- Effect on the Antioxidant Capacity of Polypropylene by Vanillin: A Natural Antioxidant Extracted from Cotton Stalk Lignin
Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education and Xinjiang Uyghur Autonomous Region, College of Chemical Engineering, Xinjiang University, Urumchi 830046, China
- Vanillin의 Polypropylene 항산화 효과 연구: Cotton Stalk Lignin 추출 천연 항산화제
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Vanillin (VA) was extracted from cotton stalk lignin by UV photocatalysis method using Co2+/Ni2+ as a catalyst, the yield of which was quantitative analyzed through gas chromatography-mass spectrometer and high performance liquid chromatography. The structure and thermal properties were compared between extracted and standard VA, which showed basically consistent. PP/VA materials characterized by fourier transform infrared spectroscopy were prepared by melt intercalation and subjected to 20 days thermal oxygen aging. Thermal gravimetric analysis and oxidation induction time results showed VA (0.9 wt%) could improve the thermal stability of polypropylene (PP) by 33.4 ℃ and 49.03%. Differential scanning calorimetry confirmed that VA increased the crystallinity of PP reaching to 15.70%. After 20 days aging, mechanical test showed the impact strength and breaking elongation retention of PV-3 (0.9 wt%) were 10.0% and 14.8% higher than those of PP; surface scanning electron microscopy showed that PV-3 had the most smooth surface.
This study provided a simple, environmentally friendly and high selectivity extraction method of vanillin (VA) with excellent yield; it also showed a new choice of natural and harmless antioxidant for improving the antioxidant capacity of polypropylene (PP). Moreover, it was of great significance to the recycling of bio-based resources and the sustainable development of natural environment.
Keywords: vanillin, UV photocatalysis, polypropylene, antioxidant capacity, mechanical properties.
This work was funded by the National Natural Science Foundation of China (No. 21764013) and the Postgraduate Research Innovation Project of Xinjiang Uygur Autonomous Region (XJ2020G033 and XJ2021G032).
Information is available regarding the experimental procedure for the extraction of LG from cotton stalk, extraction of VA by UV photocatalysis from LG, and characterization between exracted VA and standard VA. The materials are available via the Internet at http://journal.polymer-korea.or.kr
PK_2021_045_05_717_Supporting_Information_template.pdf (485 kb)
Supplementary Information
Polypropylene (PP) is one of the five most widely used general plastics in the world, which is white granules, odorless and non-toxic.1 While because of the special tertiary carbon structure, the tertiary hydrogen reaction activity of PP is very reactive, so that PP is easy to be degraded by thermal oxygen and light in processing, storage and using.2 Antioxidants are often added to avoid this phenomenon during the processing of PP.3 At present, the most commonly used antioxidants are mainly blocked phenols.4 However, in recent years more and more safety problems such as easy migration and toxicity of antioxidants have appeared in food packaging materials. It is of great significance to find a kind of healthy and safe natural antioxidants with good antioxidant capacity for PP.5 Nanni et al.6,7 studied the effects of natural extracts from grape skins, seeds and stems produced in the process of wine production on the thermal stability of PP through UV and thermal aging, and compared them with common industrial antioxidants. The results showed natural extracts from grape seeds, which was able to protect PP against UV aging, presented the best antioxidant effect, and the oxidation induction time (OIT) value of which had been the highest among all the investigated samples. This had been due by the ability of working both as radical scavenging and UV absorber.
Cotton stalk lignin (LG) is a kind of complex polyphenolic natural amorphous polymer compound, which is composed of phenylpropane structural unit connected by ether bond and C-C single bond and has an amorphous three-dimensional network molecular structure.8 Because of many phenolic hydroxyl groups and alcohol hydroxyl groups, LG structure produces strong hydrogen bond and intermolecular force, which has thermoplasticity and high glass transition temperature.9 In the molecular structure of LG, there are phenolic hydroxyl groups and other active groups, so it can produce downstream aromatic products such as syringyl, guaiacyl, p-hydroxyphenyl and vanillin through redox, alcoholysis, photolysis, sulfonation, alkylation or graft copolymerization reaction. These active groups and reaction performance of LG are the important way to expand its application. He et al.10 prepared PP/LG composite materials and carried out artificial accelerated aging test on it to explore the influence of LG as the antioxidant on PP. The results showed LG could effectively prevent the surface cracks and furrows of PP after aging, and the mechanical properties of PP were well preserved. China is a big producer of cotton and Xinjiang's cotton output accounts for about 70% of the national cotton output. However, the comprehensive utilization rate of cotton stalk in China is low, and a large amount of cotton stalk is burned, which pollutes the environment and wastes resources.11 If the cotton stalk rich in LG could be fully utilized, its economic and social benefits are very great. With the speeding up of industrialization and resources crisis, LG as a kind of green biomass resources would get to be studied in depth.
Vanillin (VA, 4-hydroxy-3-methoxy-benzaldehyde) is one of the most important degradation product of LG and widely used spices nowadays, which is cheap and easy to obtain. Because of the phenolic hydroxyl group, aldehyde group and other chemical functional groups, VA has good thermal stability, oxidation resistance and reactivity, which it is not easy to decompose after heating sublimation.12 The demand for VA is increasing rapidly due to its wide application prospects in antioxidant, anticancer, and anti-mutagenic activities. Nowadays, the main source of VA is the conversion of natural biomass resources, such as eugenol, guaiacol and ferulic acid, into VA by chemical conversion and synthesis,13 besides, there are a few reports on the extraction of VA by UV photocatalysis method from LG. Kansal et al.14 used TiO2 and ZnO as two kinds of catalysts to degrade LG, and found that ZnO had better photocatalytic effect. The combination of catalyst and oxidant could promote the degradation of LG and the experimental results showed the laden ZnO could also effectively catalyze the degradation of LG in pulp and paper waster. Erdocia et al.15 used 320 W UV lamp and nano TiO2 as a catalyst to photodegrade LG in the presence of NaOH, to explore the effects of reaction time on the molecular weight of LG and the distribution of degradation products. The results found the degradation products were mainly VA, syringaldehyde, catechol, and p-hydroxyphenylbutanone. However, the selectivity of the above studies was not good, the yield of VA did not exceed 50%.
In this work, VA was extracted from LG by UV photocatalysis method using Co2+/Ni2+ as a catalyst with the yield of 84.01%. The antioxidant capacity and mechanical properties of PP/VA materials subjected to 20 days thermal oxygen aging were researched. This study provided a simple, environmentally friendly and high selectivity extraction method of VA with excellent yield; at the same time, it showed a new choice of natural and harmless antioxidant for improving the antioxidant capacity of PP. Moreover, it was of great significance to the recycling of bio-based resources and the sustainable development of natural environment.
Materials. PP (D(Y)-W0723F: homo polymer blowing film grade, Mw=100000 g/mol, Mn=80000 g/mol, MFR=3.8 g/10 min) was purchased from Sinopec Dushanzi Co., Ltd., China. Cotton stalk was collected from Kashi Prefecture, China. Toluene (C7H8, AR), ethanol (C2H6O, AR), and ethyl acetate (C4H8O2, AR) were purchased from Fuchen Chemical Reagent Factory, China. Ionic liquids ([Bmim]Cl, 99%) was purchased from Keneng Material Technology Co., Ltd., China. Cobalt(II) acetate tetrahydrate (C4H6CoO4·4H2O, 99.5%), Nickel(II) acetate tetrahydrate (NiC4H6O4·4H2O, 99.5%), and VA standard sample (commercially available, 99%) were purchased from Aladdin industrial corporation, China.
Extraction of LG. The extraction of LG from cotton stalk was according to previous literature,16 and the experimental details were shown in Supporting Information.
Extraction of VA. 1 g sodium lignosulfonate (SL), which was obtained by sulfonation of LG, was added into 45 mL distilled water with a certain amount of CO2+/Ni2+ catalyst, and was added to the photochemical reaction apparatus as shown in Figure S1 after ultrasonic dispersion for 20 min. After repeated extraction and purification of reaction solution, the extraction VA was obtained. The standard curve of concentration and peak area of VA was drawn by high performance liquid chromatography (HPLC), the peak area and the concentration of components after reaction was calculated to measure the extraction yield.
Preparation of PP/VA Materials. PP and extracted VA were vacuum dried at 50 ℃ for 24 h to remove residual moisture. PP and a certain amount of VA were mechanically mixed and manually added into the twin-screw extruder (TE-20, L/D=30, 1 cm diameter of the screw, Nanjing Rubber & Plastic Co., Ltd., China) to obtain the blending granules of PP/VA materials by melt intercalation in the way of counter rotating and intermeshing. Temperature of melting extrusion and granulation from feeding area (three kneading zones) to die head was set at 170, 190, 200, and 190 ℃ respectively, and the feed speed and screw speed of extruder were both set at 30 rpm/min. Then, the granules of PP/VA materials after vacuum drying were fused and extruded, granulated and injected by plastic injection molding machine (XL-400 VI, L/D=15, Ningbo Gaoxin Co., Ltd., China) to obtain the standard splines of PP/VA materials for tensile and impact test under 8×106 Pa at 190 ℃ and screw speed of 30 rpm/min. In PP/VA materials, the addition of VA was 0, 0.3, 0.6, and 0.9 wt% respectively, and was finally named PV-0, PV-1, PV-2, and PV-3 respectively.
Artificial Accelerated Aging Test. According to ASTM D 2565, the PP/VA materials was put into an electric heating blast drying oven (DHG-9245A, Yiheng Co., Ltd., China) for 20 days thermal and oxygen aging test. The temperature was set at 120 ℃. Materials were tested on 5, 10, 15, and 20 days respectively.
Characterization. The degradation products in reacted solution by UV photocatalysis method were qualitatively analyzed by gas chromatography-mass spectrometer (GC-MS, 7890B-5977B, Beinalde Technology Co., Ltd., China). GC-MS was equipped with HP-5 non-polar capillary column (30 m×0.32 mm×0.25 mm), nitrogen as carrier gas, 1 mL sample injection, the initial temperature was 60 ℃, the temperature was raised to 340 ℃ at 10 ℃/min, and solvent delay was 3 min. The ionization energy was 70 eV, the temperature of the ion source generator was 230 ℃, and the mass scanning range was 40-1000 amu.
The quantitative analysis of VA was determined by high performance liquid chromatography (HPLC, LC-20A, Shimadzu Corporation, Japan). The standard curve of concentration and peak area of main products could be drawn by HPLC to detect the peak area after reaction and calculate the concentration of the component to be measured in the solution after reaction. For HPLC, Shimazu HP-5 column (30 m×0.32 mm×0.25 mm) was used. The mobile phase was ethyl acetate, the column temperature was maintained at 300 ℃, the detection time was 20 min, the injection volume was 2 mL, the total flow rate was 1.000 mL/min and the detection wavelength was 254 nm.
Nuclear magnetic resonance (NMR) was used to characterize the structure of standard and extracted VA samples by nuclear magnetic resonance spectrometer (DRX-400, Bruker, Germany). The resonant frequency of 1H was 400.13 MHz, and that of 13C was 100.61 MHz.
Fourier transform infrared spectroscopic (FTIR) of the materials were recorded on a (Thermo Scientific Nicolet iS5, USA) spectrometer by using standard KBr pellet/disk technique. Spectra were recorded over a spectral range of 4000 to 400 cm-1. All the samples were dried at 50 ℃ for 24 h before analysis.
X-ray powder diffraction analysis (XRD) was performed on a Bruker D8 Advance powder diffractometer (Karlsruhe, Germany) to study the crystal morphology of VA. A Cu-Ka radiation and A P-Ni filter (U=40 kV, A=40 mA) were used for recording in the 2θ range of 10 to 60° at a scanning rate of 6°/min.
Thermogravimetric analysis (TGA) was conducted on HCT-1 thermogravimetric analyzer (Hengjiu Experimental Equipment Co., Ltd., Beijing, China). Each sample (about 10 mg) was heated at a heating rate of 10 ℃/min in the temperature range of room temperature to 600 ℃ under nitrogen atmosphere (25 mL/min).
OIT was conducted on a DSC Q-2000 differential scanning calorimeter (TA Instruments, USA). Each sample (about 10 mg) was exposed to nitrogen atmosphere (50 mL/min) and heated to 200 ℃. After maintaining for several minutes at constant 200 ℃, the nitrogen flow was immediately changed to oxygen flow (50 mL/min), and the sample was kept at a constant temperature until it was completely oxidized.
Flame retardancy analysis was conducted on FTT0077 microcalorimeter (UK) according to the ISO-5660-1. Each sample (about 10 mg) was tested in a mixed atmosphere (nitrogen 80%, oxygen 20%) under the heating rate of 1 ℃/s from 100 to 600 ℃. The samples were dried at 50 ℃ for 24 h before testing.
Differential scanning calorimetric (DSC) analysis of each sample of PP/VA materials (about 10 mg) were conducted on a DSC Q-2000 differential scanning calorimeter (TA Instruments, USA). The test was conducted under nitrogen atmosphere (50 mL/min). The samples were heated from room temperature to 200 ℃ at a heating rate of 10 ℃/min and held for 5 min to clear off the thermal history. Then, the samples were cooled to 20 ℃ at a rate of 20 ℃/min, and the DSC curves of the process were generated. Finally, the enthalpy, peak and low values of the DSC curves were calculated by TA universal software.
Mechanical tensile properties were measured according to ASTM D 412-80 by using a CMT6104 electronic universal testing machine (New Sansi Co., Ltd., Shenzhen, China) with a crosshead speed of 100 mm/min. Each sample was measured 5 times to take the average value.
Izod impact testing without notch was carried out at room temperature by using a XJJD-50 electronic impact testing machine (Jinjian Instrument Co., Ltd., Chengde, China) according to ASTM D 256. Each sample was measured 5 times to take the average value. The measuring range was 7.5 J, the length of the spline was 80 mm, the width was 10 mm and the thickness was 4 mm.
Scanning electron microscopy (SEM) (SU8010, Hitachi, Japan) at 20 kV of accelerating voltage was applied to observe the surface morphology of PP/VA materials. The samples were sputter-coated with gold to limit charging during the tests before test.
The blown film tensile properties of PP/VA materials were conducted by a Instron-5967 tensile testing machine (USA) according to ASTM D 256. The machine was equipped with the tension sensor of 0.5 level accuracy and the static extenometer (2603-085, USA) of 1.0 level accuracy. At least five measurements for each sample were recorded. The width of the blown film sample was 10 mm, the length was 10 cm, and the uniaxial tensile rate was 100 mm/min.
Extraction and Characterization of VA. In this work, the orthogonal experimental design method was used to select the optimum extraction process of VA, which could be found in Supporting Information. Therefore, (A) the reaction temperature, (B) the UV lamp wattage, (C) the reaction time, and (D) the catalyst mass ratio were selected as effect factors for the orthogonal experimental, as shown in Table S1. Range analysis was performed using the yield of VA as the objective function. The analytic results were exhibited in Table S2, in which the impact factor of each level was calculated to be 2.39, 2.95, 2.41, and 1.86, respectively. According to the results, it was clear that factor B had the greatest influence on the yield of VA. As for other factors, which also had influence on the yield of VA, were ranked as followed: the reaction time, the reaction temperature, and the catalyst mass ratio. The results of the orthogonal analysis showed the optimum reaction conditions were to be the reaction temperature of 55 ℃, the UV lamp wattage of 1000 W, the reaction time of 4 h, and the catalyst mass ratio of 15 wt%. Under the condition, the VA extraction yield was 89.01%.
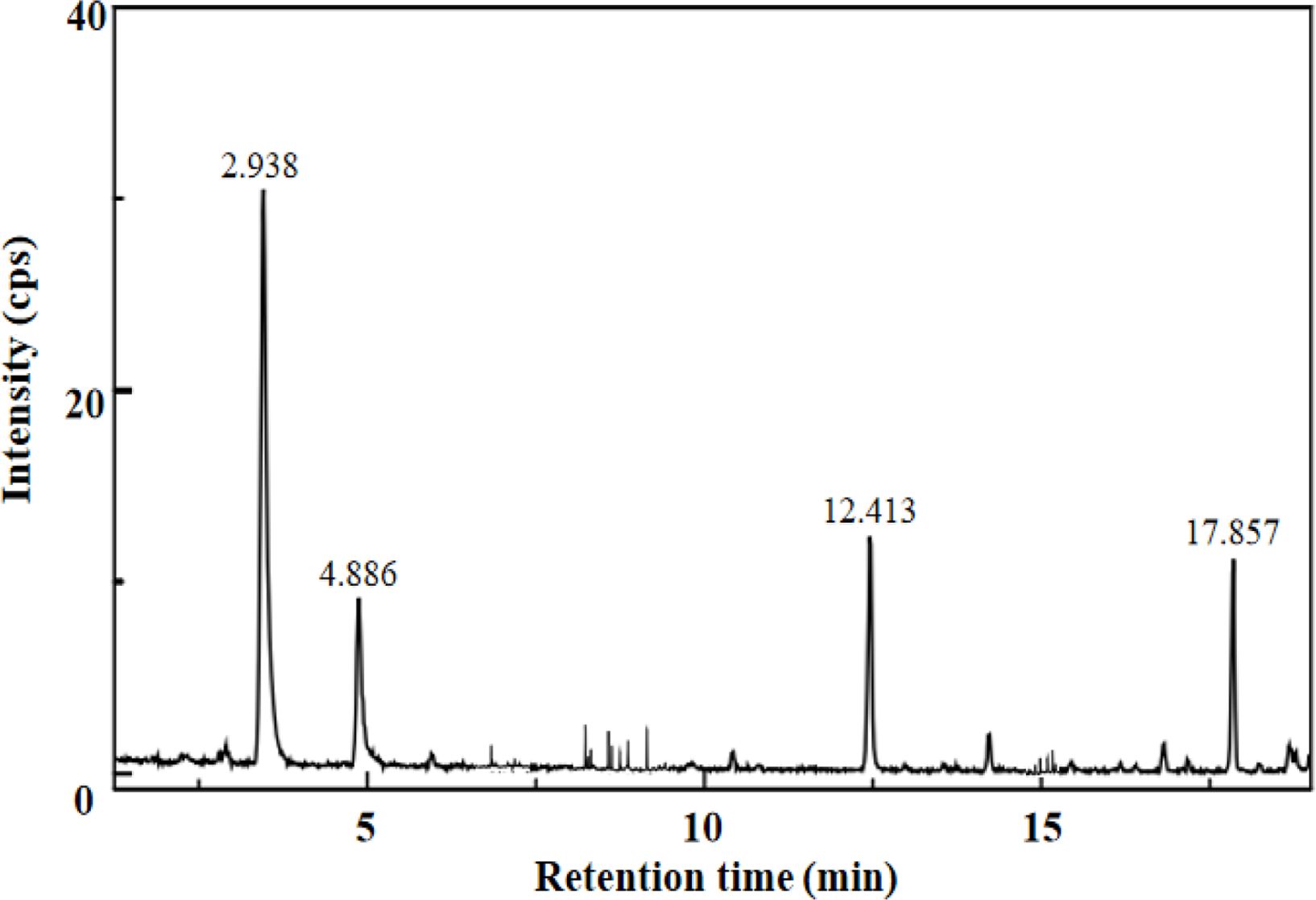
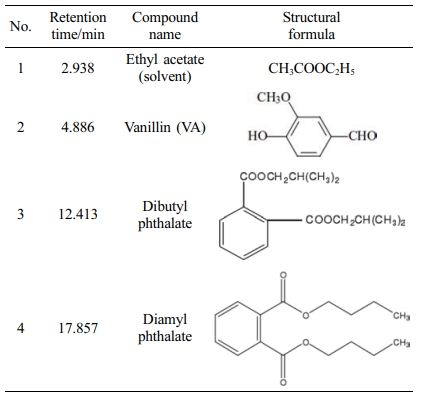
In order to conduct qualitative and quantitative analysis of the degradation products of LG by UV photocatalysis method, the reaction process under the optimal technological conditions obtained from the above orthogonal test was analyzed by GC-MS and HPLC.17 The obtained results were shown in Table 1 and Figure 1. It could be seen that the main products of LG degradation by UV photocatalysis method were VA, dibutyl phthalate and diamyl phthalate under the optimal technological conditions, and the products were relatively concent- rated, which indicated that the reaction had good selectivity to phenolic and aromatic aldehydes. The main mechanism of the reaction was that first the CO3+ oxide produced by CO2+/Ni2+ catalyst in the oxidizing environment would decay, while the Ni3+ oxide had good catalytic activity and long-term stability, and the CO-Ni oxide synergistic effect of the two would show good chemical stability and catalytic activity in the alkaline medium.18 Then, CO2+/Ni2+ catalysts seized the hydroxyl hydrogen atom of α carbon atom on LG molecule to form the carbonyl compound, which then activated β-O-4 bond, resulting in the cracking of C-O in LG molecule and the formation of VA and other aromatic aldehydes and aromatic esters.19 What’s more, the C-C bond of the branch chain of the basic unit of LG molecule was also catalytically broken. When α carbon atom lost electron and was oxidized, the enyl group in the molecule could resonate with the generated C+ ion, thus stabilizing the C+ ion, which was conducive to the rupture of the C-C bond, and finally being oxidized to VA.20
The structure and thermal properties between extracted VA and standard VA were characterized by NMR, FTIR, XRD, and TGA, which was showed Figure S2, S3 and Table S3 in Supporting Information. The results showed that the extracted VA were basically the same as the standard sample, and there was no significant difference.21-24
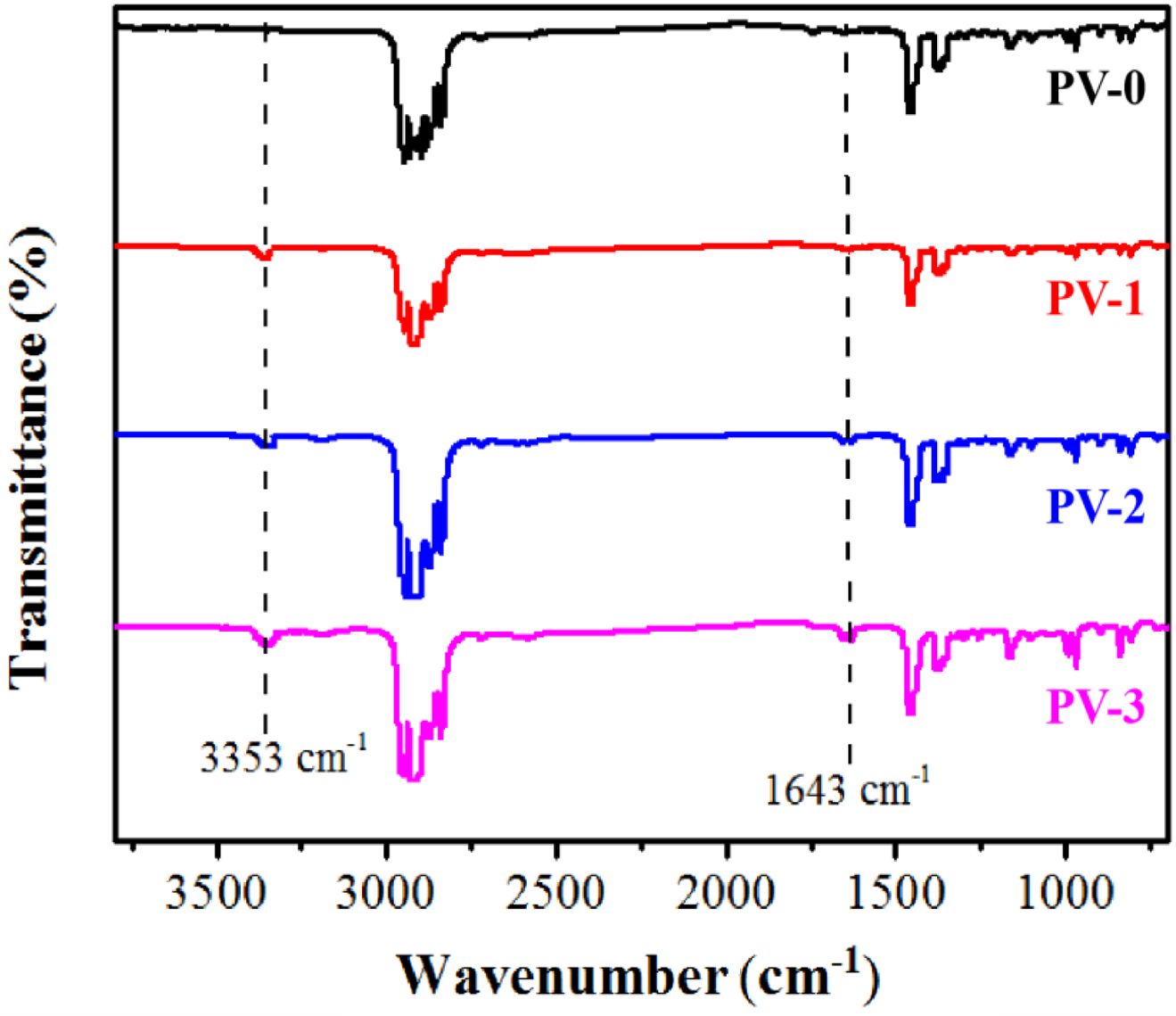
FTIR and Thermal Properties of PP/ VA Materials. The FTIR spectra of PP/VA materials was shown in Figure 2. It could be seen that the main change in the spectra of PV-0 material with VA antioxidant was the characteristic peak of hydroxyl group (-OH) at 3353 cm-1 and the stretching vibration of C=C bond of benzene ring in VA molecule at 1643 cm-1.22 The hydroxyl group (-OH) had high reactivity and was generally considered as the main group to improve the antioxidant capacity of polymers. The hydroxyl group (-OH) provided by VA could give PP/VA materials certain ability of capturing and scavenging free radicals, which could reduce the aging effect of hot oxygen, thus extending the service life.
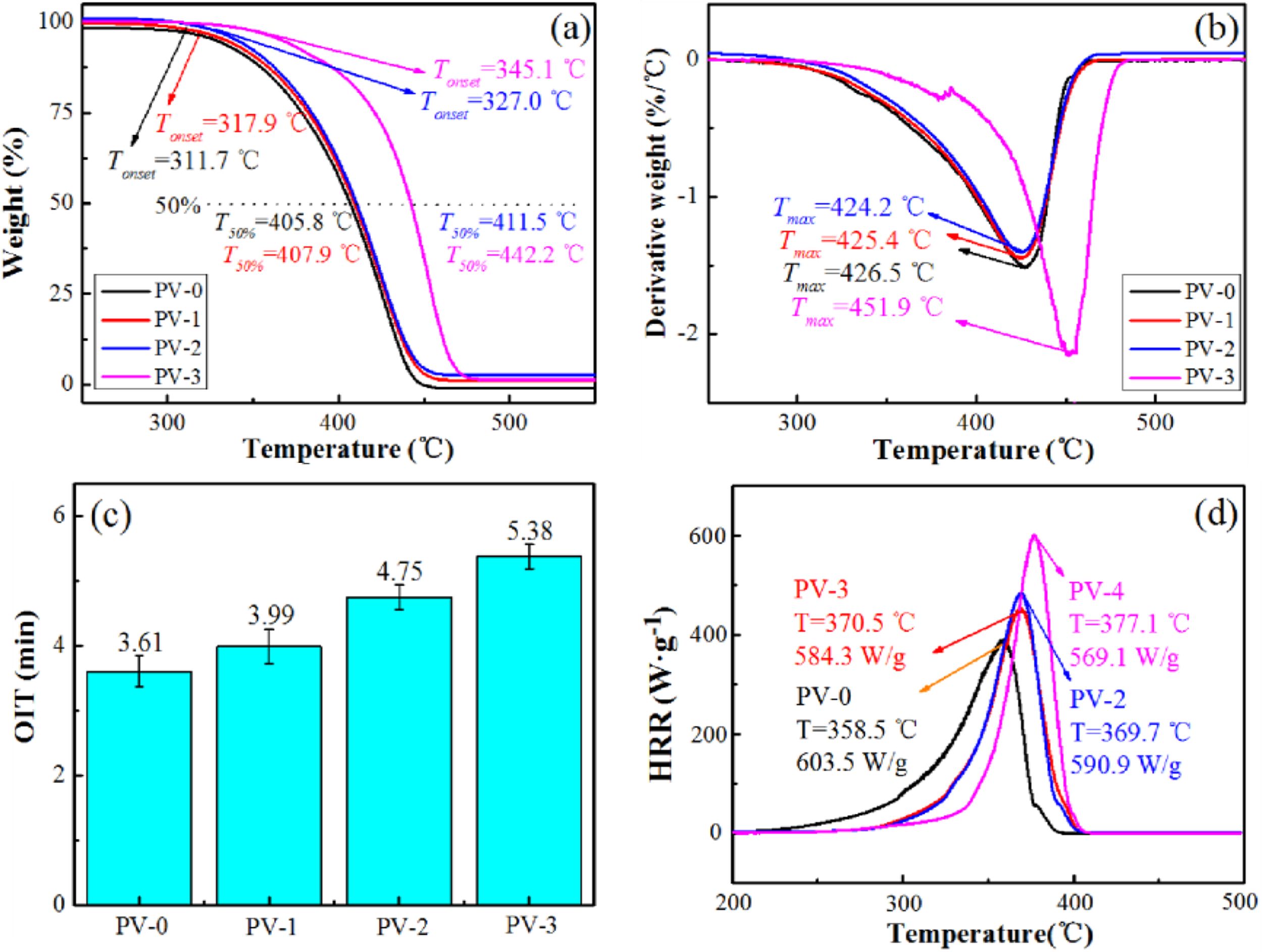
The thermal properties of PP/VA materials were shown in Figure 3. As shown in Figure 3(a) and 3(b), the initial temperature of main thermal decomposition stage (Tonset) and the maximum thermal decomposition rate temperature (Tmax) of PP/VA materials were measured by TGA. The Tonset of PP/VA material was slightly higher than that of pure PP (PV-0), and the increase of Tonset was not obvious when the VA addition amount was low. This would be due to the fact that VA had been partially decomposed by thermal oxygen aging in the processing and molding process, and the enhancement of structure activity in PP molecule was not obvious. However, the Tonset and Tmax of PV-3 were increased by 33.4 ℃ and 25.4 ℃, respectively, compared with PP (PV-0). This was because the molecular coupling and cooperation in the PP/VA materials were enhanced,25 forming a complex and interactive spatial network structure.26 The free movement of PP molecular chain segments was restricted, leading to the increase of temperature and energy required for thermal decomposition,27 which could effectively protect the weight loss of PP molecular chain degradation in the high temperature and oxygen-rich environment and delay the aging failure of PP to a certain extent, enhancing the antioxidant capacity of PP.
OIT refers to the time when materials begin to oxidize in high-temperature oxygen-rich environment, which is an index to characterize the antioxidant capacity of materials.28 Figure 3(c) exhibited the OIT of PP/VA materials. The OIT of PV-1, PV-2, and PV-3 increased by 10.53, 31.58 and 49.03%, respectively, compared with that of pure PP (PV-0). This was because VA as an antioxidant could reduce the side effects of oxygen, by capturing and neutralizing free radicals for reduction reaction to reduce the oxygen content, combined with the peroxide produced by the polymer oxidation reaction, interrupt the chain reaction, and then enhanced the antioxidant capacity of PP.29
Microcalorimetry is a testing that quantitatively characterizes the combustion behaviors of polymer materials, in which heat release rate (HRR) is the relatively important parameter.30 The HRR curves of PP/VA materials were shown in Figure 3(d). It could be seen that the HRR value of PV-0, PV-1, PV-2 and PV-3 was 603.5, 590.9, 584.3, and 569.1 W/g, respectively. What’s more, the little amount addition of VA (0.9 wt%) increased the temperature at maximum heat release from 358.5 to 377.1 ℃. This indicated that the introduction of VA improved the heat-resistant quality of PP/VA materials to a certain extent compared with that of pure PP. This result was consistent with the TGA and OIT.
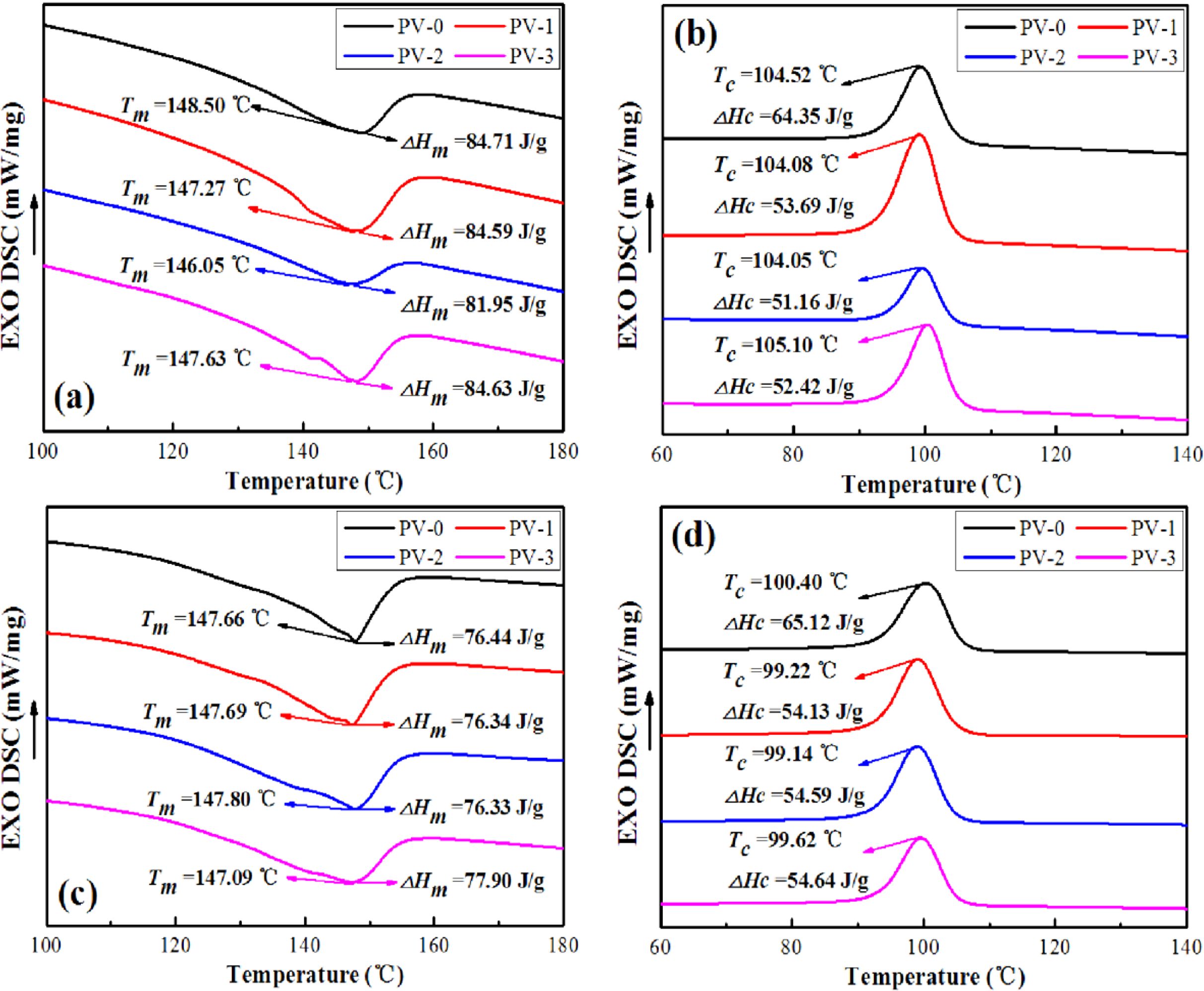
DSC Analysis of PP/VA Materials Before and After Aging. Differential scanning calorimetry (DSC) is a technology to measure the change of heat flow difference between sample and reference sample with temperature (time) at programmed temperature.31 The melting curves and crystalline curves of PP/VA materials before and after 20 days aging were shown in Figure 4, in which Tm was the measured melting temperature and Tc was the cold crystallization temperature. As shown in Figure 4, the addition of different doses of VA had little effect on the change of Tm and Tc of PP (PV-0), and the peak location was roughly between 145-150 ℃ and 100-110 ℃, respectively. This further proved that VA, as a natural antioxidant, had the potential to participate in the molding process of PP and would not affect the original basic properties of PP.
The crystallization degree (Xc) was determined from DSC analysis data employing eq. (1). Moreover, ΔHm, ΔHc, and f were the melting enthalpy, coldcrystallization enthalpy, and weight fraction of PP, respectively. The degree of crystallinity (Xc) of polymer could be calculated by using the equation given below:
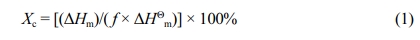
ΔHΘm was the melting enthalpy expected for a polymer with 100% crystallinity. The heat of fusion (ΔHΘm) of pure PP was 207 J/g.32 After calculation, the Xc of PV-0, PV-1, PV-2, and PV-3 before aging were 9.84, 14.97, 14.96, and 15.70%, respectively. This was because the addition of a small amount of VA played a role in heterogeneous nucleation of PP, which slightly improved the crystallinity of PP. After 20 days aging, the Xc of PV-0, PV-1, PV-2, and PV-3 were 5.47, 10.76, 10.57, and 11.34%, respectively, which decreased by 4.37, 4.21, 4.39, and 4.36% compared with those before aging, respectively. This was due to the entanglement and degradation of the molecular chains inside the material in the aging process, which resulted in the decrease of the crystallinity. The above analysis showed that VA had little effect on the molding temperature Tm and Tc of PP, and as a nucleating agent, it could slightly improve the crystallization properties of PP.33 The crystallization performance improved and the crystallinity of PV-3 after aging was the highest.
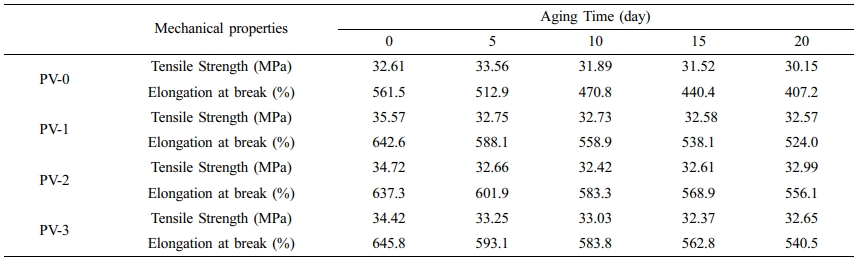
Mechanical Properties of PP/VA Materials Before and After Aging. Table 2 and Figure 5 showed the changes of mechanical properties of PP/VA materials at different times of thermal oxygen aging. As shown in Table 2, the tensile strength and elongation at break of PV-1, PV-2, and PV-3 before aging were increased by 9.1, 6.1, 5.6% and 14.4, 13.5, 15.0%, respectively, compared with PV-0. This was because the addition of a small amount of VA played the role of nucleating agent, which helped PP spherulites grow faster and denser through heterogeneous nucleation. Furthermore, the internal arrangement of PP molecules became regular and the degree of molecular orientation was improved, and the interaction force between molecules increased,34 thus enhancing the tensile strength and elongation at break of PP/VA materials, which was consistent with the results of DSC analysis. With the increase of aging time, the molecular chain of materials had different degrees of unwinding and degradation, leading to the decline of tensile strength and elongation at break.
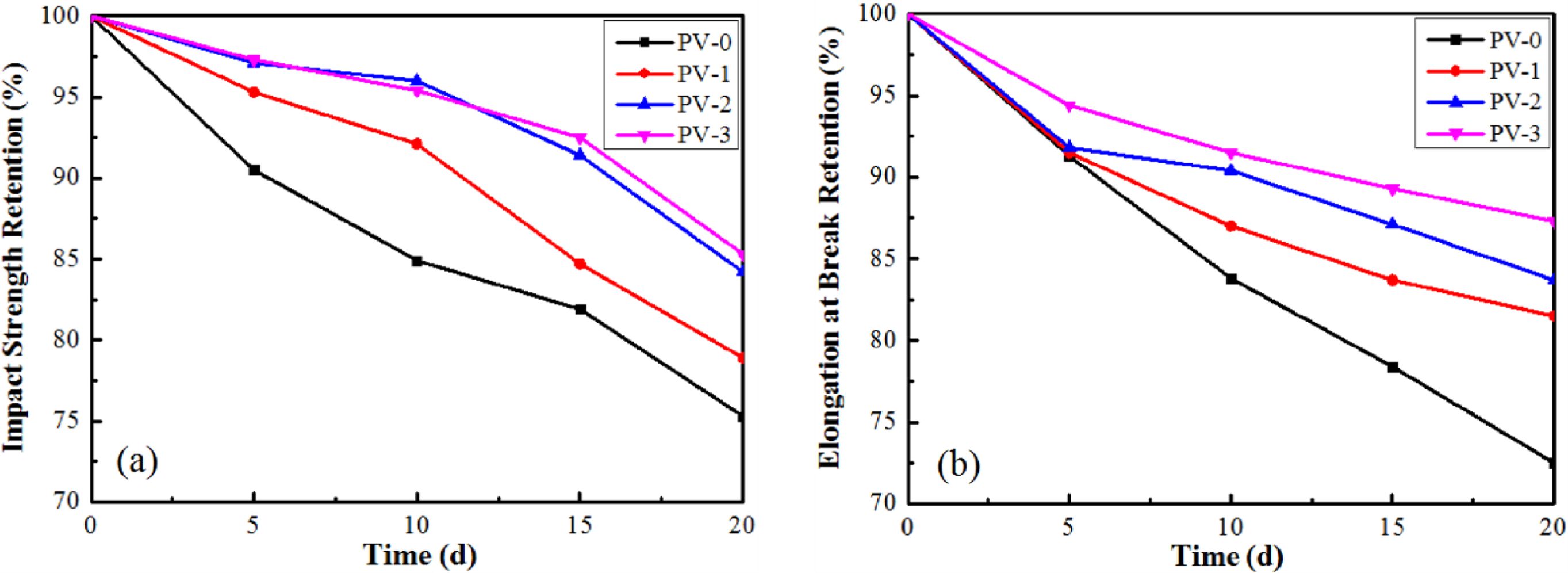
As shown in Figure 5, the impact strength retention and elongation at break retention were calculated compared with unaged samples, which were obtained by dividing the data obtained from the aged samples and the data obtained from the unaged samples. The retention of impact strength and elongation at break of PP/VA materials were both higher than that of PV-0 after aging, which proved that VA, as an antioxidant, could delay the thermal oxygen aging of PP and had good antioxidant effect. Among them, after 20 days thermal oxygen aging, the retention rate of impact strength and elongation at break of PV-3 were the highest, and the oxidation resistance was the best, which was consistent with the results of thermal performance analysis of PP/VA materials.
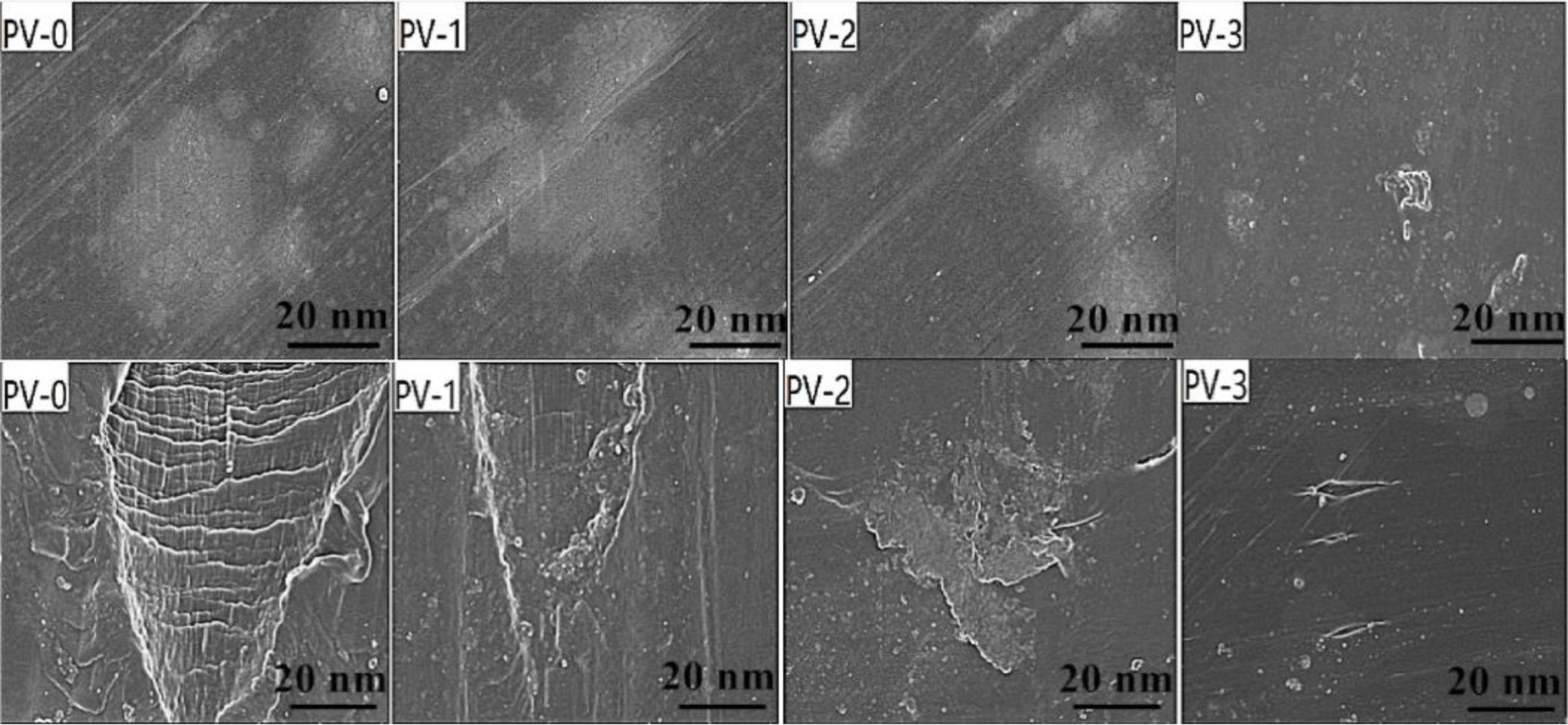
SEM of PP/VA Materials Before and After Aging. Figure 6 showed the SEM images of PP/VA materials before and after 20 days aging. As shown in Figure 6, the surface of PV-0 before aging was smooth without wrinkles. However, the surface appeared crisscross cracks and collapse after 20 days aging, which was the macroscopic expression of oxidative degradation of polymer. The mechanical properties of PV-0 with cracks decreased, and the elongation at break descended. While, the surface crack formation of PP with VA as antioxidant could be effectively reduced. After 20 days aging, the surface of PV-1 and PV-2 did not have obvious cracks, but uneven and ravines appeared to varying degrees. PV-3 only had epidermal cracking, and its surface became rough under the influence of temperature and oxygen factors. PV-3 also maintained a certain toughness after 20 days aging, so that the mechanical properties could be maintained in the hot oxygen environment for a longer time, which was consistent with the results of mechanical properties.
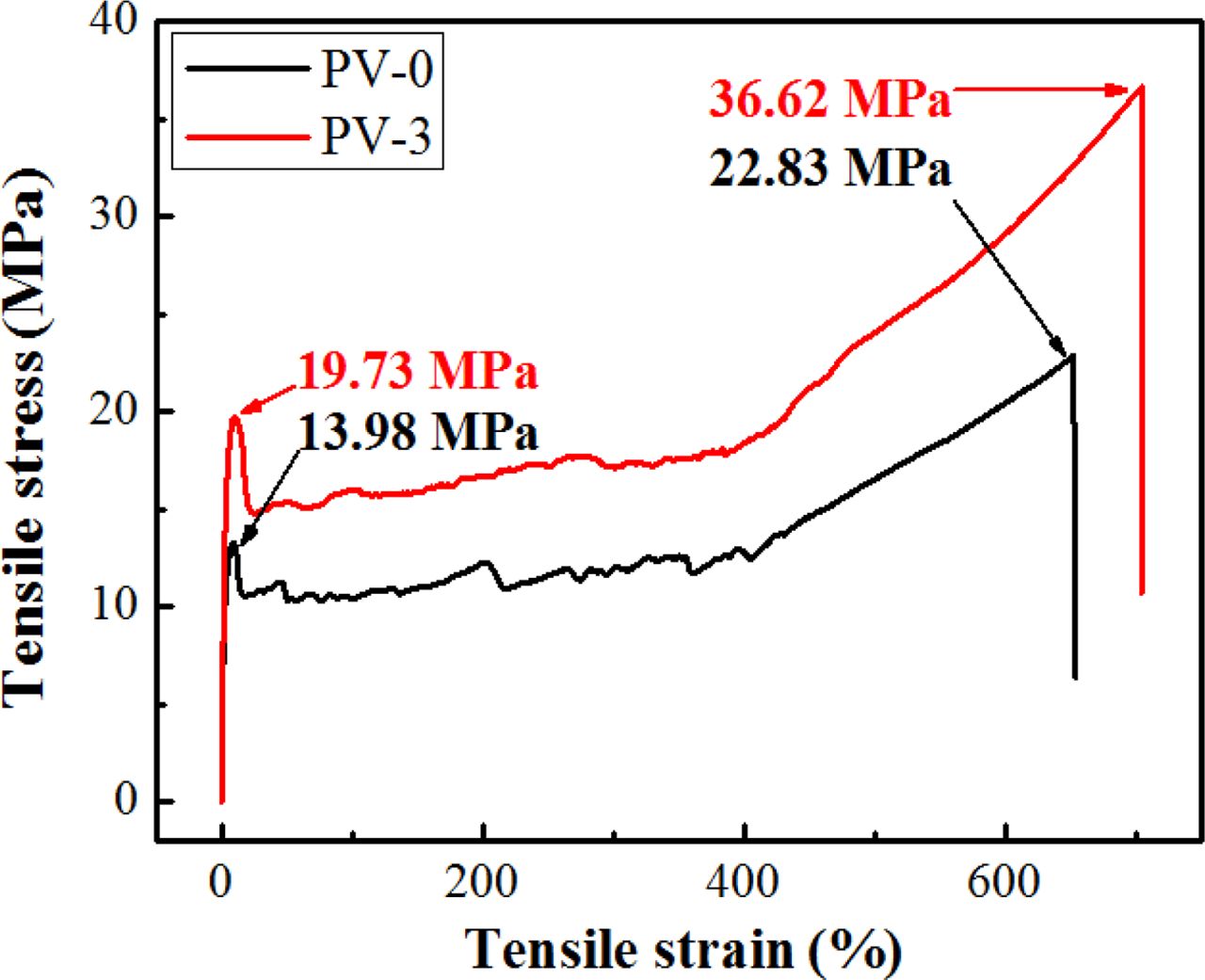
Blown Film Tensile Properties of PP/VA Materials. PV-0 and PV-3, which was of the best oxidation resistance after 20 days aging, were blown into film by blow molding. Figure 7 showed the tensile stress-strain curves of PV-0 and PV-3 blown film. It could be seen that the fracture mode of PV-0 and PV-3 membrane materials was typical ductile fracture, in which the yield strength, breaking strength and elongation at break of PV-0 were 13.98, 22.83 MPa and 652.06%, respectively. While the yield strength, breaking strength and elongation at break of PV-3 were 19.73, 36.62 MPa and 703.87%, respectively, which were all higher than that of PV-0. This was because the introduction of VA formed a large number of spherulites with finer diameter in the PP matrix, leading to a more perfect crystallinity of PP, increased molecular intermolecular force and improved molecular toughness and strength,35 which was manifested as the fortify of tensile properties of PV-3 blown film. The above analysis was consistent with DSC and mechanical properties analysis.
|
Figure 1 The HPLC spectrum of UV photocatalysis degradation of LG under the optimal technological conditions. |
|
Figure 2 FTIR spectra of PP/VA materials. |
|
Figure 3 The thermal properties of PP/VA materials: (a) TG; (b) DTG; (c) OIT; (d) HRR. |
|
Figure 4 DSC curves of PP/VA before and after 20 days aging: (a) melting curves before aging; (b) crystalline curves before aging; (c) melting curves after aging; (d) crystalline curves after aging |
|
Figure 5 Mechanical properties of PP/VA materials after different aging time: (a) impact strength retention; (b) elongation at break retention |
|
Figure 6 SEM images of PP/VA before and after 20 days aging. |
|
Figure 7 Tensile stress-strain curves of PV-0 and PV-3 blown film. |
|
Table 1 The GC-MS of Main Products After UV Photocatalysis Degradation Under the Optimal Technological Conditions |
In the work, VA was extracted from cotton stalk LG by UV photocatalysis method using Co2+/Ni2+ as a catalyst, the yield of which was quantitative analyzed through GC-MS and HPLC. The orthogonal analysis results showed the optimum reaction conditions with the yield of 84.01% to be as followed: the reaction temperature of 55 ℃, the UV lamp wattage of 1000 W, the reaction time of 4 hours, and the catalyst mass ratio of 15 wt%. The structure and thermal properties of extracted and standard VA were studied by NMR, FTIR, XRD and TGA, which showed basically consistence. This demonstrated this method of UV photocatalysis using Co2+/Ni2+ as a catalyst had high selectivity and yield. The antioxidant capacity and mechanical properties of PP/VA materials were investigated. The results showed that the crystallinity of PP was improved due to the introduction of VA, thus enhancing the antioxidant capacity and mechanical properties of PP. After 20 days thermal oxygen aging, PV-3 (0.9 wt%) had the best performance in crystallinity, the mechanical properties retention and surface morphology, which still represented well in tensile properties after blowing molding into film, thus broadening the application range of PP membrane materials. This study provided a simple, environmentally friendly and high selectivity extraction method of VA with excellent yield; it also showed a new choice of natural and harmless antioxidant for improving the antioxidant capacity of PP. Moreover, it was of great significance to the recycling of bio-based resources and the sustainable development of natural environment.
- 1. Hasegawa, N.; Okamoto, H.; Kato, M.; Usuki, A. Preparation and Mechanical Properties of Polypropylene-clay Hybrids Based on Modified Polypropylene and Organophilic Clay. J. Appl. Polym. Sci. 2015, 78, 1918-1922.
- 2. Leong, Y. W.; Bakar, M. B. A.; Ishak, Z. A. M.; Ariffin, A. Characterization of Talc/calcium Carbonate Filled Polypropylene Hybrid Composites Weathered in a Natural Environment. Polym. Degrad. Stabil. 2004, 83, 411-422.
-

- 3. Wims, A. M.; Swarin, S. J. Determination of Antioxidants in Polypropylene by Liquid Chromatography. J. Appl. Polym. Sci. 2010, 19, 1243-1256.
-

- 4. Ji, Y.; Kim, J.; Bae, J. Y. Flame-retardant ABS Resins from Novel Phenyl Isocyanate Blocked Novolac Phenols and Triphenyl Phosphate. J. Appl. Polym. Sci. 2010, 102, 721-728.
-

- 5. Zhang, J. H.; Zhang, H.; Wang, S. T.; Liu, M. J. Antioxidant-loaded Carbon Nanotube to Sustain a Long-term Aging-protection for Acrylonitrile-butadiene Rubber. Polym. Degrad. Stabil. 2017, 144, 93-99.
-

- 6. Nanni, A.; Messori, M. A Comparative Study of Different Winemaking By-products Derived Additives on Oxidation Stability, Mechanical and Thermal Proprieties of Polypropylene. Polym. Degrad. Stabil. 2018, 149, 9-18.
-

- 7. Nanni, A.; Battegazzore, D.; Frache, A.; Messori, M. Thermal and UV Aging of Polypropylene Stabilized by Wine Seeds Wastes and Their Extracts. Polym. Degrad. Stabil. 2019, 165, 49-59.
-

- 8. Zhang, L. B.; Tan, J. Y.; Xing, G. Y.; Dong, X. T.; Guo, X. Q. Cotton Stalk-derived Hydrothermal Carbon for Methylene Blue Dye Removal: Investigation of the Raw Material Plant Tissues. Bioresour. Bioprocess. 2021, 8, 10.
-

- 9. Malik, K.; Salama, E. S.; El-Dalatony, M. M.; Jalalah, M.; Harraz, F. A.; Al-Assiri, M. S.; Zheng, Y. Z.; Sharma, P.; Li, X. K. Co-fermentation of Immobilized Yeasts Boosted Bioethanol Production from Pretreated Cotton Stalk Lignocellulosic Biomass: Long-term Investigation. Ind. Crop. Prod. 2020, 159, 113122-113131.
-

- 10. He, M. Y.; Musajian, D.; Hasan, G.; Hou, G. B.; Yimit, M. Effects of Coupling Agent on Antioxidant Properties and Structure of PP/Cotton Stalk Lignin Composites. Pol. J. Chem. Technol. 2020, 22, 78-85.
-

- 11. Hasan, G.; Musajan, D.; Hou, G. B.; He, M. Y.; Li, Y.; Yimit, M. Role of Different Lignin Systems in Polymers: Mechanical Properties and Thermal Stability. Pol. J. Chem. Technol. 2020, 22, 10-16.
-

- 12. Walton, K. N.; Mayer, J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505-515.
-

- 13. Bagheri-Kalmarzi, M.; Sajedi, R. H.; Asadollahi, E.; Mahmoodi, N. O.; Hajihosseini, R. Effect of Vanillin and its Acid and Alcohol Derivatives on the Diphenolase Activity of Mushroom Tyrosinase. Mol. Biol. Res. Commun. 2012, 1, 74-82.
-

- 14. Kansal, S. K.; Singh, M.; Sud, D. Studies on TiO2/ZnO Photocatalysed Degradation of Lignin. J. Hazard. Mater. 2008, 153, 412-417.
-

- 15. Erdocia, X.; Prado, R.; Corcuera, M. A.; Labidi, J. Base Catalyzed Depolymerization of Lignin: Influence of Organosolv Lignin Nature. Biomass Bioenerg. 2014, 66, 379-386.
-

- 16. Musajian, D.; Hasan, G.; He, M. Y.; Yimit, M. Free Radical Scavenging Ability of Sodium Lignosulfonate and its Application in Food Grade Polypropylene. Pol. J. Chem. Technol. 2020, 22, 56-66.
-

- 17. Heinz, L. C.; Pasch, H. High-temperature Gradient HPLC for the Separation of Polyethylene-polypropylene Blends. Polymer. 2005, 46, 12040-12045.
-

- 18. Yuan, J. C.; Silva, L. C.; Gomes, P. T.; Valerga, P.; Campos, J. M.; Ribeiro, M. R.; Chien, J. C. W.; Marques, M. M. Living and Block Polymerization of a-olefins using a Ni(II)-a-diimine Catalyst Containing OSiPh2tBu Groups. Polymer. 2005, 46, 2122-2132.
-

- 19. Yue, X. K.; Zhang, S.; Shang, N. Z.; Gao, S. T.; Wang, Z.; Wang, C. Porous Organic Polymer Supported PdAg Bimetallic Catalyst for the Hydrodeoxygenation of Lignin-derived Species. Renew. Energ. 2020, 149, 600-608.
-

- 20. Jeon, W. J.; Choi, I. H.; Park, J. Y.; Lee, J. S.; Hwang, K. R. Alkaline Wet Oxidation of Lignin Over Cu-Mn Mixed Oxide Catalysts for Production of Vanillin. Catal. Today. 2019, 37, 352-362.
-

- 21. Brisa, P.; Louis-Charles, D. M.; Ricard, G. V.; Tania, G. Characterization of Polysulfone and Polysulfone/Vanillin Microcapsules by 1H NMR Spectroscopy, Solid-State 13C CP/MAS-NMR Spectroscopy, and N2 Adsorption-Desorption. Acs. Appl. Mater. Inter. 2011, 3, 4420-4430.
-

- 22. Tang, B.; Yue, T. X.; Wu, J. S.; Dong, Y. M.; Ding, Y.; Wang, H. J. Rapid and Sensitive Spectrofluorimetric Determination of Trace Amount of Cr(III) with o-vanillin-8-aminoquinoline. Talanta. 2004, 64, 955-960.
-

- 23. Liu, G. F.; Shi, T. S.; Zhao, Y. N. Infrared and Raman Spectra of Complexes About Rare Earth Nitrate with Schiff Base from o-vanillin and 1-naphthylamine. J. Mol. Struct. 1997, 412, 75-81.
-

- 24. Ando, D.; Nakatsubo, F.; Yano, H. Thermal Stability of Lignin in Ground Pulp (GP) and the Effect of Lignin Modification on GP's Thermal Stability: TGA Experiments with Dimeric Lignin Model Compounds and Milled Wood Lignins. Holzforschung. 2019, 73, 493-499.
-

- 25. Karathanos, V. T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N. K. Study of the Solubility, Antioxidant Activity and Structure of Inclusion Complex of Vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652-658.
-

- 26. Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of Antioxidant Activity of Vanillin by using multiple antioxidant assays. BBA-Gen Subjects 2011, 1810, 170-177.
-

- 27. Xue, Y.; Guan, Y.; Zheng, A.; Wang, H.; Xiao, H. Synthesis and Characterization of Ciprofloxacin Pendant Antibacterial Cationic Polymers. J. Biomat. Sci-Polym E 2012, 23, 1115-1128.
-

- 28. Viebke, J.; Gedde, U. W. Antioxidant Diffusion in Polyethylene Hot-water Pipes. Polym. Eng. Sci. 2010, 37, 896-911.
-

- 29. Fearon, P. K.; Whiteman, D. J.; Billingham, N. C.; Bigger, S. W. The Second Time Derivative Analysis of Chemiluminescence Emission Profiles and its Application to the Accurate Determination of Oxidative Induction Times. J. Appl. Polym. Sci. 2015, 79, 1986-1993.
-

- 30. Zhang, H.; Zhen, W. J. Performance, Rheological Behavior and Enzymatic Degradation of Poly(lactic acid)/modified Fulvic Acid Composites. Int. J. Biol. Macromol. 2019, 139, 181-190.
-

- 31. Papadopoulos, L.; Klonos, P. A.; Terzopoulou, Z.; Psochia, Z.; Bikiaris, D. N. Comparative Study of Crystallization, Semicrystalline Morphology, and Molecular Mobility in Nanocomposites based on Polylactide and Various Inclusions at Low Filler Loadings. Polymer. 2021, 217, 123457-123467.
-

- 32. Yang, L.; Zhen, W. J. Preparation and Characterization of Phosphorylated Graphene Oxide Grafted with Poly(L-lactide) and its Effect on the Crystallization, Rheological Behavior, and Performance of Poly(lactic acid). Polym. Advan. Technol. 2019, 30, 1-14.
-

- 33. Odalanowska, M.; Borysiak, S. Influence of Wood Thermal Modification on the Supermolecular Structure of Polypropylene Composites. Polym. Composite. 2021, 58, 478-490.
-

- 34. Feng, Y.; Jin, X.; Hay, J. N. Effect of Nucleating Agent Addition on Crystallization of Isotactic Polypropylene. J. Appl. Polym. Sci. 2015, 69, 2089-2095.
-

- 35. Li, J.; Chen, D. K.; Gui, B. Z.; Gu, M. H.; Ren, J. Crystallization Morphology and Crystallization Kinetics of Poly(lactic-acid): Effect of N-Aminophthalimide as Nucleating Agent. Polym. Bull. 2011, 67, 775-791.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(5): 717-726
Published online Sep 25, 2021
- 10.7317/pk.2021.45.5.717
- Received on Mar 26, 2021
- Revised on Jun 15, 2021
- Accepted on Jun 21, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Mamatjan Yimit
-
Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education and Xinjiang Uyghur Autonomous Region, College of Chemical Engineering, Xinjiang University, Urumchi 830046, China
- E-mail: mjan10@xju.edu.cn
- ORCID:
0000-0001-5260-3194










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.