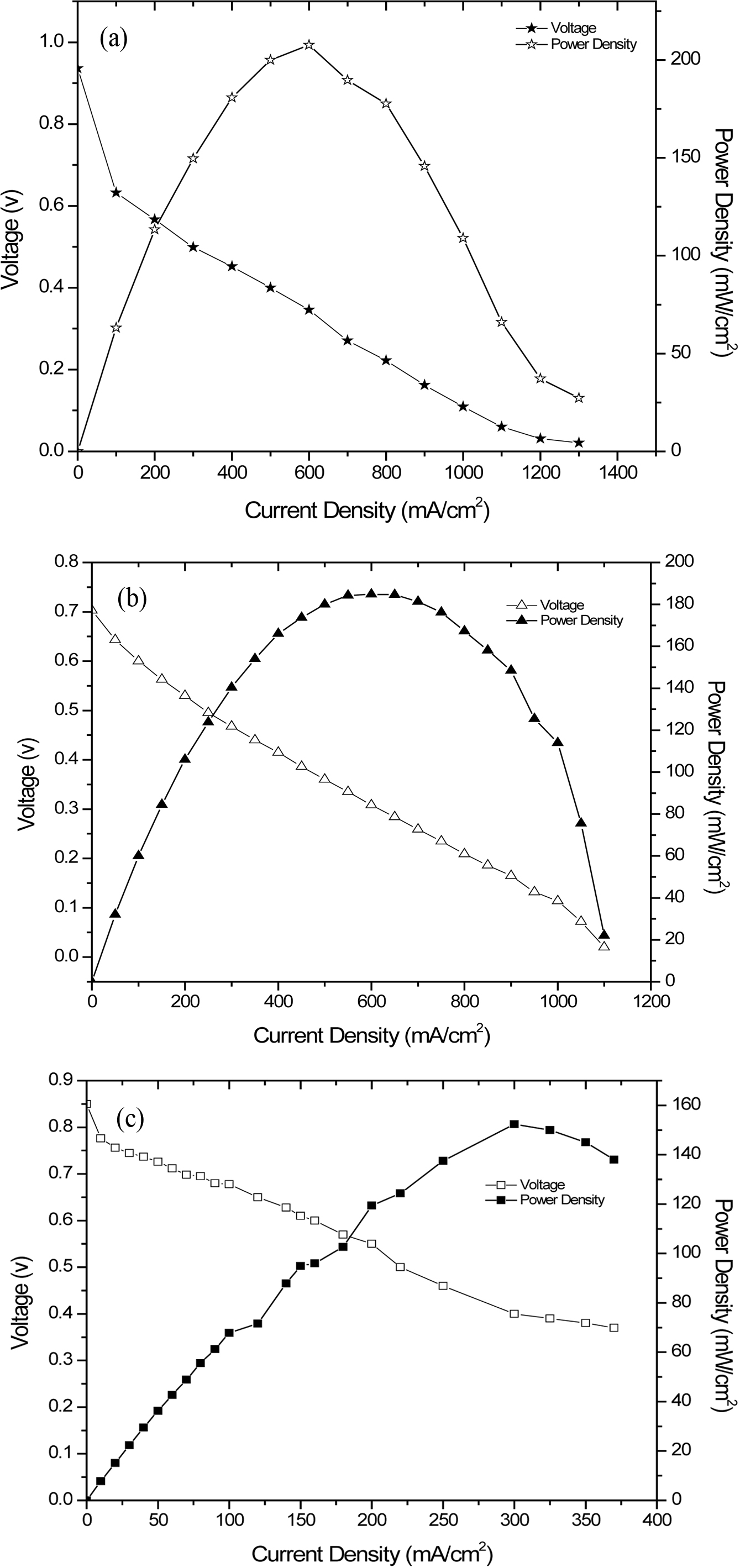
Single cell performance test was conducted for SPEEK and its nanohybrid membranes of ~500µm thickness at room temperature for 24h. The open circuit potential (OCP) obtained for SPEEK membrane is 0.703V. At 600mV, a maximum power density of 185mW/cm2 with a current density of 0.308mA/cm2 similar to Nafion 117 was achieved. Provided SPEEK/SMMT nanohybrid membrane, the achieved OCP was 0.850V with a power density of 153mW/cm2 obtained at 400mV with a current density of 300mA/cm2. Thus, the polarization curves showed in Figure 9 clearly indicate that the incorporation of SMMT inhibited the possibility of proton conductivity thereby resulting in decreased power density as compared to that of SPEEK. The current density at the anode depends on the concentration of fuel (H2). Therefore it can be inferred that the SPEEK membrane at room temperature experiences an average power density of 185mW/cm2 and further increment of power density can be achieved at elevated temperatures.
Validation of Single Cell Performance Test Using MATLAB. MATLAB software was employed to validate the fuel cell performance test and to acquire the simple polynomial equation from the OCP curves for all the 3 membranes which shows the R-square and adjusted R-square values nearly equal to 1 which confirms the suitable OCP curve fitting in the single cell performance test. The root mean squared error (RMSE) and sum of squares due to error (SSE) value for current density vs power density seems to be high because of non-linear curve and the RMSE and SSE value for current density vs voltage exhibit the value nearer to zero which reveals that the test has a negligible error for the linear curve. Where x and f(x) represents the X-Axis and Y-Axis, respectively (Table S1-3).
|
Figure 2 Consequence of reaction time on DS and IEC. |
|
Figure 3 (a) Nomenclature of the aromatic protons for SPEEK; (b) 1 H NMR spectra of SPEEK; (c) comparative 1 H NMR spectra of SPEEK samples. |
|
Figure 4 (a) DSC curves of virgin and sulfonated PEEK membranes; (b) DSC curves of SPEEK and SPEEK/MMT nanocomposite membranes; (c) DSC curves of SPEEK and SPEEK/SMMT nanocomposite membranes. |
|
Figure 5 (a) TGA curves of virgin and sulfonated PEEK membranes; (b) TGA curves of SPEEK and SPEEK/MMT nanocomposite membranes; (c) TGA curves of SPEEK and SPEEK/SMMT nanocomposite membranes. |
|
Figure 6 (a) Plot showing temperature vs water uptake for SPEEK membranes; (b) plot showing temperature vs water uptake for SPEEK and SPEEK/MMT nanocomposite membranes; (c) plot showing temperature vs water uptake for SPEEK and SPEEK/ SMMT nanocomposite membranes. |
|
Figure 7 SEM micrographs: (a) virgin PEEK; (b) SPEEK50; (c) SPEEK70; (d) SPEEK93; (e) SPEEK/MMT; (f) SPEEK/SMMT. |
|
Figure 8 Comparison of proton conductivities of SPEEK and its nanocomposite membranes. |
|
Figure 9 Current density curve (blank symbol) and power density curve (filled symbol) at room temperature of Nafion (★), SPEEK (▲), SPEEK/SMMT (■). |
SO3H functionalized PEEK/sulfonated MMT nanocomposites have been validated as potential alternate to the expensive fluoropolymer, Nafion, based polymer electrolyte for use in PEMFC. The water uptake, insolubility as well as membranes sustainability in water at higher temperatures have resulted in optimized for 93 h as optimum reaction time at room temperature. SPEEK/SMMT nanocomposites showed reliable performance in the connection with thermal reliability and proton conductivity. To a great extent, the in-service performance of SPEEK and SPEEK/SMMT nanocomposite are very much comparable with that of Nafion, tested under hydrated conditions. The results validated through MATLAB software reveal that hydrocarbon based polymer electrolyte membranes can easily replace the fluoropolymer electrolytes thereby opening up the avenue for affordable fuel cells.
- 1. Dhar, H. P. On Solid Polymer Fuel Cells. J. Electroanal. Chem. 1993, 357, 237-250.
-

- 2. Appleby, A. J. Recent Developments and Applications of the Polymer Fuel Cell. Phil. Trans. R. Soc. Lond. A 1996, 354, 1681-1693.
-

- 3. Shoesmith, J. P.; Collins, R. D.; Oakley, M. J.; Stevenson, D. K. J. Status of Solid Polymer Fuel Cell System Development. Power Sources 1994, 49, 129-142.
-

- 4. Gao, Y.; Robertson, G. P.; Guiver, M. D.; Mikhailenko, S. D.; Li, X.; Kaliaguine, S. Synthesis of Poly(arylene ether ether ketone ketone) Copolymers Containing Pendant Sulfonic Acid Groups Bonded to Naphthalene as Proton Exchange Membrane Materials. Macromolecules 2004, 37, 6748-6754.
-

- 5. Savadogo, O. Emerging Membranes for Electrochemical Systems: (I) Solid Polymer Electrolyte Membranes for Fuel Cell Systems. J. New. Mater. Electrochem. Syst. 1998, 1, 47-66.
-

- 6. Inzelt, G.; Pineri, M.; Schultze, J.; Vorotyntsev, M. A. Electron and Proton Conducting Polymers: Recent Developments and Prospects. Electrochim. Acta 2000, 45, 2403-2421.
-

- 7. Kerres, J. A. Development of Ionomer Membranes for Fuel Cells. J. Membr. Sci. 2001, 185, 3-27.
-

- 8. Kreuer, K. D. On the Development of Proton Conducting Polymer Membranes for Hydrogen and Methanol Fuel Cells. J. Membr. Sci. 2001, 185, 29-39.
-

- 9. Rikukawa, M.; Sanui, K. Proton-conducting Polymer Electrolyte Membranes Based on Hydrocarbon Polymers. Prog. Polym. Sci. 2000, 25, 1463-1502.
-

- 10. Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric Proton Conducting Membranes for Medium Temperature Fuel Cells (110–160°C). J. Membr. Sci. 2001, 185, 73-81.
-

- 11. Ulrich, H. H.; Rafler, G. Sulfonated Poly(aryl ether ketone)s. Macromol. Mater. Eng. 1998, 263, 71-78.
-

- 12. Zaidi, S. M.; Mikhailenko, S.; Robertson, G.; Guiver, M.; Kaliaguine, S. Proton Conducting Composite Membranes from Polyether Ether Ketone and Heteropolyacids for Fuel Cell Applications. J. Membr. Sci. 2000, 173, 7-34.
-

- 13. Wang, F.; Hickner, M.; Kim, Y. S.; Zawodzinski, T. A.; McGrath, J. E. Direct Polymerization of Sulfonated Poly(arylene ether sulfone) Random (Statistical) Copolymers: Candidates for New Proton Exchange Membranes. J. Membr. Sci. 2002, 197, 231-242.
-

- 14. Kerres, J.; Ullrich, A.; Meier, F.; Häring, T. Synthesis and Characterization of Novel Acid–base Polymer Blends for Application in Membrane Fuel Cells. Solid State Ion. 1999, 125, 243-249.
-

- 15. Unnikrishnan, L.; Nayak, S. K.; Mohanty, S.; Sarkhel, G. Polyethersulfone Membranes: The Effect of Sulfonation on the Properties. Polym-Plast Technol. Eing. 2010, 49, 1419-1427.
-

- 16. Genies, C.; Mercier, R.; Sillion, B.; Cornet, N.; Gebel, G.; Pineri, M. Soluble Sulfonated Naphthalenic Polyimides as Materials for Proton Exchange Membranes. Polymer 2001, 42, 359-373.
-

- 17. Guo, X.; Fang, J.; Watari, T.; Tanaka, K.; Kita, H.; Okamoto, K. Novel Sulfonated Polyimides as Polyelectrolytes for Fuel Cell Application. 2. Synthesis and Proton Conductivity of Polyimides from 9,9-Bis(4-aminophenyl)fluorene-2,7-disulfonic Acid. Macromolecules 2002, 35, 6707-6713.
-

- 18. Jones, D. J.; Rozière, J. Recent Advances in the Functionalisation of Polybenzimidazole and Polyetherketone for Fuel Cell Applications. J. Membr. Sci. 2001, 185, 41-58.
-

- 19. Asensio, J. A.; Borrós, S.; Gómez-Romero, P. Proton-conducting Polymers Based on Benzimidazoles and Sulfonated Benzimidazoles. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 3703-3710.
-

- 20. Bintou, O.; Savadogo, O. Electrophoretic Deposition and Physicochemical Characterization of Al2O3-NiO Composite Materials. J. New Mater. Electrochem. Syst. 2015, 18, 49-62.
-

- 21. Pourcelly, G.; Gavach, C. Perfluorinated Membranes. Proton Conductors. U.S. Patent 294, 1992.
-

- 22. Kobayashi, T.; Rikukawa, M.; Sanui, K.; Ogata, N. Proton-Conducting Polymers Derived from Poly(ether-etherketone) and Poly(4-phenoxybenzoyl-1,4-phenylene). Solid State Ion. 1998, 106, 219-225.
- 23. Kerres, J.; Ullrich, A.; Haring, Th. New Ionomer Membranes and Their Fuel Cell Application 1. Preparation and Characterization, Proc. of 3rd Int. Symp. on New Materials for Electrochemical Systems, Montreal: Canada, 1999; p 230.
- 24. Poppe, D.; Frey, H.; Kreuer, K. D.; Heinzel, A.; Mülhaupt, R. Carboxylated and Sulfonated Poly(arylene-co-arylene sulfone)s: Thermostable Polyelectrolytes for Fuel Cell Applications. Macromolecules 2002, 35, 7936-7941.
-

- 25. Jones, D. J.; Rozi`ere, J. Recent Advances in the Functionalisation of Polybenzimidazole and Polyetherketone for Fuel Cell Applications. J. Membr. Sci. 2001, 185, 41-58.
- 26. Soczka-Guth, T.; Baurmeister, J.; Frank, G.; Knauf, R. International Patent WO1999029763A1, 1999.
- 27. Reichert, P.; Kressler, J.; Thomann, R.; Mulhaupt, R.; Stoppelmann, G. Nanocomposites Based on a Synthetic Layer Silicate and Polyamide-12. Acta Polym. 1998, 49, 116-123.
-

- 28. Giannellis, E. P. Polymer-layered Silicate Nanocomposites: Synthesis, Properties and Applications. Appl. Organomet. Chem. 1998, 12, 675-680.
-

- 29. Lin, Y.-F.; Yen, C.-Y.; Ma, C.-C. M.; Liao, S.-H.; Hung, C.-H.; Hsiao, Y.-H. Preparation and Properties of High Performance Nanocomposite Proton Exchange Membrane for Fuel Cell. J. Power Sources 2007, 165, 692-700.
-

- 30. Lavrenčič Štangar, U.; Grošelj, N.; Orel, B.; Schmitz, A.; Colomban, P. Proton-conducting Sol–gel Hybrids Containing Heteropoly Acids. Solid State Ion. 2001, 145, 109-118.
-

- 31. Chang, J.-H.; Park, J. H.; Park, G.-G.; Kim, C.-S.; Park, O. O. Proton-conducting Composite Membranes Derived from Sulfonated Hydrocarbon and Inorganic Materials. J. Power Sources 2003, 124, 18-25.
-

- 32. Hasani-Sadrabadi, Mohammad M.; Emami, Shahriar H.; Ghaffarian, R.; Moaddel, H. Nanocomposite Membranes Made from Sulfonated Poly(ether ether ketone) and Montmorillonite Clay for Fuel Cell Applications. Energy Fuels 2008, 22, 2539-2542.
- 33. Robertson, G. P.; Mikhailenko, S. D.; Wang, K.; Xing, P.; Guiver, M. D.; Kaliaguine, S. Casting Solvent Interactions with Sulfonated Poly(ether ether ketone) During Proton Exchange Membrane Fabrication. J. Membr. Sci. 2003, 219, 113-121.
-

- 34. Gosalawit, R.; Chirachanchai, S.; Shishatskiy, S.; Nunes, S. P. Sulfonated Montmorillonite/sulfonated Poly(ether ether ketone) (SMMT/SPEEK) Nanocomposite Membrane for Direct Methanol Fuel Cells (DMFCs). J. Membr. Sci. 2008, 323, 337-346.
-

- 35. Jung, D. H.; Cho, S. Y.; Peck, D. H.; Shin, D. R.; Kim, J. S. Preparation and Performance of a Nafion®/Montmorillonite Nanocomposite Membrane For Direct Methanol Fuel Cell. J. Power Sources 2003, 118, 205-211.
-

- 36. Xing, P.; Robertson, G. P.; Guiver, M. D.; Mikhailenko, S. D.; Wang, K.; Kaliaguine, S. Synthesis and Characterization of Sulfonated Poly(ether ether ketone) for Proton Exchange Membranes. J. Membr. Sci. 2004, 229, 95-106.
-

- 37. Jin, X.; Bishop, M. T.; Ellis, T. S.; Karasz, F. E. A Sulphonated Poly(aryl ether ketone). Br. Polym. J. 1985, 17, 4-10.
-

- 38. Gaowen, Z.; Zhentao, Z. Organic/Inorganic Composite Membranes for Application in DMFC. J. Membr. Sci. 2005, 261, 107-113.
-

- 39. Kim, T. K.; Kang, M.; Choi, Y. S.; Kim, H. K.; Lee, W.; Chang, H.; Seung, D. Corrigendum To “Preparation of Nafion-sulfonated Clay Nanocomposite Membrane for Direct Menthol Fuel Cells Via a Film Coating Process”. J. Power Sources 2007, 165, 1-2.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 101-112
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.101
- Received on Aug 9, 2020
- Revised on Sep 19, 2020
- Accepted on Sep 20, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Lakshmi Unnikrishnan
-
CIPET, School for Advanced Research in Polymers (SARP)-APDDRL, Hi Tech Defence and Aerospace Park, Bengaluru 562149, India
- E-mail: lakshmipvallath@gmail.com
- ORCID:
0000-0002-6590-6052









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.