- Synthesis of Biomass-based Reactive Plasticizer and Its Effects on the Processing Properties of Poly(vinyl chloride)
Department of Polymer Science and Engineering, Chungnam National University, 99, Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
- 바이오매스 기반의 반응형 가소제 합성 및 Poly(vinyl chloride)의 가공 특성에 대한 영향
충남대학교 고분자공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Waste vegetable cooking oil (WVCO), a type of biomass, is primarily produced in large quantities within the residential and commercial sectors and is a resource with low recycling rate. WVCO has been receiving much attention as a renewable resource due to rising environmental concerns and the depletion of petrochemical resources. In this study, reactive plasticizer (BmBP) recycled with biomass-based WVCO was synthesized via a three-step process that was innovatively simplified from the conventional seven-step epoxidation process, which has high heat resistance and high strength. The synthesized BmBP was compared with corresponding specialty plasticizers, such as TINTM and DIDP, in terms of processability.
바이오매스의 일종인 폐식물유는 대부분 주거 및 상업 부문에서 대량으로 생성되며, 재활용률이 낮은 자원이다. 폐식물유는 환경에 대한 관심이 고조되고 석유화학 자원의 고갈에 따라 재생가능한 자원으로서 지대한 관심을 받아오고 있다. 본 연구에서는 바이오매스 기반의폐식물유를 재활용하여 고내열성 및 고강도 특성이 우수한 반응형 가소제(BmBP)를 기존의 7단계 에폭시화 반응 공정을 혁신적으로 단순화한 3단계 공정을 통해 합성하였다. 합성된 가소제는 대응하는 특수 가소제인 TINTM 및 DIDP와 가공성 측면에서 상대 비교를 하였다.
Reactive plasticizer recycled with waste vegetable cooking oil was synthesized via a three-step process that was innovatively simplified from the conventional seven-step epoxidation process. The synthesized BmBP was compared with corresponding specialty plasticizers, such as TINTM and DIDP, in terms of processability.
Keywords: biomass, reactive plasticizer, poly(vinyl chloride), thermal property, mechanical properties
This work was supported by research fund of Chungnam National University.
Billions of tons of food and oil are wasted globally every year. Waste or used vegetable cooking oils (WVCOs) are biobased oils that have been used for cooking, frying, and other processing types in households, restaurants, fast foods, and the food manufacturing industry. Most industrial, commercial, and domestic WVCO is disposed of landfill sites or the ocean without the particular utilization. In fact, it is a renewable resource and can be reused to produce fuel oils and biobased products such as lubricants in the replacement of petroleum-based mineral oils. However, the major obstacle in reusing it as energy and chemical sources is its high cost of collection, transportation, pretreatment (e.g., purification), and commercialization.1-4
WVCO is primarily composed of triglycerides, monoglycerides, diglycerides, and variable quantities of free fatty acids (5~20% w/w) generated during the frying process. This process causes physical and chemical changes in cooking oil due to chemical reactions, such as hydrolysis, thermal degradation, oxidation, and polymerization. The main components of triglycerides are saturated and unsaturated fatty acids, which can be used as platform chemicals in the manufacturing of added-value chemicals and polymer materials.5-8
The presence of unsaturated fatty acids in oil waste allows for the formation of epoxy groups through the epoxidation of double bonds. Epoxidized fatty acids are used in plasticizers and lubricants to improve the flexibility and workability of polyvinyl chloride (PVC), polyolefins, epoxy resins, and biodegradable polymers, such as poly(lactic acid) (PLA), poly(butylene succinate) (PBS), and so on. Furthermore, not only epoxidized fatty acids but also saturated fatty acids can undergo esterification with polyglycerol for the formation of fatty acid polyglycerol esters used as surfactants in various types of food and technical processes. The presence of epoxy groups allows for further chemical modification via ring-opening reactions to increase the performance of synthesized surfactants and create polyurethane and other polymers.9-15
According to the reported literature, edible oils were used for epoxide synthesis, as plasticizer application and the epoxidation of vegetable oils focused primarily on soybean,16 palm,17 castor,18 tung,19 sunflower,20 rice bran,21 palm kernel,22 linseed,23 olive,24 corn,24 safflower,25 melon seed26 (citrullus lanatus seed), cotton seed,27 and rubber seed oil.28 Currently, epoxidized soybean oil (ESO) has been used on the market widely only as secondary heat stabilizer rather than a plasticizer due to its poor compatibility with base polymers, such as PVC resin in particular. However, no information is available in the literature regarding plasticizer synthesis via epoxidation reaction using purely WVCO. In most cases, the epoxidation synthetic route using auxiliary materials, such as citric acid, terephthalic acid, and benzoic acid, is predominant.29-32
With the recent strict regulation of the European Union and US on six phthalates, such as DINP, DEHP, DBP, BBP, DIDP, and DnOP, there is a strong demand for the development of alternatives to commodity, including specialty plasticizers. Currently, phthalate-based commodity plasticizers have been replaced considerably with terephthalic-based plasticizers such as DOTP, but there are no proactive actions to replace high-functional plasticizers. In the ECHA (European Chemical Agency), many types of wire and cable-range plasticizers are currently being evaluated for possible controls, such as restriction or designation, as an SVHC, which is identified in the “Candidate List of substances of very high concern for Authorisation.” Typically, the plasticizer content in thermoplastic PVC insulation and jacket compounds is approximately 30%, so unlike most other organic additives in many polymer systems, plasticizers represent a significant proportion of the total plastic. Some of the main plasticizers under consideration in wire and cable PVC insulation and jacketing applications include C8-11 phthalates, linear acid diesters, and especially trimellitates.33 In addition, securing the technology to lower the hardness while maintaining high strength and heat resistance, which are the unique characteristics of specialty plasticizers, is becoming a key issue.
This study aims to develop a biomass-based reactive plasticizer (BmBP) with high functionality using WVCO. The reactive BmBP was synthesized through a process that simplified the current complex seven epoxidation steps into only three steps, leading to a mechanically strong and thermally stable macromolecular network via stabilization and partial crosslinking reaction with structurally soft and long hydrocarbon chains. Then, this study makes a relative comparison in terms of processability, such as strength, heat resistance, and hardness, with specialty plasticizers such asDIDP and TINTM, which are used in wire, cable, and/or electrical insulation applications.
Materials. For the synthesis of BmBP, biomass (WVCO) was purchased from Daejimulsan Co., Ltd. (Korea), and decolorization and deodorization were performed using a distillation apparatus. Formic acid and hydrogen peroxide (35% w/v) were purchased from Daejung Chemical & Metals Co., Ltd. (Korea), while the PVC resin with a K value of 1000 was obtained from Hanwha Chemical Co., Ltd. (Korea). The commercial plasticizers, diisodecyl phthalate (DIDP) and triisononyl trimellitate (TINTM) were supplied by Aekyung Petrochemical Co., Ltd. (Korea) and Hanwha Chemical Co., Ltd. (Korea). Finally, the heat stabilizer, LOX-7200, was obtained from KD Chemical Co., Ltd. (Korea).
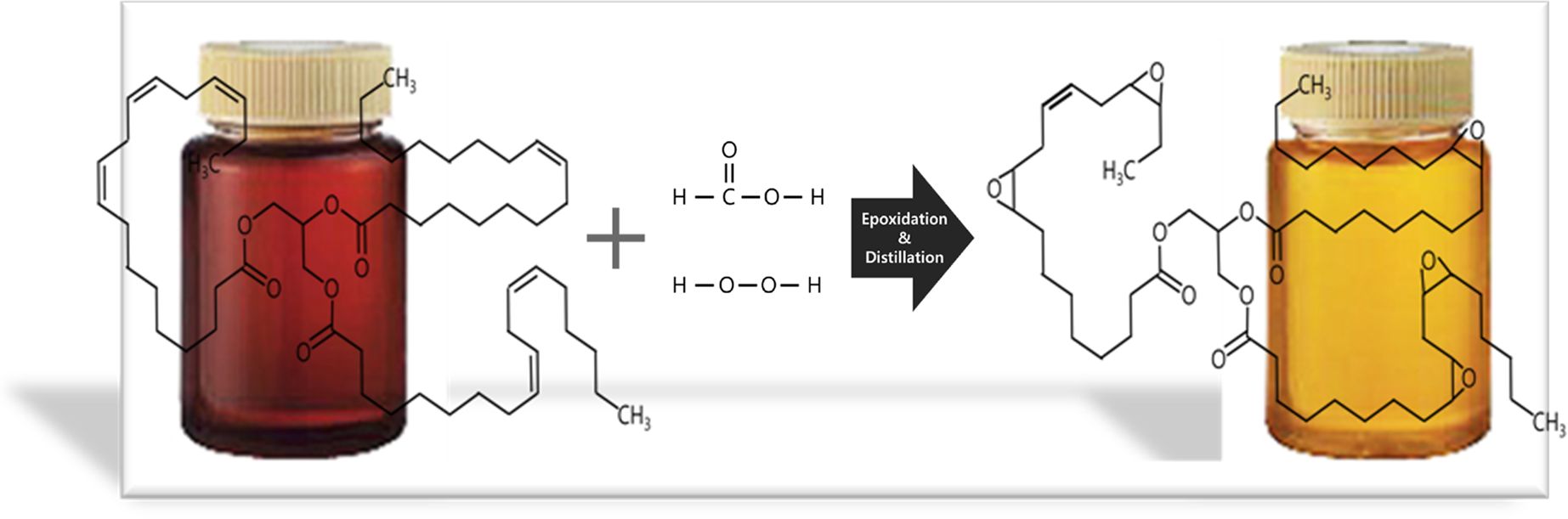
Synthesis of the BmBP. The BmBP was synthesized via a three-step process that innovatively simplified the conventional seven-step epoxidation process, which consists of pretreatment, reaction, separation, neutralization, bleaching, filtration, and distillation steps.34
In the first pretreatment step, biomass was decolorized and deodorized using distillation apparatus at 60 oC under vacuum. Following the second reaction step, biomass (220 g) was first mixed with formic acid (26 g) in a 500 mL four-neck flask equipped with a mechanical stirrer, a thermostat, a reflux condenser, and a hydrogen peroxide inlet. The mixture was slowly heated to 50 oC in a nitrogen atmosphere and was then kept constant for 30 min. Hydrogen peroxide (136 g) was then added in drops by a funnel while maintaining low speed. At this time, the temperature of the mixture was maintained below 50 oC to avoid the formation of byproducts due to the exothermic reaction between peroxy-formic acid and unsaturated sites of biomass. In addition, the epoxidation at temperature higher than 50 oC increases the possibility of ring-opening of oxirane groups. The mixture was finally maintained at 50 oC for 5 h after the injection of the formic acid was completed. After the reaction, the reaction product was transferred to a distillation apparatus for the third step. BmBP was separated using a distillation process at 50 oC for 8 h under vacuum. A conversion rate of 90% can be obtained via a simplified three-step epoxidation process and precise temperature control. Overall, the scheme of a simplified epoxidation process for the synthesis of BmBP is indicated in Figure 1.
Preparation of the PVC Sheets. Three types of flexible PVC dry blends were prepared by mixing the PVC powder with three different plasticizers-BmBP, DIDP, and TINTM-in the standard proportion of 36 parts plasticizer and 2 parts heat stabilizer to 100 parts resin by weight; however, PVC dry blend with BmBP used a stabilizer of 0.5 parts. These mixtures were then thoroughly blended using a laboratory-type super mixer. PVC powder was first placed into the super mixer, after which the temperature was raised to approximately 60 oC. Afterward, the mixture of plasticizer and heat stabilizer were added at moderate speed. The compound was finally obtained after the volume of the mixture increased and then decreased. The PVC sheets were obtained from the melts, which were fused at 180 oC for 7 min using HAAKE internal mixer, using a press apparatus.
Characterization. The biomass (WVCO) and BmBP were identified using gas chromatography (GC) with a GC–FID HP7890A apparatus (Agilent Technologies, USA), Fourier transform infrared spectroscopy (FTIR) with a VERTEX 80v FTIR spectrometer (Bruker, Germany), and 1H NMR spectroscopy with an AVANCE III 600 spectrometer (Bruker BioSpin, Germany) at 600 MHz.
The fusion properties of PVC dry blends were examined using a HAAKE MARS-I Rheometer (Thermo Electron GmbH, Germany) with an internal mixer, and the evaluation of the fusion behavior was performed at 170 oC for 7 min. The effects on the processability were predicted based on the fusion behavior analysis. The analysis of PVC sheets to correlate the processability with the mechanical and thermal properties was then performed. The Young’s modulus, tensile strength, and elongation at break were determined at room temperature using a universal testing machine LS 1 (LLOYD, UK) at a constant rate of 500 mm/min by applying a 20 N load cell according to ASTM D-638. The analysis of the thermal stability was performed using a thermogravimetric analyzer (TGA) (Thermo plus EVO II, Rigaku Co., Japan) at a 10 oC/min heating rate from room temperature to 600 oC under nitrogen flow, while the Fourier transform infrared spectroscopy (FTIR) used a VERTEX 80v FTIR spectrometer (Bruker, Germany).
|
Figure 1 Simplified epoxidation process for the synthesis of BmBP. |
Synthesis Characterization of the BmBP. Gas Chro-matography (GC-FID):
The biomass used in this study was refined waste vegetable cooking oil (WVCO) that was discarded after being used in fried foods in Korea. The primary source of domestic WVCO are establishments that use cooking oil in large-scale restaurants and fast foods. WVCO is of no value to food, but can be recycled as soap or biodiesel.
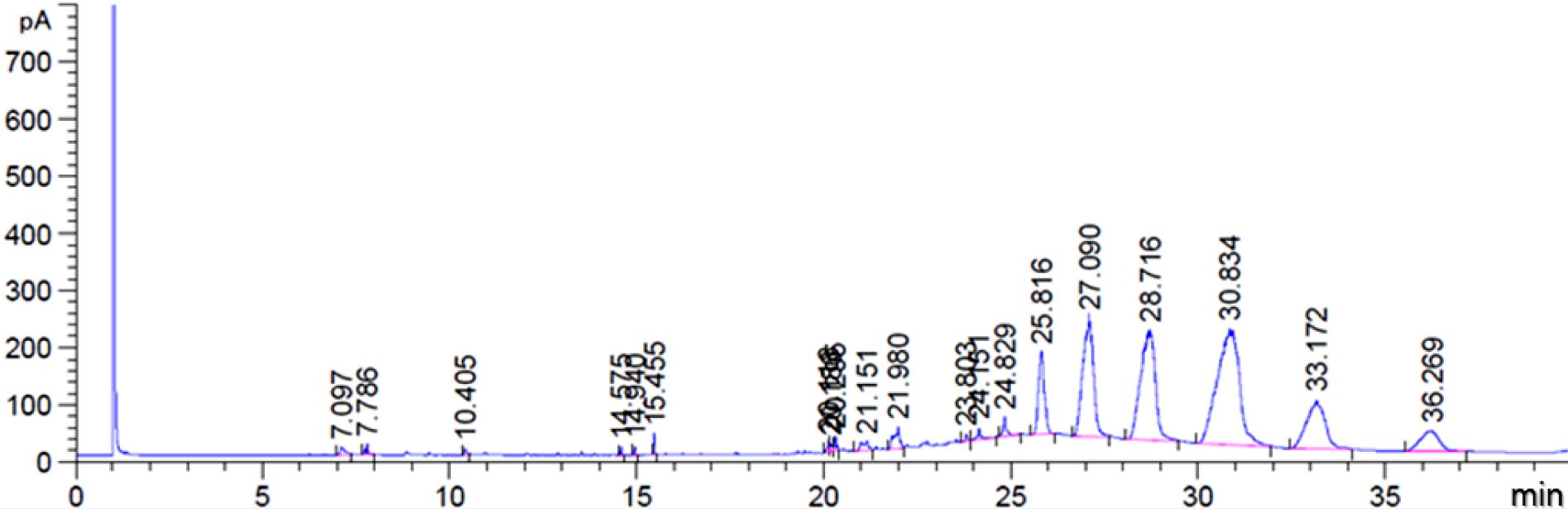
The accurate characterization of such biomass was performed through classical gas chromatography (GC).35,36 Figure 2 illustrates the GC-FID chromatogram of biomass after decolorization and deodorization in addition to the approximate concentrations of the fatty acids in biomass calculated by each area ratio as follows: 2.98% palmitic saturated C16, 1.56% stearic saturated C18, 22.2% oleic mono-unsaturated C18:1, 22.4% linoleic di-unsaturated C18:2, 26.1% linolenic tri-unsaturated C18:3, and 24.7% eicosapentaenoic penta-unsaturated C20:5 fatty acids, and 0.06% the other fatty acids.
According to the GC analysis, it was found that approximately 95.5% of the biomass was composed of vegetable oils with unsaturated groups. Additionally, it can be seen that the biomass has the structure of given triglycerides that are detected in 25 min or more. Since its molecular structures are bulky and incompatible with PVC resin, their use as plasticizers may be generally limited due to migration issue and poor compatibility with PVC resin. However, these problems can be prevented by modifying the structure of the unsaturated site through an epoxidation reaction.
In this study, some of the unsaturated groups in the biomass structure were left intact to introduce crosslinked structures that are strong macromolecular networks both mechanically and thermally act as a reactive plasticizer. Meanwhile, other epoxy groups were introduced for use as a primary plasticizer and a thermal stabilizer; in other words, an epoxy group was introduced to improve the thermal stability by increasing the compatibility with PVC resin and the unsaturated group caused crosslinking with PVC resin to improve mechanical properties.
In addition, due to the characteristics of the GC equipment used in this experiment, there was a limitation in the analysis of the polar epoxidized material, and FTIR and NMR analyses were performed to characterize BmBP.
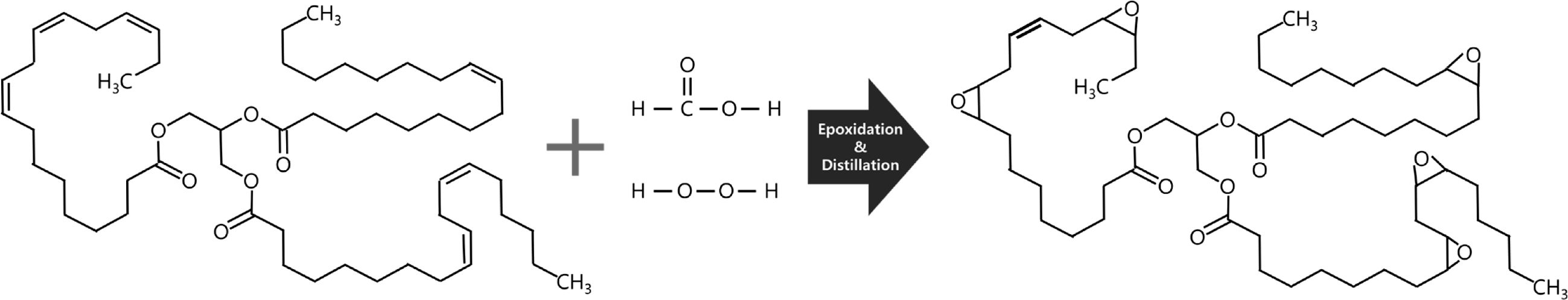
Fourier Transform Infrared (FTIR): The conversion of the unsaturated groups of WVCO structure to the epoxy group was analyzed through ATR-FTIR spectroscopy. The frequencies and intensities confirm the presence of the epoxy groups in WVCO according to the disappearance of or noticeable decrease in the double bonds in the BmBP. The various absorption bands of the spectra were assigned based on data provided in the literatures.37-39
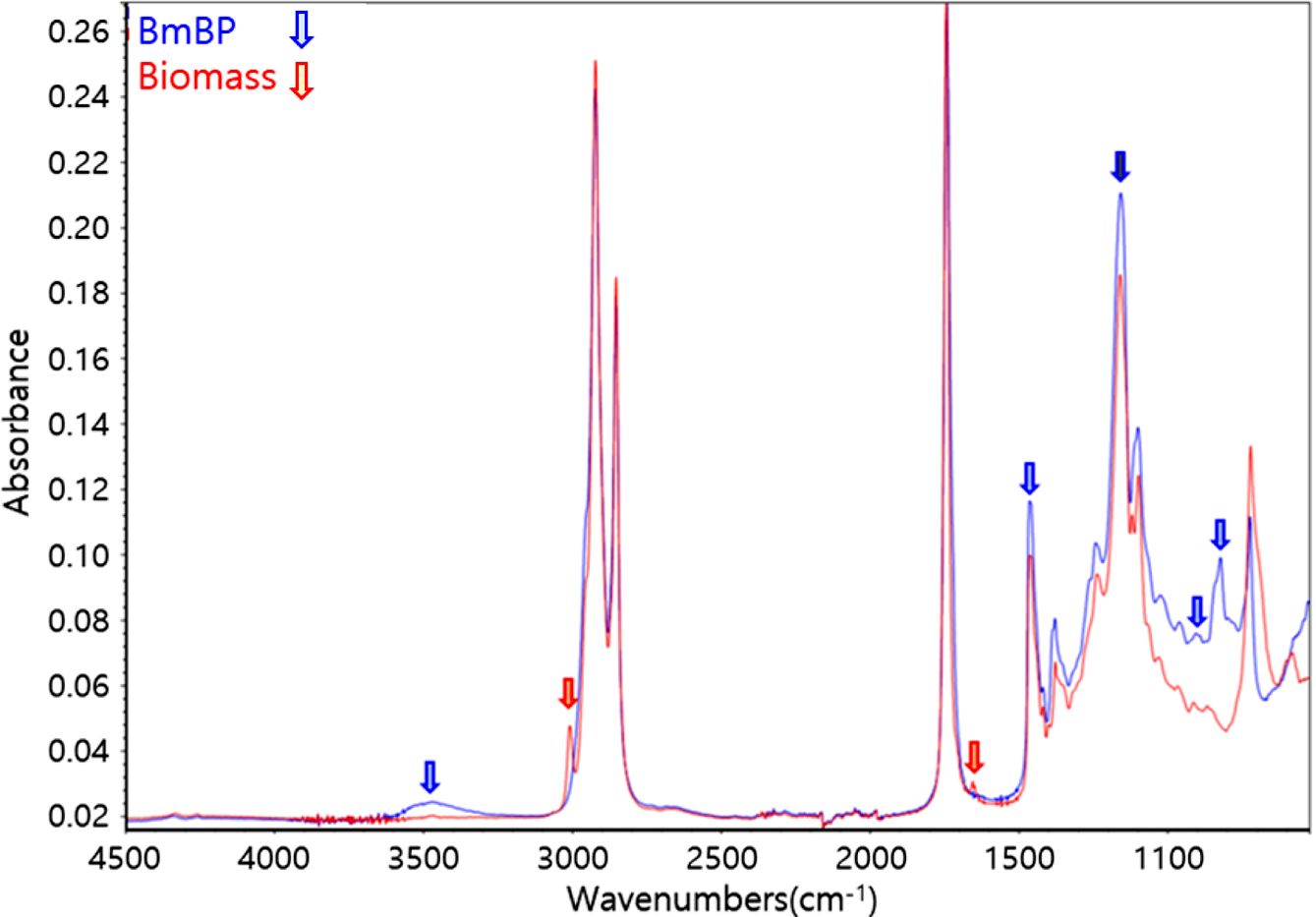
Figure 3 displays the ATR-FTIR spectra of biomass and BmBP. The presence of double bonds in the biomass is visible as the absorption peaks of above 3000 cm-1, which is frequently diagnostic of unsaturation and 1680~1620 cm-1 of alkenyl C=C stretching vibration. Several indicators reveal that the double bonds in the biomass were converted into epoxy and hydroxy groups. Namely, the formation of epoxy groups at 830 cm-1 (stretching C-O-C of oxirane group), 910 cm-1 (stretching C-O of oxirane group), and 1400~1500 cm-1 (stretching C-H of oxirane ring) were confirmed by the transformation of (unsaturated) double bonds into a three-membered oxirane ring via epoxidation reaction. In addition, ATR-FTIR spectra exhibited a trace of broad OH absorption peak at approximately 3000~3500 cm-1, which represents oxirane cleavage during or after the epoxidation reaction.
These results suggest that some of the double bonds are converted into epoxy groups that act as a primary plasticizer and heat stabilizer. Furthermore, a trace of double bond remains unreacted, which acts as a reactive plasticizer by chemically bonding with PVC resins and preventing the continuous thermal degradation of PVC resin [Figure 8(d)]. Both greatly contribute to improving the thermal stability and mechanical properties of PVC resins.
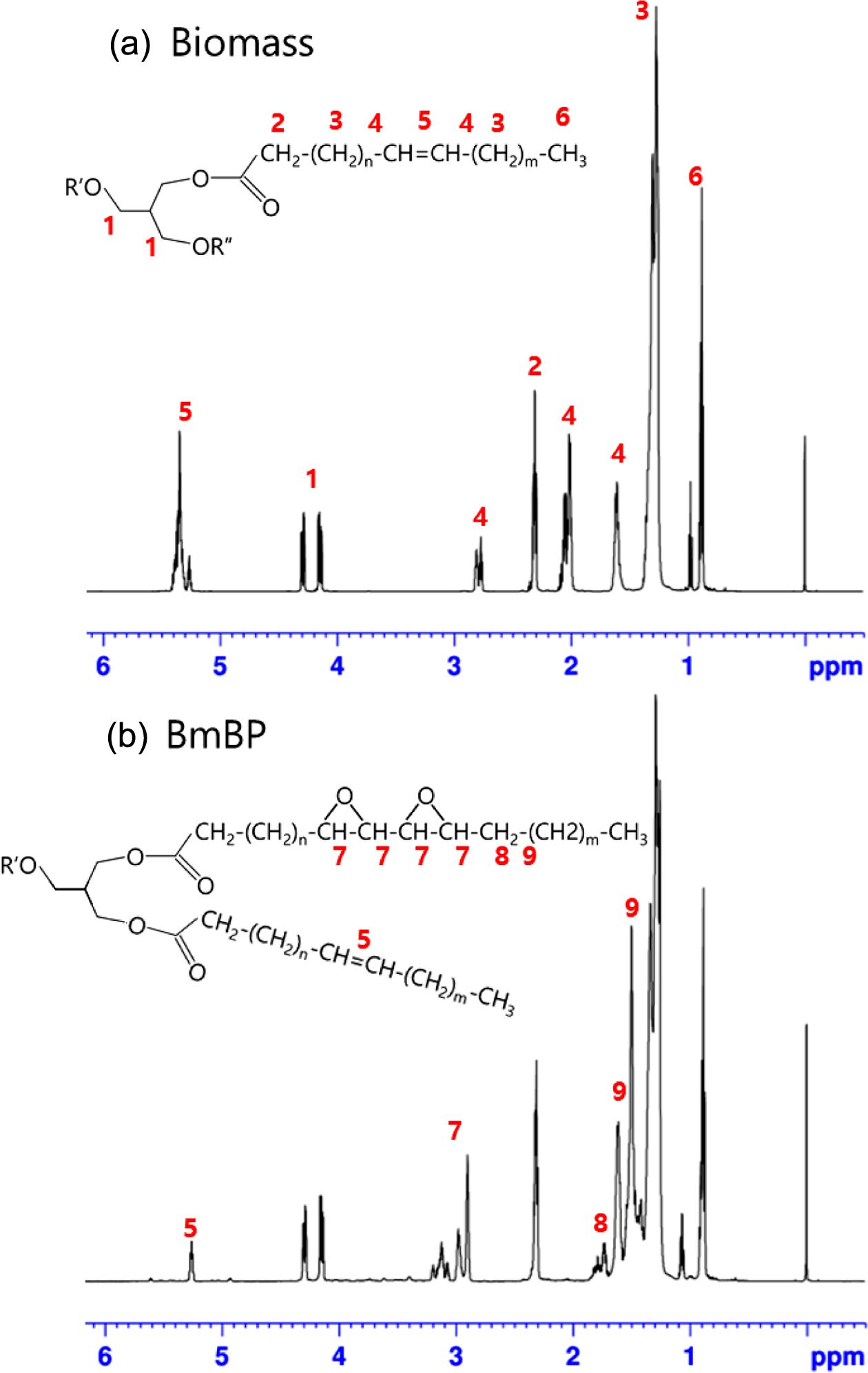
1H NMR: The chemical compositions of the biomass and the BmBP were characterized by 1H NMR spectroscopy. The presence of double bonds in the biomass and epoxy groups in the BmBP is illustrated in Figure 4. The characteristic peaks of the biomass were observed clearly in Figure 4(a). The two sharp peaks at 4.1 and 4.3 ppm were attributed to the methylene protons on the glycerol backbone, while the peaks at ~0.9, ~1.3, ~1.6, and ~2.3 ppm were assigned to the terminal methyl protons, the proton of the methylene group adjacent to the methyl terminal, the proton of the methylene group adjacent to the carbonyl, and the proton of the methylene alpha to the carbonyl, respectively. The presence of double bonds in the biomass was confirmed by the peaks at ~2.0 ppm (-CH2CH=CHCH2-) and 5.2 to 5.5 ppm (-CH=CH-). The characteristic peaks of the BmBP, as displayed in Figure 4(b), were observed at 2.9~3.2, 1.7~1.8, and 1.5~1.6 ppm, which were assigned to the oxirane ring (CHOCH), the proton of the methylene group adjacent to epoxy ring, and the OCOCH2CH2. Moreover, the peak at 5.2~5.3 ppm indicated the presence of some double bonds in the BmBP.
These results indicate that the BmBP, that is comprised of 90% epoxy groups and 10% double bonds, is successfully synthesized from the biomass through the calculation of area ratio.
Processing Properties of PVC Sheets with BmBP, DIDP, and TINTM. Fusion Behavior of the PVC Compounds: The information on fusion behavior plays an important role in determining the processability and predicting the mechanical and thermal properties in PVC compounds.40 Processability is defined as the ease with which various processes combine the plasticizer and other additives with the PVC resin to compound and form the end product. The processability in PVC processing depends on its viscoelastic behavior, which is related to the gelation and fusion of the PVC compounds. The characterization method for the progress of continuous gelation and fusion over the temperature or time range of interest was developed using viscoelastic or torque measurements.41-43 Gelation refers to a process in which liquid additives, such as a plasticizer and a stabilizer, completely penetrate the PVC resin particles in a mixture system of PVC resin, plasticizer, and stabilizer; the process in which entangled PVC macromolecular chains inside PVC particles dissociate is called fusion. In general, when observed with the naked eye, the color of the PVC mixture in the gelation process is white and becomes transparent as fusion progresses.
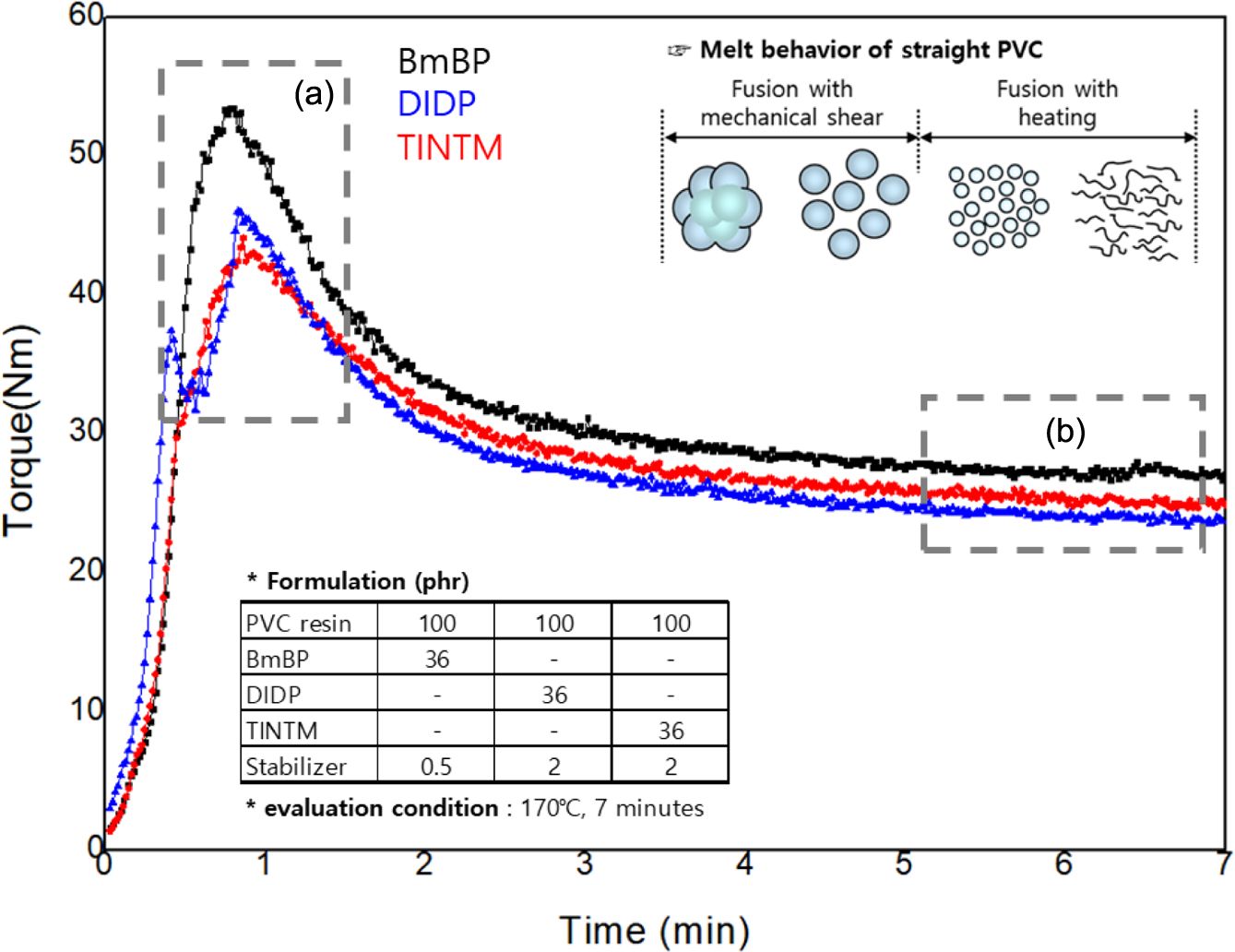
Figure 5 displays the fusion behaviors of PVC dry blends with BmBP, DIDP, and TINTM. The curves illustrate the thermomechanical history experienced by PVC dry blends under mixing and provide practical information on the prediction of the mechanical and thermal properties. When PVC dry blends are fed into the heated mixing chamber, they offer a certain resistance to the free rotation of the blades, after which the solid particles break into sub-grains or primary particles and simultaneously melt the surface of them, therefore causing the torque to increases to a maximum in Figure 5(a). PVC dry blends are gelled during the formulation process using a super mixer. Thus, agglomerated tertiary particles of PVC dry blends are weakened by the gelation process and are easily dissociated into primary particles by processing temperature and mechanical shear. In addition, the fusion behavior up to the maximum occurs primarily within the particles, after which the heating acts as the main factor, and the primary particles become the macromolecular chains. The torque consequently decreases and reaches a steady-state condition as the fusion of PVC dry blends continues, after which it may increase or decrease depending on whether crosslinking or degradation phenomena occurs. The property of the plasticizer sorption of PVC dry blends can be characterized based on the torque maximum, as presented in Figure 5(a). The fact that the torque is the highest indicates that the plasticizer absorption proceeded more ideally, indicating that the thermal stabilizer easily penetrates the PVC particles together with the plasticizer. Therefore, it contributes simultaneously to the thermal stabilization of the PVC resin and the improvement of mechanical properties. If the plasticizer absorption proceeds incompletely, the double peaks in the torque are observed, and the primary and tertiary particles coexist in the melt state, causing deterioration in the thermal and mechanical properties. The plasticizer absorption of BmBP had the highest level, and the levels for the DIDP and TINTM followed. The thermal stability of the PVC dry blends is determined based on whether the heat stabilizer penetrates the internal structure of the PVC particles with the plasticizer as quickly as possible to stabilize chlorine atoms vulnerable to heat. Therefore, PVC compound using BmBP can be estimated to exhibit better thermal stability than those using DIDP and TINTM with benzene, which is a thermal resistant structure, even though BmBP has no benzene structure and a relatively small content of heat stabilizer. In addition, the final torque serves as a measure of the melt viscosity, which is an important characteristic for optimizing the actuating variables of a production line and provides a clue about the mechanical property, as presented in Figure 5(b). In general, the higher the melt viscosity, the better the mechanical property. Therefore, the PVC sheet using BmBP can be the predicted to exhibit a better mechanical property than those using DIDP and TINTM, due to the effect of the complete solvation and fusion and the crosslinking in the reactive sites, such as the oxirane group and double bond of BmBP.
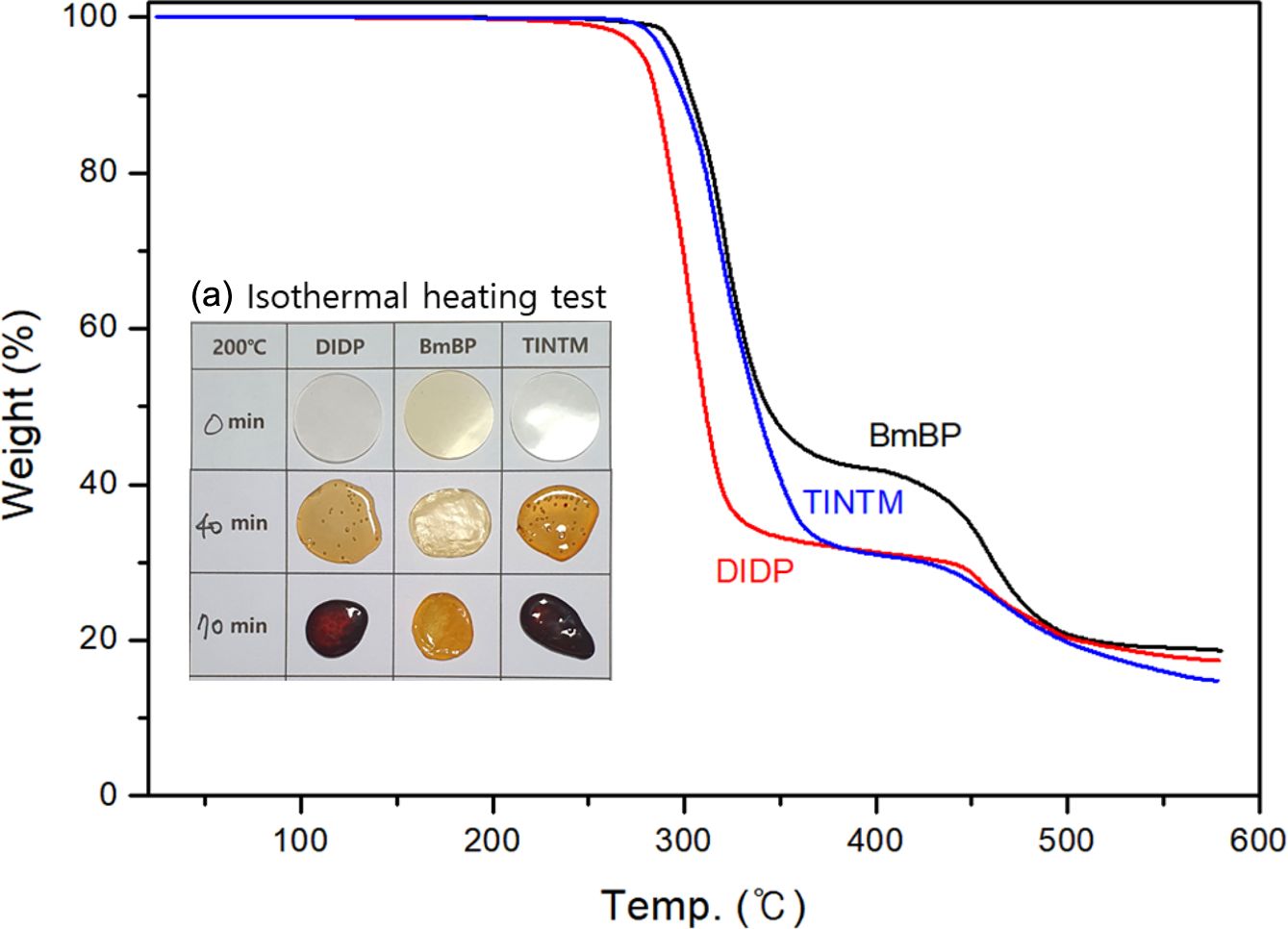
Thermal Properties of the PVC Sheets: Figure 6 indicates the thermal stabilities of PVC sheets with BmBP, DIDP, and TINTM. The thermal stability of PVC sheets was confirmed by the weight loss, indicated by the TGA analysis and isothermal heating test, as illustrated in Figure 6(a). As previously stated, the thermal stability of PVC sheet with BmBP is superior to those with DIDP and TINTM. Additionally, the weight loss of PVC sheets begins at 295, 283, and 284 oC for BmBP, TINTM, and DIDP, respectively. In general, the sharp weight loss in the temperature range of 250 to 350 oC indicated the dehydrochlorination of the PVC resin, which involves progressive “unzipping” of neighboring chlorine and hydrogen atoms along the PVC chain. The development of color is attributed to the conjugated double bonds formed in this dehydrochlorination process. Therefore, the color of the PVC sheet sequentially changed to yellow, red, brown, and finally black as the thermal degradation proceeded. It is noted that vegetable oils have a unique yellowish color, and the content of heat stabilizer in the PVC sheet with BmBP is reduced to a quarter compared with those with DIDP and TINTM. The thermal stability of the PVC sheet with BmBP composed of pure hydrocarbon is superior to those with DIDP and TINTM composed of a benzene structure because it is associated with the excellent plasticizer absorption and the reactive sites, such as oxirane group and carbon-carbon double bond. In particular, excellent plasticizer absorption of BmBP permeates the PVC particles with heat stabilizer more quickly than DIDP and TINTM and prevents the dehydrochlorination of PVC resin and changes into thermally stable structures such as chlorohydrin compounds. Moreover, the fact that the first plateau of the PVC sheet with BmBP is higher than those with DIDP and TINTM indicates that crosslinking and stabilization reaction occurred at the reactive sites, which contributes to improving the thermal stability of BmBP. This result is consistent with the fusion behavior in Figure 5(a).
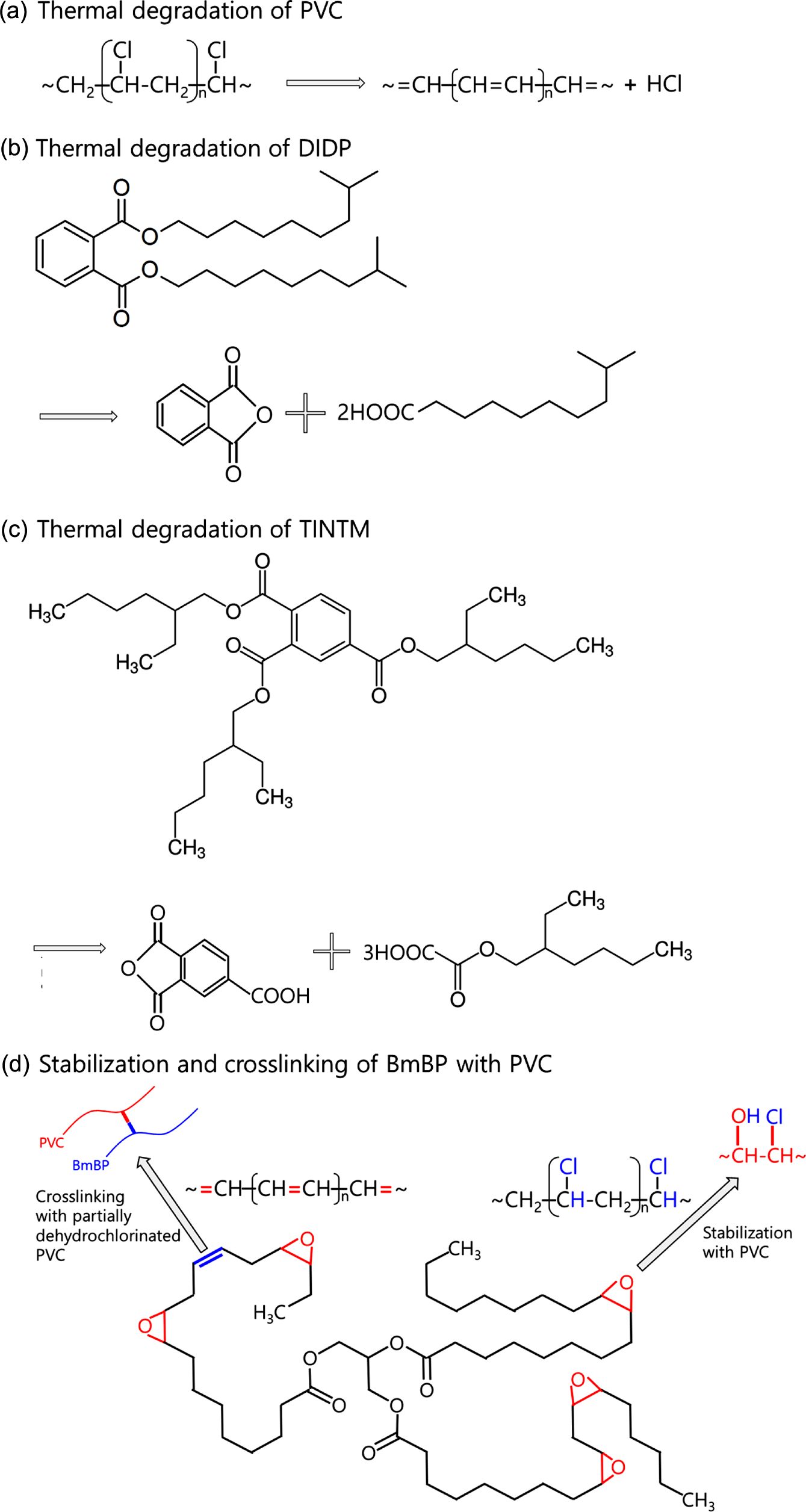
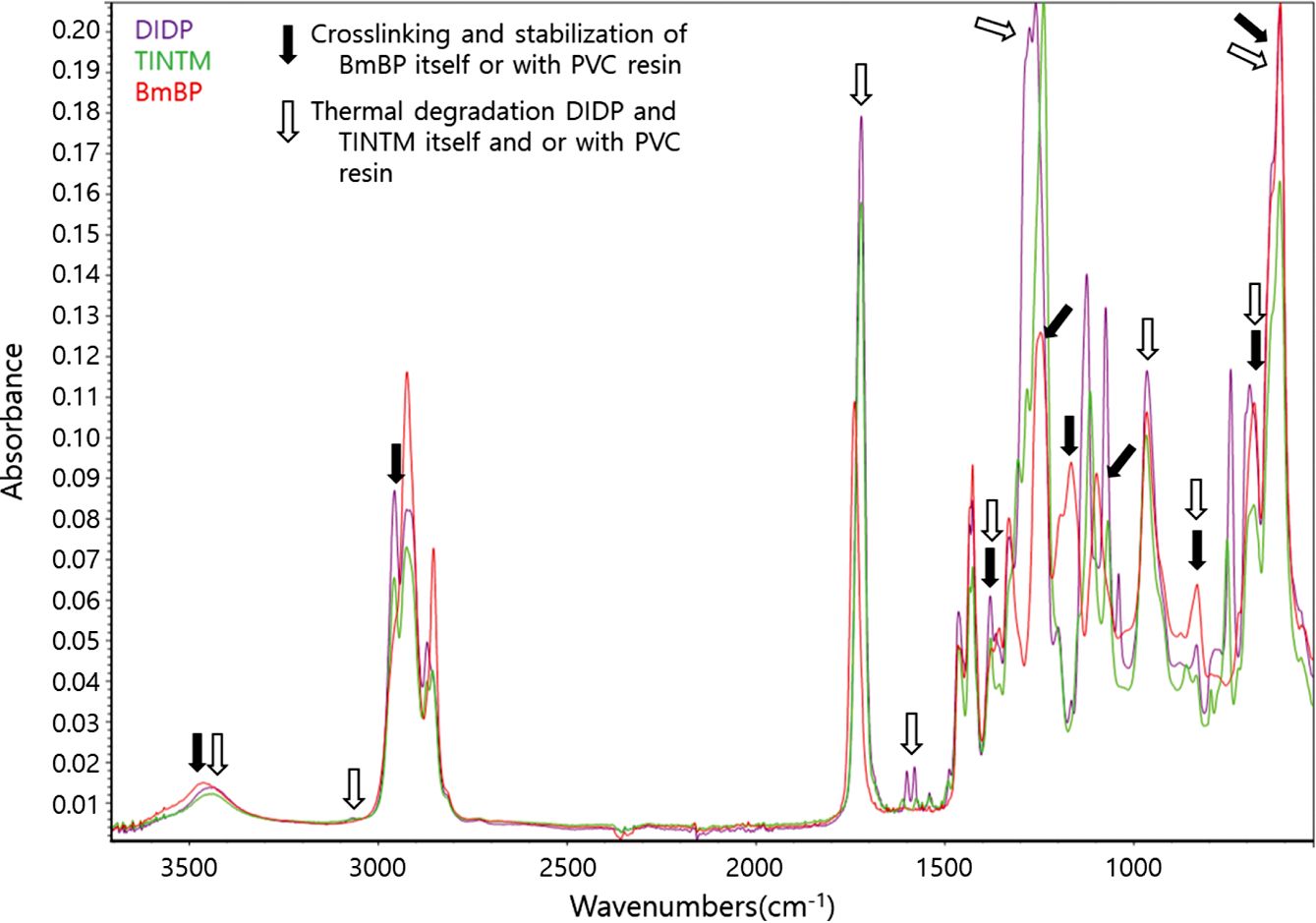
Figure 7 presents the FTIR analysis of PVC sheets with BmBP, DIDP, and TINTM, while Figure 8 indicates the thermal degradation, stabilization, and crosslinking of PVC sheets with DIDP, TINTM, and BmBP. The assignment of the spectral bands was performed according to previously published literatures.44-46
There exists much evidence of the crosslinking and stabilization reaction of BmBP and thermal degradation of DIDP and TINTM with PVC resin. Most of all, PVC sheets with BmBP, DIDP, and TINTM exhibit a broad band at approximately 3450 cm-1. In the case of BmBP, the stabilization reaction between the oxirane group of BmBP and hydrogen chloride of PVC resin occurs to yield the hydroxyl group [Figure 8(d)]. Additionally, strong peaks of C-OH stretching vibrations are observed near 1100 and 1170 cm-1. On the other hand, DIDP and TINTM are thermally decomposed to form hydrogen phthalate by cis-elimination and phthalic anhydride and alcohol by further thermal decomposition [Figure 8(b) and 8(c)]. In this case, a strong peak appears near 1750 cm-1 that corresponds to hydrogen phthalate. Furthermore, new extra- ordinarily strong peaks appear near 1260 and 1030 cm-1 in particular due to the presence of cyclic C-O-C asymmetric and symmetric stretching vibrations that correspond to phthalic anhydride, respectively [Figure 8(b) and 8(c)].
Near 3000 cm-1 of the carbon-hydrogen stretching absorption, a new band of PVC sheets with DIDP and TINTM is formed at approximately 3050 cm-1 and may be associated with the carbon-hydrogen stretching vibration from an unsaturated carbon-carbon linkage. Moreover, the weak absorption band near 1610 cm-1 is assigned to the carbon-carbon double bond stretching vibration for conjugated aliphatic bonds, which areformed as a result of some dehydrochlorination reaction [Figure 8(a)]. Regarding the PVC sheet with BmBP, the C-H stretching band on neighboring CHCl groups at 2970 cm-1 disappears after the PVC stabilization process [Figure 8(d)]. The presence of double bonds and chlorine atoms suggests that the stabilization of PVC sheets with DIDP and TINTM did not function normally and was followed by degradation [Figure 8(a)]. In addition, the absence of peaks corresponding to double bonds within the PVC sheet with BmBP indicates that the crosslinking was formed successfully [Figure 8(d)].
Some specific peaks are found in the PVC sheets with BmBP, DIDP, and TINTM: CH-Cl out of plane angular deformation near 1380 and 1250 cm-1 and C-Cl stretching vibration near 830, 690, and 620 cm-1. The peaks in the former disappear as the main chains of PVC resin are destroyed and the chlorine atoms are removed by the thermal degradation or the chlorine atoms of PVC resin are stabilized with heat stabilizer and a reactive plasticizer such as BmBP to form metal chloride complexes and chlorohydrin compounds, respectively; these results are consistent with data presented in Figure 6. In the latter case, the presence of chlorine in PVC sheets using DIDP and TINTM indicates that the stabilization reaction did not proceed smoothly. On the other hand, the presence of chlorine in the PVC sheet with BmBP indicates that the reaction between oxirane groups of BmBP and chlorine atoms of PVC resin occurred to create new -CHCl- structures [Figure 8(d)].
Mechanical Properties of the PVC Sheets: The analysis of the fusion characteristic is a measure of the ability of a plasticizer to fuse with the polymer and cause further structural rearrangement (namely entanglement) that may improve its melt viscosity. In general, the mechanical properties of the PVC sheets improve as PVC dry blends are fully solvated and fused or crosslinked.
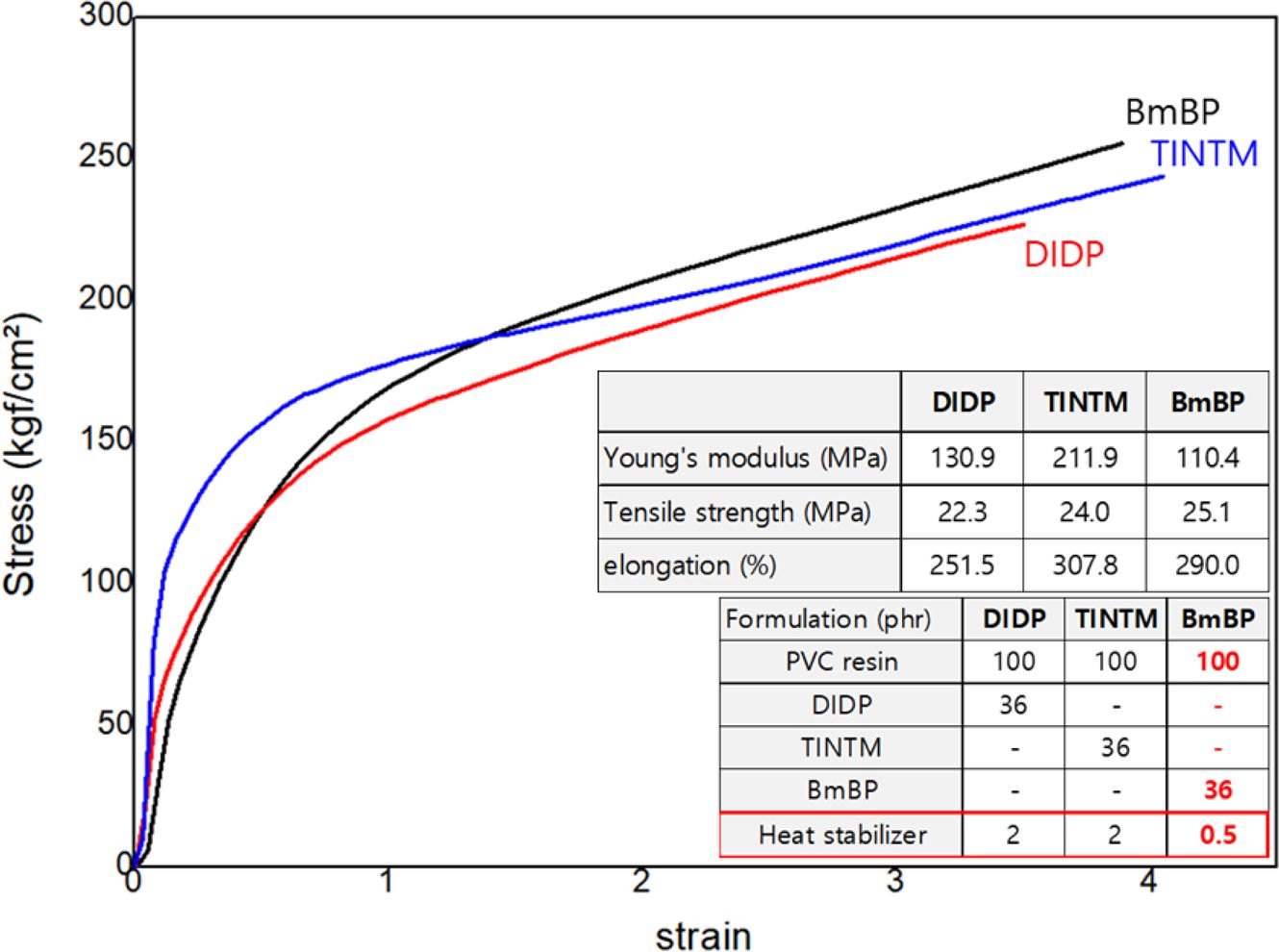
Figure 9 illustrates the measurement of the mechanical properties of PVC sheets with DIDP, TINTM, and BmBP using UTM. Considering the slopes of stress-strain curves and the data of Young’s modulus, the PVC sheet with BmBP is much softer than those with TINTM and DIDP, due to the difference between the soft hydrocarbon structure of BmBP and the rigid benzene segment of DIDP and TINTM. Furthermore, the data above reveals that the plasticization efficiency and reduction effect of plasticizer content for PVC sheet with BmBP are better than those with DIDP and TINTM. Moreover, the tensile strength of the PVC sheet with BmBP is superior to those with DIDP and TINTM, which is consistent with the prediction in Figure 5(b). This result is caused by the presence of the reactive sites, such as double bonds and oxirane groups, which can lead to a mechanically strong and stable crosslinking structure. The elongation of the PVC sheet with BmBP is slightly inferior to that with TINTM but is superior to that with DIDP, which is caused by the partial crosslinking despite the high molecular weight of BmBP. In addition, if the complete crosslinking occurred, the elongation of PVC sheet with BmBP is greatly inferior to those with DIDP and TINTM.
|
Figure 2 GC-FID chromatogram of biomass after decolorization and deodorization. |
|
Figure 3 ATR-FTIR spectra of biomass and BmBP. |
|
Figure 8 Thermal degradation, stabilization, and crosslinking of
PVC sheets with DIDP, TINTM, and BmBP. |
|
Figure 4 1H NMR spectra of (a) Biomass; (b) BmBP |
|
Figure 5 Fusion behaviors of PVC dry blends with BmBP, DIDP,
and TINTM |
|
Figure 6 Thermal stabilities of PVC sheets with BmBP, DIDP, and
TINTM. |
|
Figure 7 FTIR analysis of PVC sheets with BmBP, DIDP, and
TINTM |
|
Figure 9 Measurement of the mechanical properties of PVC sheets
with DIDP, TINTM, and BmBP using UTM. |
BmBP was successfully synthesized via three epoxidation steps that were innovatively simplified from the conventional seven steps, which were otherwise comprised of complex and expensive manufacturing processes. In addition, BmBP consisted of 90% oxirane groups and 10% unsaturated groups reinforce the thermal and mechanical properties of the PVC sheet and obtain low hardness through partial crosslinking, which is a major obstacle of DIDP and TINTM. The continuous gelation and fusion behaviors were characterized by torque measurement and used as a predictive model for correlating the thermal and mechanical properties. BmBP demonstrates excellent plasticizer absorption compared with DIDP and TINTM; therefore, the permeation of BmBP with the thermal stabilizer into the PVC particles was faster than those of DIDP and TINTM, resulting in superb thermal and mechanical properties through stabilization with thermally sensitive chlorine atoms. Moreover, reactive sites of BmBP, such as oxirane groups and double bonds, played a major role in strengthening both PVC resin and BmBP via stabilization reaction and partial crosslinking.
As a result, BmBP is a powerful alternative to specialty plasticizers such as C10-11 phthalates and trimellitates, which can be extensively used in wire, cable, and electrical insulation parts.
- 1. Mazubert, A.; Poux, M.; Aubin, J. Intensified Processes for FAME Production from Waste Cooking Oil. Chem. Eng. J. 2013, 233, 201-223.
-

- 2. Cordero, R. V.; Schallenberg, R. J. Biodiesel Production as a Solution to Waste Cooking Oil (WCO) Disposal. Will any Type of WCO do for a Transesterification Process? a Quality Assessment. J. Environ. Manag. 2018, 228, 117-129.
-

- 3. Gupt, Y.; Sahay, S. Review of Extended Producer Responsibility: a Case Study Approach. Waste Manag. Res. 2015, 33, 595-611.
-

- 4. Kumar, S.; Negi, S. Miscanthus as Cellulosic Biomass for Bioethanol Production. Biotech. 2015, 10, 847-851.
-

- 5. Pleissner, D.; Kwan, T. H.; Lin, C. S. K. Fungal Hydrolysis in Submerged Fermentation for Food Waste Treatment and Fermentation Feedstock Preparation. Bioresour. Technol. 2014, 158, 48-54.
-

- 6. Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538-1558.
-

- 7. Firdaus, M.; Meier, M. R. A.; Biermann, U.; Metzger, J. O. Renewable Co-polymers Derived from Castor Oil and Limonene. Eur. J. Lipid Sci. Technol. 2014, 116, 31-36.
-

- 8. Pleissner, D.; Lam, W. C.; Sun, Z.; Lin, C. S. K. Food Waste as Nutrient Source in Heterotrophic Microalgae Cultivation. Bioresour. Technol. 2013, 137, 139-146.
-

- 9. Petrovic, Z. S.; Zlatanic, A.; Lava, C. C.; Sinadinovic, S. Epoxidation of Soybean Oil in Toluene with Peroxoacetic and Peroxoformic Acids - Kinetics and Side Reactions. Eur. J. Lipid Sci. Technol. 2002, 104, 293-299.
-

- 10. Akintayo, E. T.; Ziegler, T.; Onipede, A. Gas Chromatographic and Spectroscopic Analysis of Epoxidized Canola Oil. Bull. Chem. Soc. Ethiop. 2006, 6, 75-81.
-

- 11. Campanella, A.; Fontanini, C.; Baltans, M. A. High Yield Epoxidation of Fatty Acid Methyl Esters with Performic Acid Generated. In Situ Chem. Eng. J. 2008, 144, 466-475.
-

- 12. Faria-Machado, A. F.; da Silva, M. A.; Adeodato Vieira, M. G.; Beppu, M. M. Epoxidation of Modified Natural Plasticizer Obtained from Rice Fatty Acids and Application on Polyvinylchloride Films. J. Appl. Polym. Sci. 2013, 127, 3543-3549.
-

- 13. Doll, K. M.; Erhan, S. Z. Synthesis and Performance of Surfactants Based on Epoxidized Methyl Oleate and Glycerol. J. Surfactants Deterg. 2006, 9, 377-383.
-

- 14. Puyou, J.; Haoyu, X.; Kehan, T.; Yonghong, Z. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303.
-

- 15. Borugadda, V. B.; Goud, V. V. Synthesis of Waste Cooking Oil Epoxide as a Bio-lubricant Base Stock: Characterization and Optimization Study. J. Bioprocess Eng. Biorefinery 2014, 3, 57-72.
-

- 16. Jia, P.; Zhang, M.; Hu, L.; Zhou, Y. Green Plasticizers Derived from Soybean Oil for Poly(Vinyl Chloride) as a Renewable Resource Material. Korean J. Chem. Eng. 2016, 33, 1080-1087.
-

- 17. Mulla, E. A. Z. A.; Yunus, W. M. Z. W.; Ibrahim, N. A.; Rahman, M. Z. A. Properties of Epoxidized Palm Oil Plasticized Polylactic Acid. J. Mater. Sci. 2010, 45, 1942-1946.
-

- 18. Chen, J.; Liu, Z.; Wang, K.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Epoxidized Castor Oil-based Diglycidyl-phthalate Plasticizer: Synthesis and Thermal Stabilizing Effects on Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2018, 135, 47142.
-

- 19. Chen, J.; Wang, Y.; Huang, J.; Li, K.; Nie, X. Synthesis of Tung-oil-based Triglycidyl Ester Plasticizer and its Effects on Poly(Vinyl Chloride) Soft Films. ACS Sustain. Chem. Eng. 2018, 6,642-651.
-

- 20. Benaniba, M. T.; Bensemra, N. B.; Gelbard, G. Stabilizing Effect of Epoxidized Sunflower Oil on the Thermal Degradation of Poly(Vinyl Chloride). Polym. Degrad. Stab. 2001, 74, 501-505.
-

- 21. Nihul, P. G.; Mhaske, S. T.; Shertukde, V. V. Epoxidized Rice Bran Oil (ERBO) as a Plasticizer for Poly(Vinyl Chloride) (PVC). Iran. Polym. J. 2014, 23, 599-608.
-

- 22. Dutta, K.; Das, S.; Kundu, P. P. Epoxidized Esters of Palm Kernel Oil as an Effective Plasticizer for PVC: A Study of Mechanical Properties and Effect of Processing Conditions. Int. Polym. Proc. 2014, 29, 495-506.
-

- 23. Fenollar, O.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Lopez, J.; Balart, R. Effect of the Epoxidized Linseed Oil Concentration as Natural Plasticizer in Vinyl Plastisols. J. Mater. Sci. 2010, 45, 4406-4413.
-

- 24. Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer Contamination in Edible Vegetable Oil in a U.S. Retail Market. J. Agric. Food Chem. 2013, 61, 9502-9509.
-

- 25. Sun, T.; Thom, R. The Effect of Epoxidized Safflower Oil on The Properties of Polyvinyl Chloride Films. J. Elastomers Plast. 2010, 42, 129-137.
-

- 26. Patil, S. S.; Jena, H. M. Synthesis of Epoxidized Citrullus Lanatus Seed Oil: Experimental Investigation and Statistical Optimization. Arab. J. Sci. Eng. 2019, 44, 9965-9976.
-

- 27. Alfredo, C. V.; David, G. S.; Amparo, J. V.; Lourdes, S. N.; Rafael B. A New Biobased Plasticizer for Poly(Vinyl Chloride) Based on Epoxidized Cottonseed Oil. J. Appl. Polym. Sci. 2016, 133, 43642.
-

- 28. Joseph, R.; Alex, R.; Vinod, V. S.; Premalatha, C. K. Studies on Epoxidized Rubber Seed Oil as Plasticizer for Acrylonitrile Butadiene Rubber. J. Appl. Polym. Sci. 2003, 89, 668-673.
-

- 29. Suzuki, A. H.; Botelho, B. G.; Oliveira, L. S.; Franca, A. S. Sustainable Synthesis of Epoxidized Waste Cooking Oil and its Application as a Plasticizer for Polyvinyl Chloride Films. Eur. Polym. J. 2018, 99, 142-149.
-

- 30. Feng, G.; Ma, Y.; Zhang, M.; Jia, P.; Liu, C.; Zhou, Y. Synthesis of Bio-base Plasticizer Using Waste Cooking Oil and its Performance Testing in Soft Poly(Vinyl Chloride) Films. JB&B 2019, 4, 99-110.
-

- 31. Zheng, T.; Zhenyu, W.; Xie, Q.; Fang, J. Structural Modification of Waste Cooking Oil Methyl Esters as Cleaner Plasticizer to Substitute Toxic Dioctyl Phthalate. J. Clean. Prod. 2018, 186, 1021-1030.
-

- 32. Silviana, S.; Anggoro, D. D.; Kumoro, A. C. Waste Cooking Oil Utilization as Bio-plasticizer Through Epoxidation Using Inorganic Acids as Homogenous Catalysts. Chem. Eng. Trans. 2017, 56, 1861-1866.
-

- 33. https://echa.europa.eu/regulations/reach.
- 34. Hosney, H.; Nadiem, B.; Ashour, I.; Mustafa, I.; El-Shibiny, A. Epoxidized Vegetable Oil and Bio-based Materials as PVC Plasticizer. J. Appl. Polym. Sci. 2018, 135, 46270.
-

- 35. Tsimidou, M.; Macrae, R. Authentication of Virgin Olive Oils Using Principal Component Analysis of Triglyceride and Fatty Acid Profiles: Part 2-Detection of Adulteration with Other Vegetable Oils. Food Chem. 1987, 25, 251-258.
-

- 36. Rezanka, T.; Rezankova, H. Characterization of Fatty Acids and Triacylglycerols in Vegetable Oils by Gas Chromatography and Statistical Analysis. Anal. Chim. Acta 1999, 398, 253-261.
-

- 37. Chen, J.; Liu, Z.; Li, K.; Huang, J.; Nie, X.; Zhou, Y. Synthesis and Application of a Natural Plasticizer Based on Cardanol for Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2015, 132, 42465.
-

- 38. Omrani, I.; Ahmadi, A.; Farhadian, A.; Shendi, H. K.; Babanejad, N.; Nabid, M. R. Synthesis of a Bio-based Plasticizer from Oleic Acid and its Evaluation in PVC Formulations. Polym. Test. 2016, 56, 237-244.
-

- 39. Gupta, N. K.; Yadav, P. K. S.; Eadara, R.; Singh, R. P. Synthesis of Epoxy Resin from Waste Ricebran Oil. Polym. Renew. Resour. 2016,7, 21-32.
-

- 40. Wilkes, C. E.; Summers, J. W.; Daniels, C. A. PVC Handbook; Hanser Publications: Munchen, 2005.
- 41. Yu, B. Y.; Kwak, S. Y. Viscoelastic Behavior of PVC Plastisol with Cyclodextrin Derivative and Antimigration of Plasticizers in Flexible PVC. Annual Transactions of the Nordic Rheology Society,2011, 19.
- 42. Yu, B. Y.; Lee, A. R.; Kwak, S. Y. Gelation/Fusion Behavior of PVC Plastisol with a Cyclodextrin Derivative and an Anti-migration Plasticizer in Flexible PVC. Eur. Polym. J. 2012, 48, 885-895.
-

- 43. Verdu, J.; Zoller, A.; Marcilla, A. Plastisol Gelation and Fusion Rheological Aspects. J. Appl. Polym. Sci. 2013, 129, 2840.
-

- 44. Chen, J.; Nie, X. A.; Jiang, J. C.; Zhou, Y. H. Thermal Degradation and Plasticizing Mechanism of Poly(Vinyl Chloride) Plasticized with a Novel Cardanol Derived Plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012008.
-

- 45. Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256.
-

- 46. Yoshioka, T.; Kameda, T.; Ieshige, M.; Okuwaki, A. Dechlorination Behaviour of Flexible Poly(Vinyl Chloride) in NaOH/EG Solution. Polym. Degrad. Stabil. 2008, 93, 1822-1825.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(3): 380-389
Published online May 25, 2021
- 10.7317/pk.2021.45.3.380
- Received on Nov 17, 2020
- Revised on Dec 29, 2020
- Accepted on Jan 4, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jae-Koo Kim
-
Department of Polymer Science and Engineering, Chungnam National University, 99, Daehak-ro, Yuseong-gu, Daejeon 34134, Korea
- E-mail: jack70@megen.co.kr
- ORCID:
0000-0001-8081-4861










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.