- Reproducible Green Radiation-based Synthesis of Poly(glycidyl methacrylate)-Grafted Non-woven Polypropylene Adsorbent for Removal of a Heavy Metal Ion
In-Tae Hwang*, Tae-Yeon Kim*, **, Joon-Yong Sohn*, Kwanwoo Shin**, Junhwa Shin*, and Chan-Hee Jung*,†

*Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup, Jeollabuk-do 56212, Korea
**Department of Chemistry, Sogang University, Mapo-gu, Seoul 04107, Korea- 중금속 이온 제거를 위한 재현성 있는 친환경 방사선 기반의 폴리글리시딜메타크릴레이트 접목 폴리프로필렌 부직포 흡착제의 합성
*한국원자력연구원 첨단방사선연구소, **서강대학교 화학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this study, we demonstrated that well-defined poly(glycidyl methacrylate)-grafted non-woven polypropylene (PP-g-PGMA) can be reproducibly achieved by pre-irradiation at the current-adjusted higher dose rate, and further used as an efficient heavy metal adsorbent by functionalization using ethylenediamine (EDA). The results of grafting degree and conversion rate revealed that the desired degree of reproducible grafting of PP-g-PGMA (while maintaining the conversion rate above 90%) can be achieved under the optimized conditions. The successful synthesis of PP-g-PGMA was clearly confirmed using Fourier transform infrared spectrometer spectroscopy (FTIR), thermogravimetric analysis (TGA), and field emission scanning electron microscopy (FE-SEM). The PP-g-PGMA-EDA adsorbents produced by the amine reaction exhibited grafting degree-dependent amine density ranging from 2.8 to 3.5 mmol/g. It also had good tensile properties suitable for application to metal ion adsorption. Noticeably, the PP-g-PGMA-EDA adsorbent exhibited better Cu2+ ion-adsorbing performance than did the commercial resin.
본 연구에서는 전류로 조정된 높은 선량율에서의 전자선 전조사 및 접목중합 반응을 통해 poly(glycidyl methacrylate) 접목 polypropylene 부직포(PP-g-PGMA)를 높은 재현성으로 제조하고 이를 ethylenediamine(EDA)을 이용한 기능화를 통해 효율적인 중금속 흡착제로 적용해 보고자 하였다. 접목중합 수율 및 전환율 평가 결과, PP-g-PGMA의 제조에 있어 최적조건에서 90% 이상의 높은 전환율을 유지함으로써 높은 재현성을 보이며 원하는 접목수율을 얻을 수 있었다. Fourier transform infrared spectrometer spectroscopy(FTIR), thermogravimetric analysis(TGA) 및 field emission scanning electron microscopy(FE-SEM) 분석을 통해 PP-g-PGMA가 성공적으로 합성되었음을 명확히 확인하였다. 아민 반응에 의해 제조된 PP-g-PGMA-EDA 흡착제는 접목중합 수율에 의존적인 2.8에서 3.5 mmol/g 범위의 아민 밀도를 나타내었고 금속이온 흡착제에 적용하기에 적합한 인장특성을 나타내었다. 특히, 제조된 PP-g-PGMA-EDA 흡착제는 상용 수지보다 더욱 우수한 Cu2+ 이온 흡착 성능을 보였다.
A PP-g-PGMA-EDA was successfully prepared from poly(glycidyl methacrylate) (PGMA)-grafted non-woven polypropylene (PP), using controlled radiation-induced emulsion graft polymerization (RIEGP) and the amine reaction with ethylenediamine (EDA). Moreover, it was demonstrated that the resulting PP-g-PGMA-EDA serves well as an efficient adsorbent for the removal of hazardous heavy metal ions.
Keywords: polypropylene nonwovens, radiation emulsion graft polymerization, glycidyl methacrylate, copper ion, adsorption.
This work was supported by the Basic Research Program of the Korea Atomic Energy Research Institute Grant funded by the Korean government.
Heavy metal pollutants in wastewaters have become of global concern due to their persistence in nature and their harmful effect on human beings and animals.1-3 Therefore, the removal of metal ions from wastewater is a critical environmental issue to be resolved.4,5 Commonly, a variety of technologies including adsorption, membrane separation, ion ex- change, precipitation, and reverse osmosis have been applied for the removal of metal ions.6,7 Among these options, the adsorption method has demonstrated an effective process in terms of cost-effectiveness, productivity, and recyclability.8-10
To achieve high-performance adsorption, non-woven, polymer-fabric-based adsorbents have been widely explored because they have a variety of desirable features that include chemical inertness, good mechanical properties, and low cost.11-13 Noticeably, their unique features of both high adsorption efficiency and liquid flux capacity (stemming from their large surface area with high-density surface functional groups) make them better than other types of materials (activated carbons, chelating resins, etc.).14,15 This non-woven polymer fabric-based adsorbent has been mainly produced using heat, plasma, UV, and other high energy radiation-induced surface polymerization and post functionalization.16-19 However, there has still been a high demand for more efficient and environmentally friendly surface polymerization in the industrial area.
In this respect, radiation-induced emulsion graft polymerization (RIEGP) (consisting of pre-irradiation of non-woven fabrics and graft polymerization in monomer micelle-containing emulsion solution in water) has been considered a promising option.16,20 This strategy offers a lower absorbed dose, a lower monomer concentration, and a shorter reaction duration in comparison to conventional radiation-induced graft polymerization.21,22 This is because monomer micelles allow provision of a high concentration of monomer at radical-active sites and prevent such side reactions as homo-polymerization.21 Due to these distinct merits, the functionalization of non-woven fabric has been much studied using RIEGP to produce a precursor for the metal adsorbents.21-26 However, although the usefulness of RIEGP has been proved in numerous studies, there is a lack of reliability and reproducibility in RIEGP due to difficulty in controlling the constant formation of radical initiators in polymer fabrics caused by irradiation.21,27 Therefore, there is still great demand for more reliable and reproducible processing conditions for highly efficient RIEGP with a higher monomer-to-grafted polymer conversion rate of 90% to constantly achieve the desirable grafting degree for the application.21
In this study, a reproducible preparation of poly(glycidyl methacrylate)-grafted non-woven polypropylene (PP) (PP-g-PGMA) was investigated using controlled electron-beam irradiation-induced emulsion graft polymerization. Ethylene diamine-functionalized PP-g-PGMA (PP-g-PGMA-EDA) was further demonstrated to serve as a highly efficient metal ion adsorbent. Glycidyl methacrylate (GMA) was selected as the monomer because of its aptness for the formation of micelles in water and epoxy group providing versatile chemistry to render the desirable functionality for the metal ions.28-32 To establish the relationship between the processing parameters, grafting degree, and conversion rate for the controlled RIEGP, the emulsion graft polymerization of GMA on the PP (at a certain monomer concentration with a given reaction time) was executed under different conditions of dose rate, absorbed dose, and temperature. The resulting PP-g-PGMA was chemically functionalized via amine reaction to produce PP-g-PGMA-EDA adsorbent. Finally, the adsorption performance of PP-g-PGMA-EDA was evaluated to demonstrate the feasibility of its use as a metal ion adsorbent.
Materials. The polypropylene (PP) non-woven fabric (30 g/m2) with a thickness of 190 μm and pore size of 10 μm used as a substrate for RIEGP was obtained from Absfil Co. (Korea). The glycidyl methacrylate (GMA, 97%) as the monomer, polyoxyethylene sorbitan monooleate (Tween 80, Tw-80) as the surfactant, ethylenediamine (EDA, 99%) as the amination reagent, and copper(II) sulfate (CuSO4) used in the adsorption experiments were purchased from Sigma Aldrich Co. (USA). Polystyrene-divinylbenzene-based AW90 resin (particle size 500-600 µm, amine functionality of 2.34 mmol/g, Samyang Co., Korea) was used as a reference comparative adsorbent. All the other solvents were purchased from Samchun Co. (Korea). All the chemicals were used without further purification.
Preparation of PP-g-PGMA by Electron Beam Irradiation-induced Emulsion Graft Polymerization. To generate the radicals by electron beam pre-irradiation as initiators, well-rolled PP non-woven fabrics with the dimensions 45×5 cm2 were put into 50 mL glass vials and sealed completely. After 10 min purging with nitrogen gas, the vials were irradiated using the ELV-8 electron beam accelerator at the Advanced Radiation Technology Institute (ARTI) of the Korea Atomic Energy Research Institute (KAERI). The energy of the electron beams was fixed at 2.5 MeV. Dose rates of 20, 40, and 60 kGy/s were controlled by adjusting the beam current to 10, 20, and 30 mA at the fixed conveyor speed of 10 m/min; or by adjusting the conveyor speed to 3, 5, and 10 m/min at the fixed beam current of 10 mA, respectively. The total adsorbed dose was 30, 60, 90, and 120 kGy. To avoid the thermal effect on the samples during irradiation, a sample stage was used as a cooling plate and kept at 5 °C. The cellulose triacetate dosimetry was carried out following ISO/ASTM 51650, and the uncertainty of the doses was less than 5%. For emulsion graft polymerization, GMA emulsion solution ranging from 2.5 to 6 wt% was prepared by mixing GMA, Tw-80, and deionized water at the weight ratio of 10 GMA:1 Tw-80:89 water. After homogenizing for 20 min, the GMA emulsion solution was de-aerated with nitrogen gas for 30 min. A consistent portion (30 mL) of GMA solution was transferred to cover completely the PP non-woven fabrics in 50 mL irradiation vials, without an excess solution. The emulsion graft polymerizations were carried out by placing the vials in a temperature-constant water bath at various temperatures (from 25 to 60 °C) for various reaction times ranging from 10 to 120 min. The organic solvent graft polymerization was carried out in methanol for a comparative study between RIGP and RIEGP at the 6 wt% of GMA concentration. After the graft polymerization was done, the product was taken out of the vials, washed repeatedly with methanol and tetrahydrofuran, and finally dried in a vacuum oven for 24 h. The grafting degree and conversion rate of the PP-g-PGMAs were determined by the following equations:
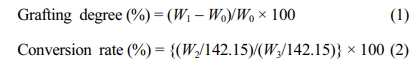
Where W0, W1, W2, and W3 are the weight of the control PP, PP-g-PGMA, grafted PGMA, and the GMA monomer used, respectively. The molecular weight of the GMA was 142.15. The samples were named in the form PP-g-PGMA-X, where X stands for the grafting degree.
Preparation of PP-g-PGMA-EDA Adsorbents. To introduce amine functional groups, 1.7 g of PP-g-PGMA was put into a 100 mL vial containing 10 wt% EDA solution in dimethylsulfoxide. The reaction was carried out in a temperature-constant water bath at 80 ℃ for 120 min. After the reaction, the PP-g-PGMA-EDA product was taken out of the vials, washed repeatedly with methanol and tetrahydrofuran, and finally dried in a vacuum oven for 24 h. The respective conversion rate and amine group density were calculated as follows:
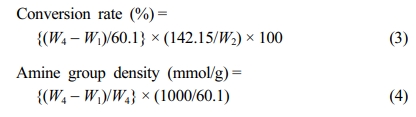
Where W1 and W4 are the respective weights of PP-g-PGMA and PP-g-PGMA-EDA, and 60.1 is the molecular weight of the EDA. The samples were named in the form PP-g-PGMA-X, where X stands for the amine group density.
Cu2+ Ion Adsorption Test. The Cu2+ ion adsorption performance of the PP-g-PGMA-EDA adsorbent was quantified through a batch-type adsorption test. PP-g-PGMA-EDA adsorbent (0.5 g) was put into 100 mL vials containing 70 mL of aqueous CuSO4 solution (pH 5) at different initial concentrations ranging from 100 to 1000 ppm. These were stirred for 20 h at ambient temperature. The Cu2+ ion concentration before and after the adsorption was measured using an inductively coupled plasma mass spectrometer (ICP-MS, 7500, Agilent, USA). The amount of Cu2+ ions adsorbed by the adsorbent was calculated using the following equation:
Where Co and Cf are the concentration (ppm) of the Cu2+ ion before and after the adsorption, respectively, V is the volume of the solution (mL), and W is the weight of the PP-g-PGMA-EDA. For comparison, an adsorption test using commercially available AW90 resin was performed under the same conditions.
To compare the Cu2+ ion adsorption performance between the PP-g-PGMA-EDA-3.5 and AW90 resin with immersion time, a batch-type adsorption test was performed. The 0.38 g of PP-g-PGMA-EDA and AW90 resin were immersed in 70 mL of 10 ppm Cu2+ ion solution (pH 5) and stirred at 30 ℃. After the immersion times of 5, 10, 20, 30, 60, and 120 min, each solution was taken, passed through a 0.2 mm filter, and the remaining Cu2+ ion concentration was measured using an ICP-MS. The Cu2+ removal rate was calculated using the following equation:
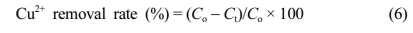
Where Co is the initial ion concentration and Ct is the ion concentration of the solution at each immersion time.
Characterization. The chemical structure was investigated using an Attenuated total reflectance Fourier transform infrared spectrometer spectroscopy (ATR FTIR, Varian 640, USA) equipped with an ATR accessory containing a ZnSe crystal. A thermogravimetric analysis (TGA) was performed using a Q500 analyzer (TA Instrument, USA) under nitrogen atmosphere at a heating rate of 10 ℃/min in the range of 50-600 ℃. High resolution field emission scanning electron microscopy (FE-SEM) was carried out using an analytical HR-SEM (SU-70, Hitachi, Japan). Tensile properties (tensile strength and elongation-at-break) were measured by using a universal testing machine (UTM, EZ-SX, Shimadzu, Japan) according to the ASTM standard D638.
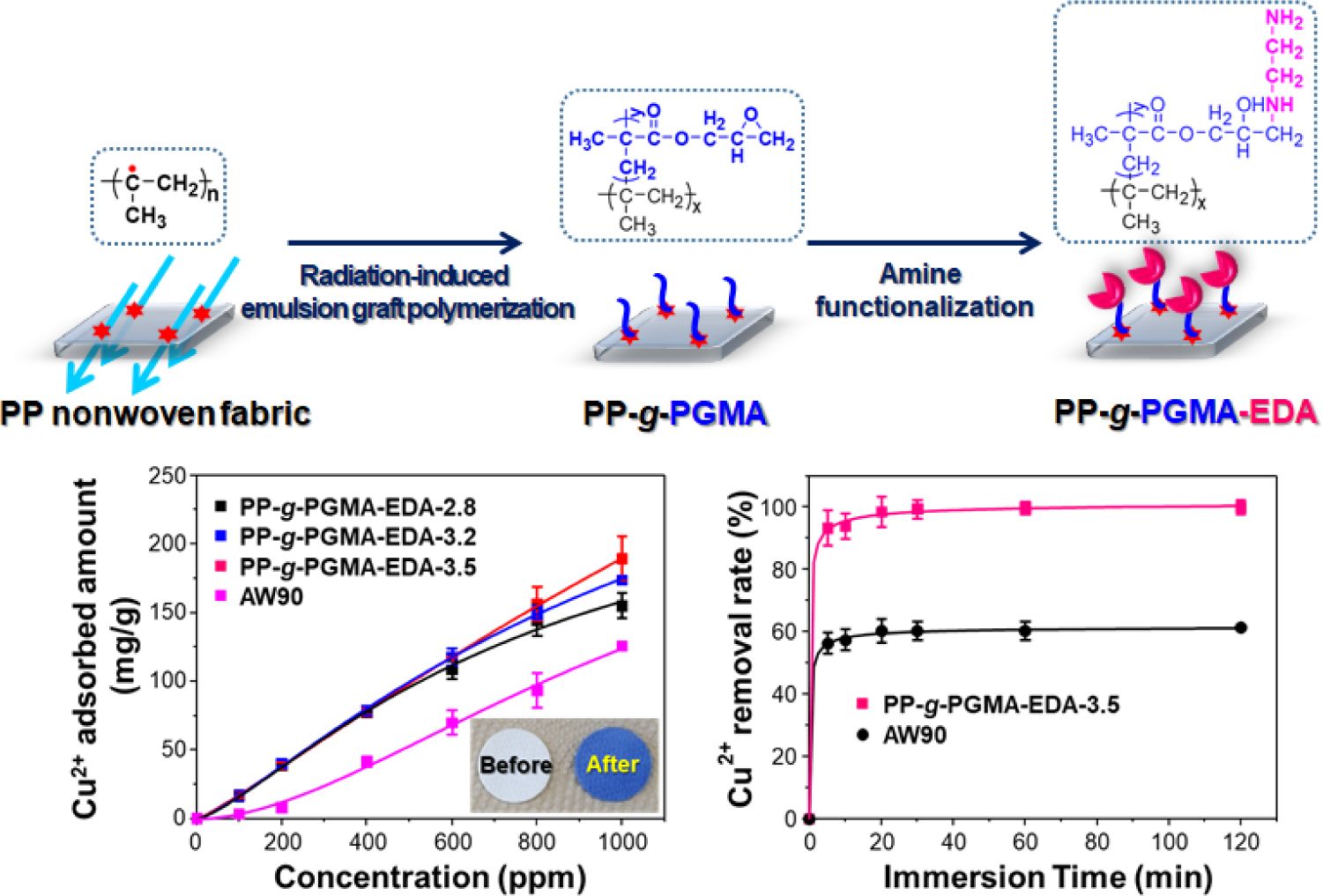
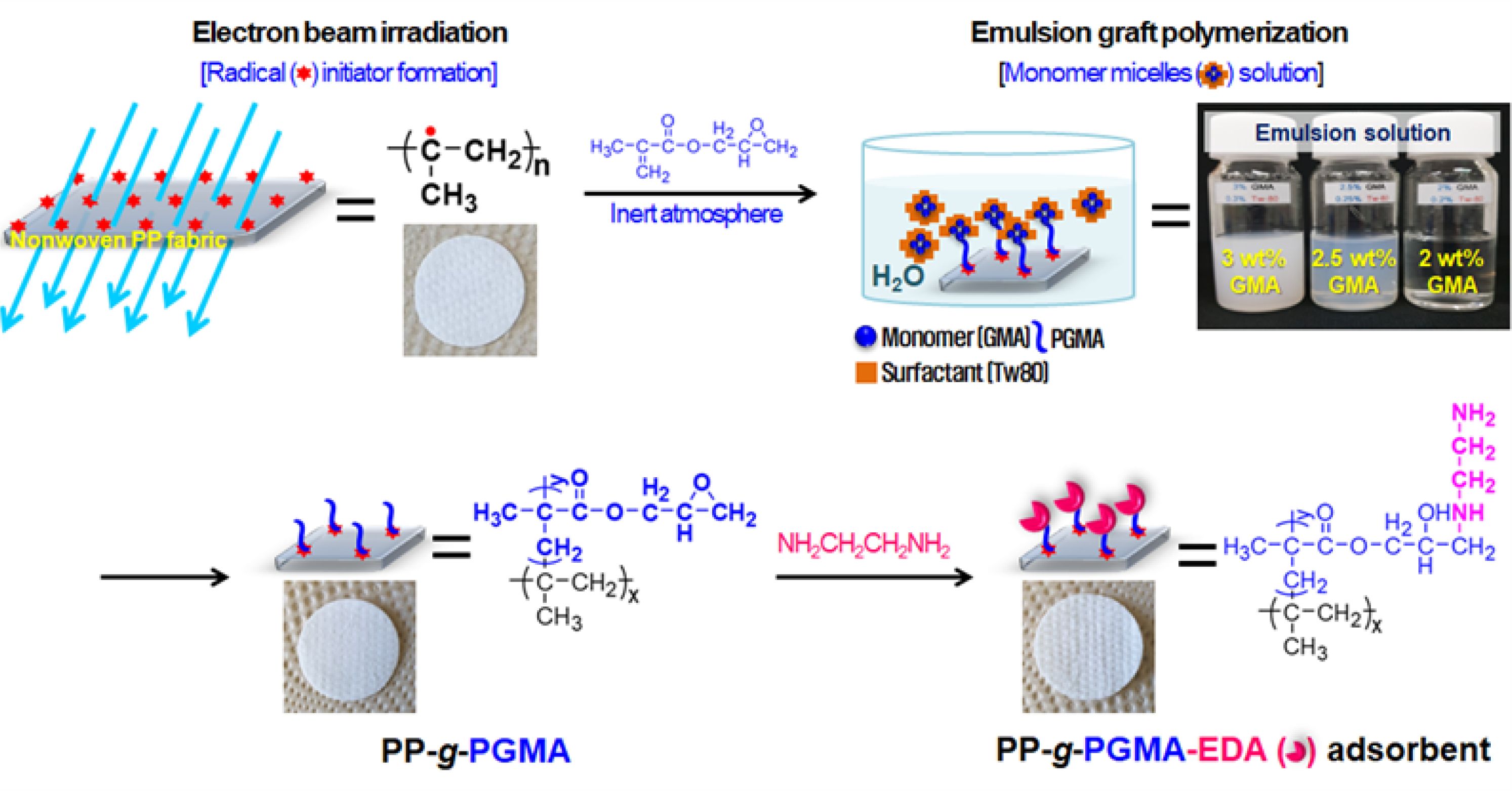
The preparation of PP-g-PGMA-EDA adsorbent was performed incrementally, as depicted in Figure 1. To form the radical initiators, the PP fabric was irradiated by electron beam irradiation under the inert condition. To produce the PP-g-PGMA, the irradiated fabric was placed into the emulsion solution in water containing monomer micelles, and the formed radicals at the tertiary carbons of the PP backbone structure readily initiated graft polymerization of GMA. As shown in the inset for the emulsion solutions, the 2.5 wt% GMA at the monomer-to-surfactant ratio of 10, was determined to be the minimum concentration for the stable emulsion solution in water. Finally, to introduce the metal-ion adsorbing functionality, the resulting PP-g-PGMA was functionalized through an amine reaction between epoxy groups of PP-g-PGMA and the amine groups of EDA, thereby producing the PP-g-PGMA-EDA adsorbents. Furthermore, there was no significant change in its physical appearance (compared to those of the pure PP and PP-g-PGMA) as shown in the inset of the photographs of the PP, PP-g-PGMA, and PP-g-PGMA-EDA adsorbent.
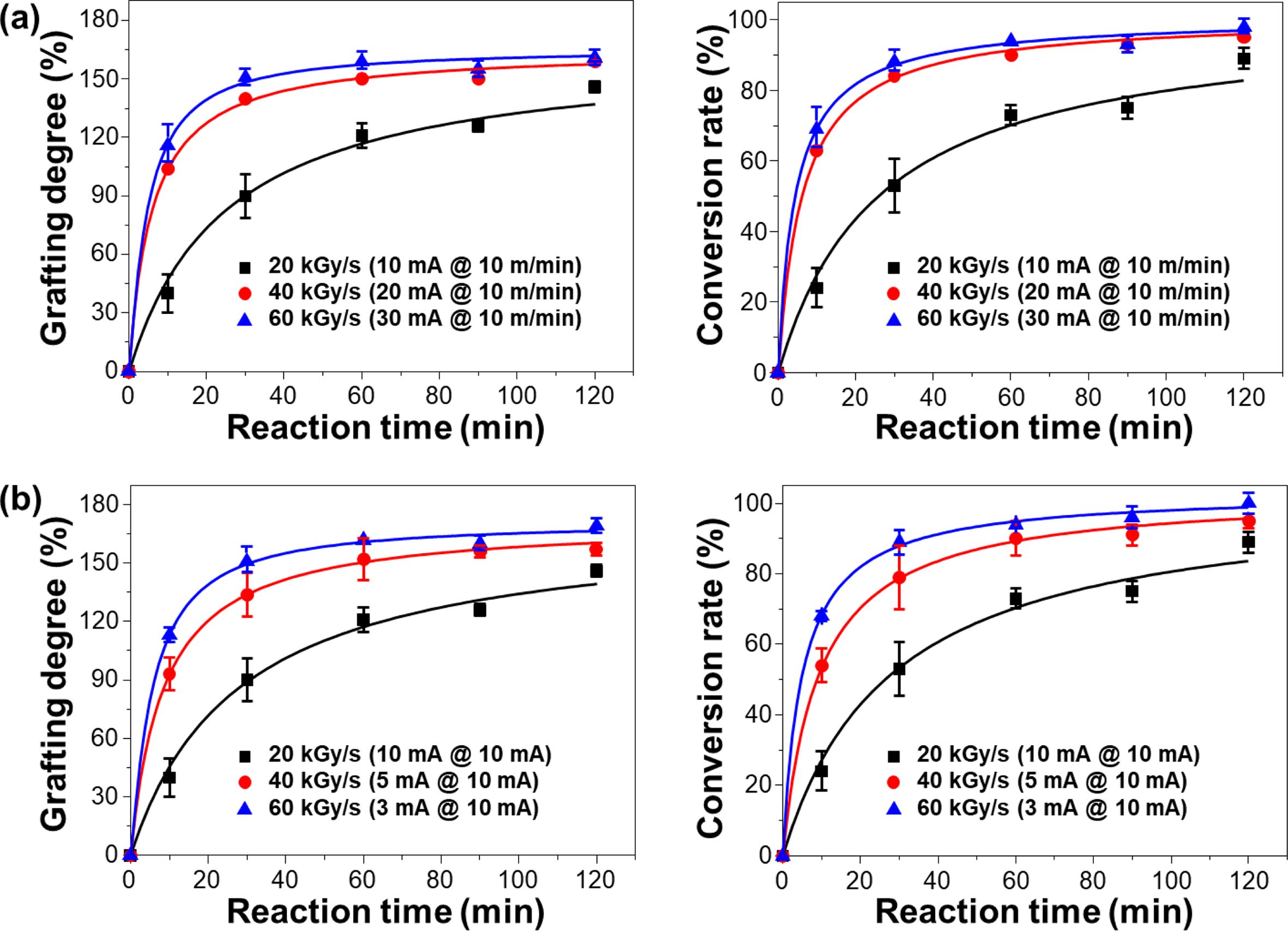
Preparation of PP-g-PGMA by Controlled Electron Beam Irradiation-induced Emulsion Graft Polymerization. To investigate the effect of the dose rate on the grafting degree and conversion rate, electron beam-induced emulsion polymerization was carried out at different dose rates (20, 40, and 60 kGy/s; corresponding to the absorbed dose of 10, 20, and 30 kGy) by either adjusting the beam current from 10 to 30 mA at the fixed conveyor speed of 10 m/min, or by adjusting the conveyor speed from 3 to 10 m/min at fixed beam current of 10 mA. As shown in the Figure 2(a), the grafting degree and conversion rate abruptly increased with increasing reaction time of up to 30 min, and then leveled off after 60 min when irradiated at 40 and 60 kGy/s by adjusting the beam current from 10 to 30 mA at a fixed conveyor speed (except for 20 kGy/s). In the case of 40 and 60 kGy/s, the respective grafting degree and corresponding conversion rate at the reaction time of 60 min tended to level off at ~160±7% and 90±3%. This higher grafting degree and conversion rate above the absorbed dose of 40 kGy/s, is probably attributable to the fact that the radicals needed to initiate the graft polymerization were efficiently generated at above absorbed dose of 20 kGy at the dose rate of above 40 kGy/s, induced quicker monomer consumption, and eventually leading to a higher grafting degree and conversion rate.33,34 Likewise, as shown in Figure 2(b), the effect of dose rate by adjusting conveyor speed at fixed beam current exhibited a similar tendency. However, comparing the deviations in the grafting degree and conversion rate at the reaction time of 60 min for 40 and 60 kGy/s, the current-adjusted dose rate is relatively smaller than the conveyor speed-adjusted one, on average (grafting degree = 160±11% and conversion rate = 90±5%). This result indicates that the current-adjusted way (even at the same dose rate) could provide more efficient and reliable grafting degree and conversion rate than the conveyor speed-adjusted one. This better efficiency of the current-adjusted way is presumably ascribable to the fact that, although the dose rates achieved by adjusting the current or conveyor speed are equal, current-adjusting way (namely, the shorter irradiation with the higher numbers of accelerated electrons to break the chemical bonds) improved the radiolysis efficiency of PP. This resulted in the efficient formation of polymerization-initiating radicals.35 Therefore, the condition to achieve the reliable level of conversion rate (average 93±2%) was the absorbed dose of 30 kGy at the dose rate of 60 kGy/s (by adjusting beam current of 30 mA at the fixed conveyor speed of 10 m/min, and a reaction time of over 60 min).
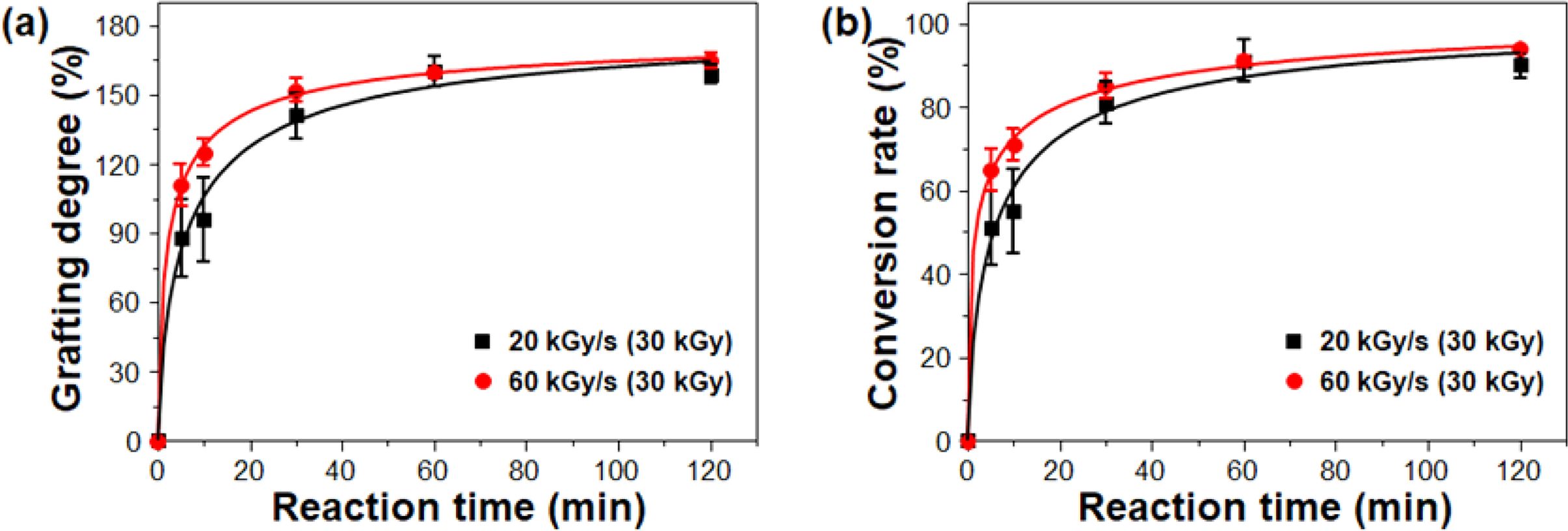
To further confirm the efficacy of the high dose rate on the grafting degree and conversion rate, change in the grafting degree and conversion rate of PP-g-PGMA with the current-adjusting different absorbed doses (corresponding to the fixed absorbed dose) was investigated, and the results are shown in Figure 3(a) and 3(b). As shown, the dose rate of 60 kGy/s produced relatively the higher grafting degree and conversion rate with smaller deviation at the initial reaction time (between 10 and 30 min) in comparison to that for 20 kGy/s, even at the same dose of 30 kGy.36-39 As expected, after 60 min, those seemed to be saturated. However, the dose rate of 60 kGy/s produced more reproducible grafting degree (160±5%) and conversion rate (93±2%) than the dose rate of 20 kGy/s (grafting degree 160±7% and conversion rate 90±5%). This result strongly supported that the higher dose rate (with higher number of accelerated electrons) at the same absorbed dose efficiently generated the polymerization-initiating radicals in the PP. This produced a reliable grafting degree and conversion rate as mentioned in the discussion of the efficiency of the current-adjusted way. Therefore, the optimized absorbed dose of 30 kGy, dose rate of 60 kGy/s, and reaction time of 60 min is good enough to achieve a relatively-constant grafting degree with a reliable conversion rate above 90%.
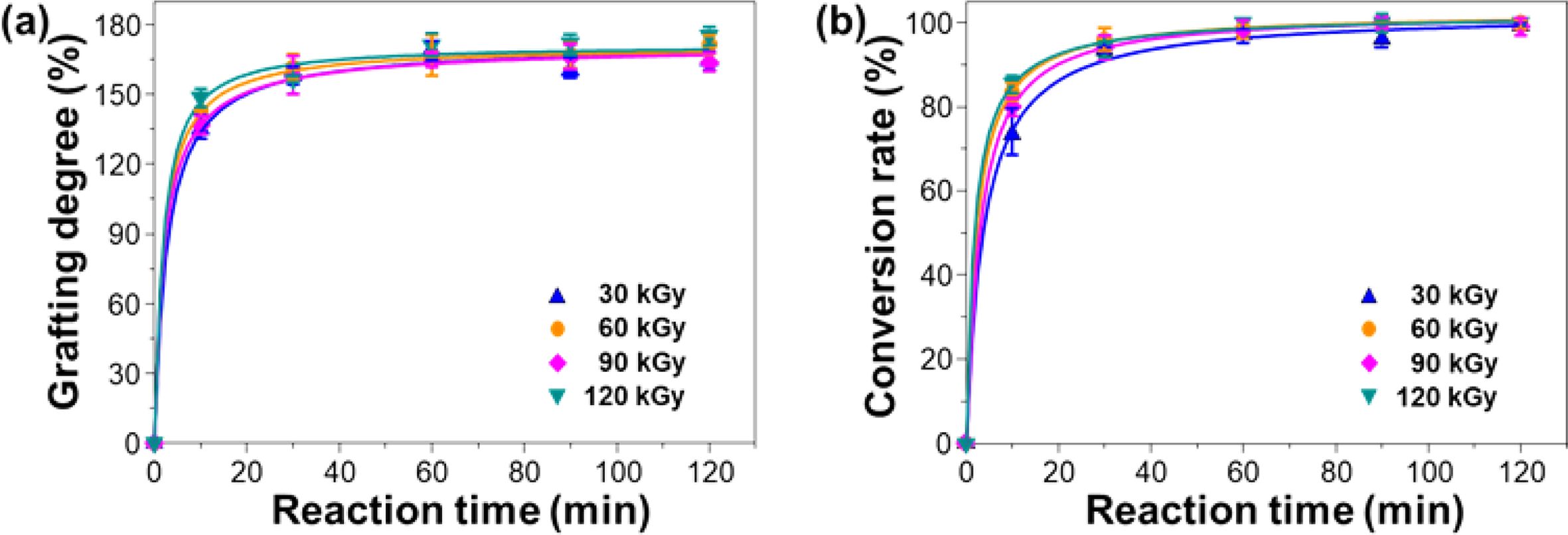
The effect of absorbed dose on the grafting degree and conversion rate was investigated, and the results are shown in Figure 4(a) and 4(b). As can be seen, the grafting degree and conversion rate at the reaction times between 10 and 30 min were slightly increased with the absorbed dose.3 However, after 60 min, both were almost saturated to the maximum (grafting degree of average 160±5% and the corresponding conversion rate of 93±2%) irrespective of the given absorbed doses. This result implies that the absorbed dose of 30 kGy and reaction time of 60 min is good enough to achieve the constant grafting degree with conversion rate above 90%.
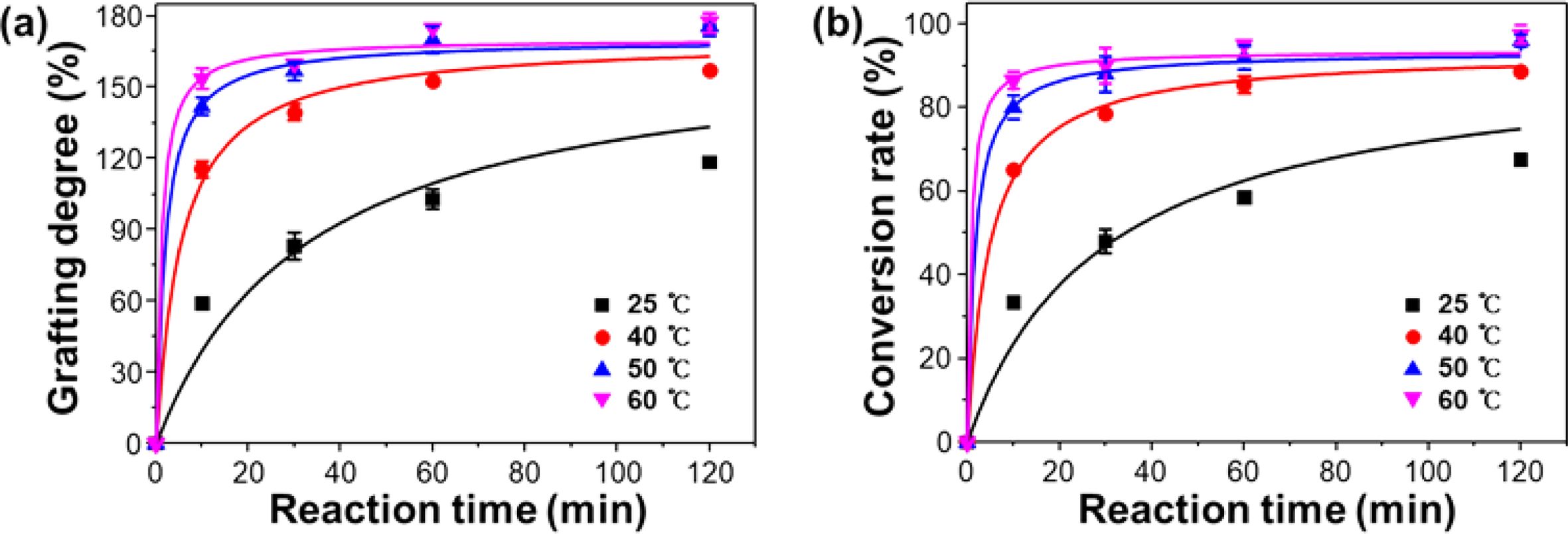
The effect of reaction temperature on grafting degree and conversion rate was investigated, and the results are presented in Figure 5(a) and 5(b). As shown, even if emulsion graft polymerization occurred at the ambient temperature of 25 ℃, the grafting degree at the reaction time of 60 min reached just ~100±6% with a conversion rate of 60±4%. At the relatively higher temperature of 40 ℃, the higher grafting average degree of 140±1% with the conversion rate of 80±2% was obtained for the reaction time of 60 min due to the heat energy effect.40,41 On the other hand, for the temperatures of 50 and 60 ℃, the average grafting degree of 160±5% with a conversion rate of 93±2% was achieved at the reaction time of 60 min although there is a thermal energy-induced discrepancy in the grafting degree and conversion rate at the reaction time of 10 and 30 min.42 Therefore, the temperature of 50 ℃ and the reaction time of 60 min is quite satisfactory for gaining the constant grafting degree with conversion rate above 90%. Therefore, it is confirmed from the overall results of the relationship between the RIEGP parameters, grafting degree, and conversion rate that the established RIEGP condition in this system (the current-adjusting dose rate of 60 kGy/s, the absorbed dose of 30 kGy, the reaction time of 60 min and the reaction temperature of 50 ℃) can achieve the constant grafting degree desired, with a conversion rate of 93±2%.
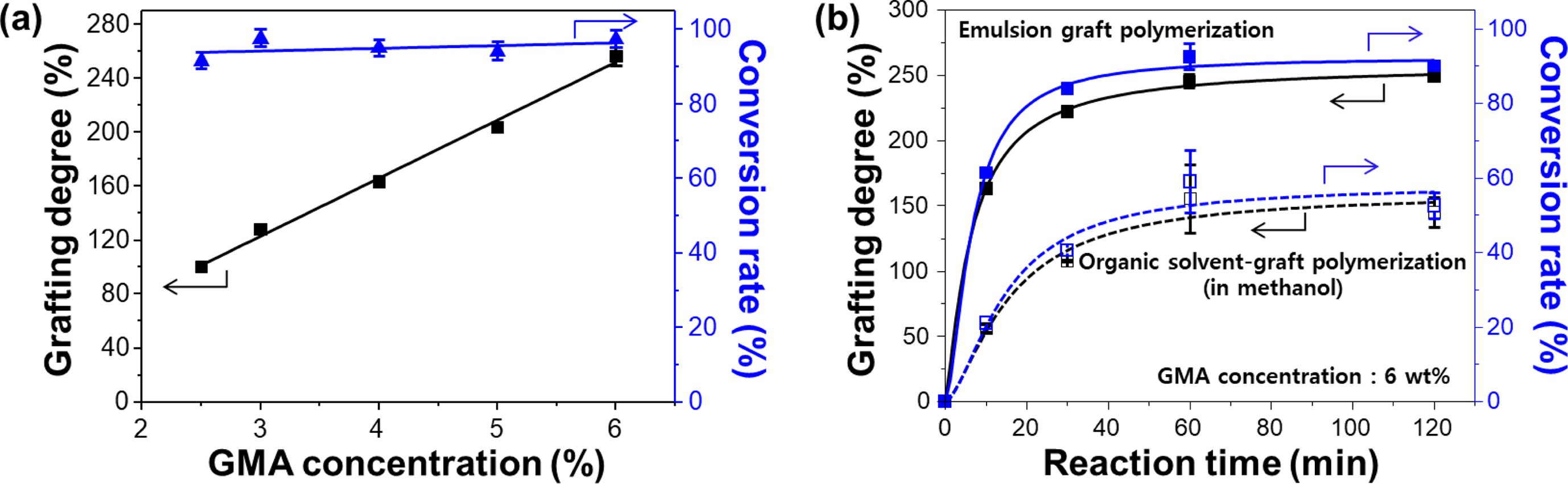
To provide further evidence of the reliability and reproducibility of the above-mentioned RIEGP condition, the relationships between monomer concentration, grafting degree, and conversion rate were investigated, and the results are presented in Figure 6(a). As expected, the grafting degree was linearly increased from average 101±2% to average 256±5% with the increase in monomer concentration from minimal concentration of 2.5 wt% to 6 wt%. Even so, the conversion rate of averaged 93±2% was nearly steady. Moreover, as shown in Figure 6(b), the grafting degree and conversion rate in emulsion graft polymerization are much higher than those in organic solvent graft polymerization.21,22 These results indicate that the grafting degree of PP-g-PGMA can be precisely controlled in emulsion graft polymerization with excellent grafting efficiency by manipulating a monomer concentration. This allows customization of its physicochemical properties to those desirable for the application of metal adsorbent.
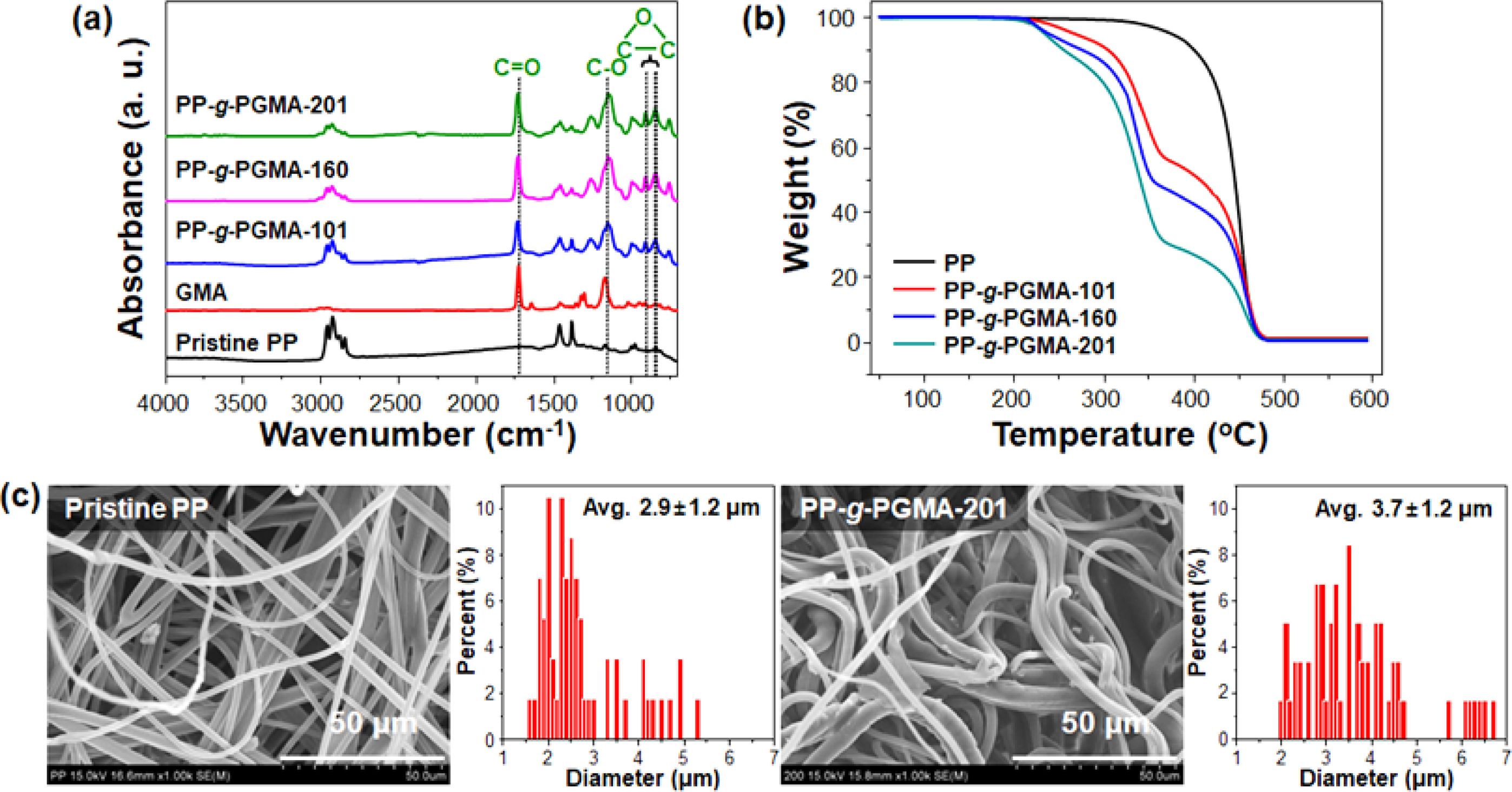
To confirm the successful formation of PP-g-PGMA by the PP fabrics by electron beam irradiation-induced emulsion graft polymerization, diverse analyses of chemical structure, thermal decomposition, and morphology were carried out, as presented in Figure 7. The FTIR spectra give clear insight into the change in the chemical structure of pristine PP fabric (Figure 7(a)): the PP spectrum showed characteristic peaks at 2918 cm-1 (aliphatic CH), 1437 cm-1 (-CH2), and 1376 cm-1 (-CH3), which were assigned to the chemical structure of PP backbone.43,44 On the other hand, in all the spectrum of PP-g-PGMA-101, PP-g-PGMA-151, and PP-g-PGMA-201, new peaks appeared clearly at 1721 cm-1 (C=O of (C=O)O) and 1180 cm-1 (C-O of (C=O)O), 905 and 843 cm-1 (tri-cyclic C-O-C). These were identical to the GMA spectrum except for the double bond, appeared along with the characteristic peaks for the pristine PP. The intensities of the peaks for the C=O, C-O, and C-O-C were relatively much increased with an increase in the grafting degree.45 As shown in the thermal decomposition profiles as other evidence for the successful introduction of PGMA on the PP (Figure 7(b)), the typical weight loss of pristine PP occurred at the temperatures between 300 and 480 ℃ in the thermal decomposition profile.46 In contrast, all the PP-g-PGMA-101, PP-g-PGMA-160, and PP-g-PGMA-201 exhibited the weight losses over two temperature spans (200-350 and 350-480 ℃). These results were due to the well-known thermal decomposition of PGMA and PP, respectively.9 The PGMA thermal decomposition-induced weigh losses were clearly dependent on the grafting degree (quite good agreement with those using the weight-based measurement). Furthermore, as shown in FE-SEM images and in the fiber diameter distributions of the fiber dimension (Figure 7(c)), pristine PP showed randomly flat fibers with randomly entangled morphology and the fiber diameter in the FE-SEM image was average 2.9±1.2 μm. On the other hand, PP-g-PMGA-201 exhibited the round fibers with three-dimensional shapes (unlike those of pristine PP), and its fiber average diameter was 3.7±1.2 μm (thicker than that of pristine PP). Therefore, it is confirmed from these overall analytic results that the PP-g-PGMA was successfully produced through emulsion graft polymerization of GMA onto the irradiated PP fabric under controlled conditions.
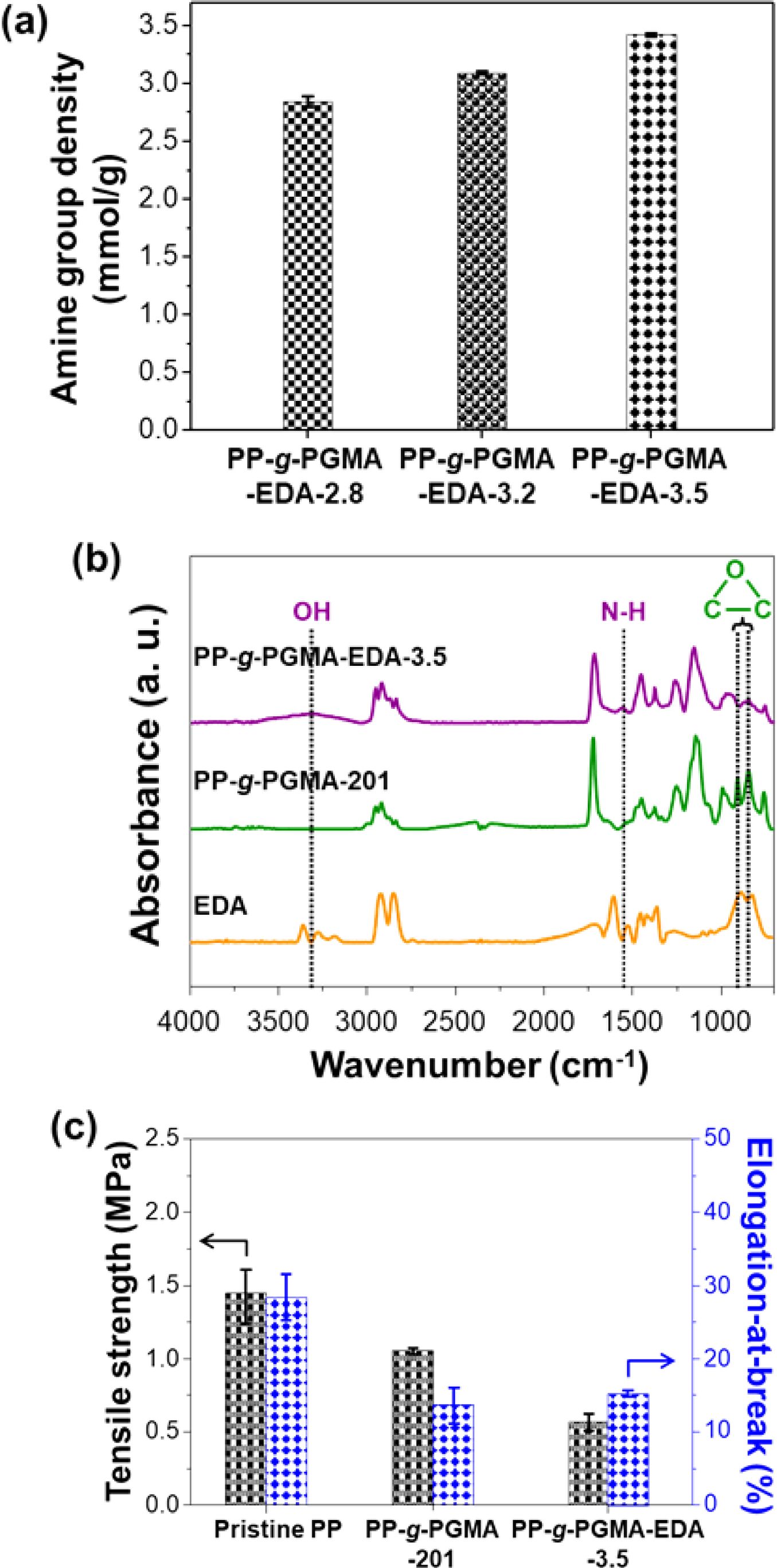
Preparation of PP-g-PGMA-EDA Adsorbent and its Copper Ion Adsorbing Performance. To investigate the formation of PP-g-PGMA-EDA adsorbent via the amine reaction between epoxy groups of PP-g-PGMA and the amine groups of EDA, amine group density measurement, FTIR, and UTM were carried out, and the results are shown in Figure 8. As shown in Figure 8(a), the prepared PP-g-PGMA-EDAs exhibited the amine group density ranging from 2.8 to 3.5 mmol/g (dependent on the grafting degree of the PP-g-PGMA) and the corresponding conversion rates of 88% were achieved under the given condition of 20 wt% EDA in dimethyl sulfoxide (DMSO) solution for 80 ℃ for 1 hr. As shown in FTIR spectra as a clear evidence for the successful preparation of PP-g-PGMA-EDA (Figure 8(b)), PP-g-PGMA-EDA-3.5 spectrum shows new peaks at 3360 cm-1 (-OH: due to ring opening reaction of epoxy with EDA) and 1605 cm-1 (-NH: corresponding to EDA spectrum) along with the near disappearance of the peaks at 905 and 843 cm-1, in comparison with those of PP-g-PGMA-201 as a starting material.47 These indicate the successful functionalization of PP-g-PGMA-201 with EDA, producing the PP-g-PGMA-EDA-3.5 adsorbent. Moreover, as presented in tensile strength and elongation-at-break results for the sequential introducing influence of PGMA and EDA on the mechanical properties of pristine PP (important for the practical application (Figure 8(c)), the pristine PP exhibited a tensile strength of 1.4±0.20 MPa and elongation-at-break of 28±2%. The tensile strength (1.0±0.02 MPa) and elongation-at-break (14±2%) of PP-g-PGMA-201 was slightly reduced in comparison to those of pristine PP. The PP-g-PGMA-EDA-3.5 (prepared by amine reaction of PP-g-PGMA-201 with EDA) exhibited the lower tensile strength (0.6±0.06 MPa) and lower elongation-at-break (15±1%) in comparison to those of PP-g-PGMA-201. The reduction of tensile properties by the incremental introduction of PGMA and EDA is probably attributable to the reduction in the flexibility of PP chain. This is induced by PGMA and further deteriorated by hydrogen bonding formation of EDA.48,49 Therefore, the PP-g-PGMA-EDA adsorbents with amine-group density ranging from 2.8 to 3.5 mmol/g were successfully produced through amine reaction with EDA. Moreover, they still retained the mechanical property suitable for application toward the metal ion adsorption, although their tensile properties were lower than those of pristine PP.9
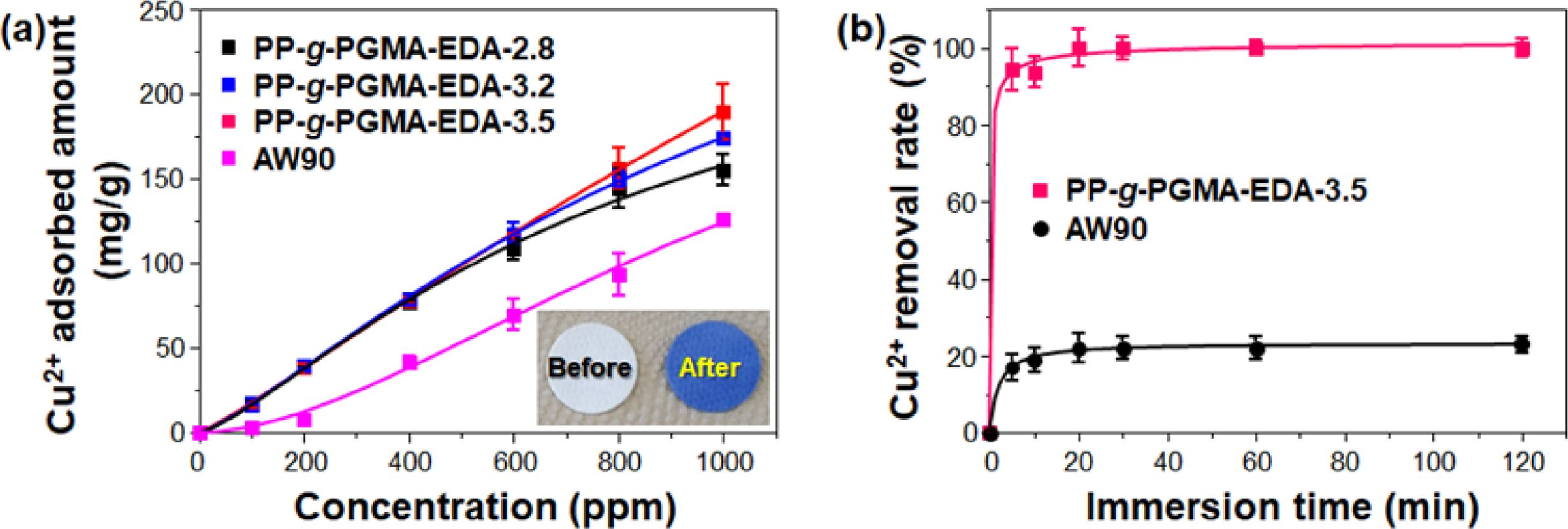
To demonstrate practically the feasibility of adopting Cu2+ ion as a new adsorbent for the removal of heavy metal ion, the adsorption performance of PP-g-PGMA-EDA adsorbents was evaluated in terms of Cu2+ ion concentration and immersion time. Cu2+ ions were selected as a model of hazardous metal ions and commercially-available AW90 resin with amine groups (industrially used for the removal of the heavy metal ion) was employed as a reference for comparison. As presented in Figure 9(a), the amount of copper adsorbed by PP-g-PGMA-EDA-2.8, -3.2 and -3.5 adsorbents all increased with increase in the Cu2+ concentration. At concentration over 600 ppm, PP-g-PGMA-EDA-3.5 with the highest amine group density showed the higher Cu2+ adsorption than PP-g-PGMA-EDA-2.8 and -3.2 did. The PP-g-PGMA-EDA-3.5 exhibited the highest amount of adsorbed Cu2+ (189±16 mg/g), which is 51% higher than that of AW90 resin (125±2 mg/g). As presented in the inset in Figure 9(a), the white PP-g-PGMA-EDA-3.5 adsorbent turned an even blue color after the adsorption of Cu2+ at the concentration of 1000 ppm. This clearly indicates efficient Cu2+ adsorption. In addition, as shown in kinetic comparison in Cu2+ removal rate between developed PP-g-PGMA-EDA-3.5 and commercial AW90 (critical to determine the removal capability for the practical continuous system; see Figure 9(b)), the PP-g-PGMA-EDA-3.5 showed a removal rate of 90±6% even at the immersion time of 5 min and then a removal rate of 100±3% at the time of >20 min. This removal rate is much higher than that of commercial AW90 (that is, removal rate of 61±2% even at the time of 120 min). This more rapid removal ability of PP-g-PGMA-EDA-3.5 is possibly attributable to its evenly distributed fibrous structure of amine groups (allows more rapid adsorption).22 The amine group is generally regarded as one of the most favorable functionality for adsorption of heavy metal ions due to its strong chelation ability towards positive charged metal ions, easily forming amine-metal complex.50,51 Therefore, it is firmly believed that the PP-g-PGMA-EDA prepared by controlled RIEGP could be considered as an efficient adsorbent for the removal of heavy metal ions from diverse wastewaters.
|
Figure 1 Schematic diagram for the preparation of PP-g-PGMA-EDA fabric by electron beam radiation-induced emulsion graft polymerization and subsequent amine reaction. |
|
Figure 2 Changes in grafting degree and conversion rate of PP-g-PGMA at the different dose rates (respective absorbed doses of 10, 20, and 30 kGy): (a) by adjusting beam current at a fixed conveyor speed; (b) by adjusting conveyor speed at a fixed beam current. |
|
Figure 3 Changes in (a) grafting degree; (b) of PP-g-PGMA at the different dose rates of 20 and 60 kGy/s (corresponding to fixed absorbed doses of 30 kGy) by adjusting the beam current at fixed conveyor speed. |
|
Figure 4 Changes in (a) grafting degree; (b) conversion rate of PP-g-PGMA at different absorbed doses ranging from 30 to 120 kGy with a fixed current-adjusting dose rate of 60 kGy/s. |
|
Figure 5 Changes in (a) grafting degree; (b) conversion rate of PP-g-PGMA at different temperature ranging from 25 to 60 ℃ at fixed absorbed dose of 30 kGy. |
|
Figure 6 (a) Change in grafting degree and corresponding conversion rate as a function of GMA concentration at fixed absorbed dose of 30 kGy; (b) comparative study on grafting efficiency between organic solvent- and emulsion-graft polymerization at the 6 wt% of GMA concentration. |
|
Figure 7 Chemical structure, thermal decomposition, and morphology of PP-g-PGMA: (a) FT-IR spectra of PP, GMA, PP-g-PGMA-101, - 161, and -201; (b) Thermal decomposition profile of PP, PP-g-PGMA-101, -160, and -201; (c) FE-SEM images and corresponding fiber diameter distributions of PP and PP-g-PGMA-201. |
|
Figure 8 Chemical and tensile properties of PP-g-PGMA-EDA adsorbents: (a) amine group density of PP-g-PGMA-EDA-2.8, -3.2, and -3.5; (b) FTIR spectra of EDA, PP-g-PGMA-201, and PP-gPGMA-EDA-3.5; (c) tensile strength and elongation-at-break of PP, PP-g-PGMA-201, and PP-g-PGMA-EDA-3.5. |
|
Figure 9 Copper (Cu2+) ion adsorbing performance of PP-g-PGMA-EDA adsorbents: (a) Cu2+ adsorbed amount of PP-g-PGMA-EDA-2.8, -3.2, -3.5, and commercial AW90 resin (inset shows the photograph of PP-g-PGMA-EDA-3.5 before and after the adsorbing test at 1000 ppm); (b) Cu2+ removal rate of PP-g-PGMA-EDA-3.5 and commercial AW90 resin. |
PP-g-PGMA-EDA was successfully prepared from PP-g-PGMA, using controlled RIEGP and the amine reaction. Moreover, it was demonstrated that PP-g-PGMA-EDA serves well as an efficient adsorbent for the removal of hazardous heavy metal ions. From the overall results of the relationship between the RIEGP parameters, grafting degree, and conversion rate, it was shown that the RIEGP conditions in this system could provide a reproducible grafting degree with a conversion rate >90%. The established RIEGP conditions used were: current-adjusted dose rate of 60 kGy/s, adsorbed dose of 30 kGy, reaction time of 60 min, and reaction temperature of 50 °C. The PP-g-PGMA-EDA produced by amine reaction with EDA exhibited the grafting degree-dependent amine group density ranging from 2.8 to 3.5 mmol/g with the tensile properties suitable for the application toward the metal ion adsorption. As a result, the PP-g-PGMA-EDA-3.5 showed the highest amount of adsorbed Cu2+ (189±16 mg/g). This is 51% higher than that of AW90 resin (125±2 mg/g). It also exhibited a faster rate of Cu2+ removal (100±3%) after 20 min, than that commercial AW90 (removal rate of 61±2%) even after 120 min. This ability to achieve more rapid removal by PP-g-PGMA-EDA-3.5 is possibly attributable to the evenly distributed fibrous structure of its amine groups. This clearly authenticates the notion that the functionalization of non-woven fabric could be more reliably and reproducibly achieved using the established RIEGP, and that it might be further customized to produce a desirable adsorbent via simple chemical treatment to remove hazardous heavy metal ions from diverse wastewaters.
- 1. Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted Magnetic Porous Powder for Highly Effective Adsorption of Heavy Metal Ions. Desalination 2011,281, 278-284.
-

- 2. Kavakli, P. A.; Korpayev, S.; Kavakli, C.; Tilki, S. Novel Cotton Fabric Adsorbent for Efficient As (V) Adsorption. Environ. Sci. Pollut. Res. 2018, 25, 34610-34622.
-

- 3. Zhang, M.; Gao, Q.; Yang, C.; Pang, L.; Wang, H.; Li, H.; Li, R.; Xu, L.; Hu, J.; Wu, G. Preparation of Amidoxime-based Nylon-66 Fibers for Removing Uranium from Low-concentration Aqueous Solutions and Simulated Nuclear Industry Effluents. Ind. Eng. Chem. Res. 2016, 55, 10523-10532.
-

- 4. Cheballah, K.; Sahmoune, A.; Messaoudi, K.; Drouiche, N.; Lounici, H. Simultaneous Removal of Hexavalent Chromium and COD from Industrial Wastewater by Bipolar Electrocoagulation. Chem. Eng. Process. 2015, 96, 94-99.
-

- 5. Coelho, G. F.; Jr, A. C. G.; Tarley, C. R. T.; Casarin, J.; Nacke, H.; Francziskowski, M. A. Removal of Metal Ions Cd II, Pb II, and Cr III from Water by the Cashew Nut Shell Anacardium Occidentale L. Ecol. Eng. 2014, 73, 514-525.
-

- 6. Shannag, M. A.; Qodah, Z. A.; Melhem, K. B.; Otaishat, M. R.; Alkasrawi, M. Heavy Metal Ions Removal from Metal Plating Wastewater Using Electrocoagulation: Kinetic Study and Process Performance. Chem. Eng. J. 2015, 260, 749-756.
-

- 7. Salehizadeh, H.; Shojaosadati, S. A. Removal of Metal Ions from Aqueous Solution by Polysaccharide Produced from Bacillus Firmus. Water Res. 2003, 37, 4231-4235.
-

- 8. Anirudhan, T. S.; Nima, J.; Divya, P. L. Adsorption of Chromium VI from Aqueous Solutions by Glycidyl Methacrylate-grafted-densified Cellulose with Quaternary Ammonium Groups. Appl. Surf. Sci. 2013, 279, 441-449.
-

- 9. Yang, Y.; Ma, N.; Zhang, Q.; Chen, S. Adsorption of Hg2+ on a Novel Chelating Fiber Prepared by Preirradiation Grafting and Amination. J. Appl. Polym. Sci. 2009, 113, 3638-3645.
-

- 10. Neghlani, P. K.; Rafizadeh, M.; Taromi, F. A. Preparation of Aminated-polyacrylonitrile Nanofiber Membranes for the Ad- sorption of Metal Ions: Comparison with Microfibers. J. Hazard. Mater. 2011, 186, 182-189.
-

- 11. Dafader, N. C.; Rahman, N.; Majumdar, S. K.; Khan, M. M. R.; Rahman, M. M. Preparation and Characterization of Imino- diacetate Group Containing Nonwoven Polyethylene Fabrics and Its Application in Chromium Adsorption. J. Polym. Environ. 2018, 26, 740-748.
-

- 12. Chen, L.; Yang, G.; Zhang, J. A study on the Exchange Kinetics of Ion-exchange Fiber. React. Funct. Polym. 1996, 29, 139-144.
-

- 13. Zeng, Z.; Wei, Y.; Shen, L.; Hua, D. Cationically Charged Polyamidoxime-grafted Polypropylene Nonwoven Fabric for Potential Uranium Extraction from Seawater. Ind. Eng. Chem. Res. 2015, 54, 8699-8705.
-

- 14. Nasef, M. M.; Ting, T. M.; Abbasi, A.; Moghaddam, A. L.; Alinezhad, S. S.; Hashim, K. Radiation Grafted Adsorbents for Newly Emerging Environmental Applications. Radiat. Phys. Chem. 2016, 118, 55-60.
-

- 15. Zhu, Y.; Wei, J.; Zhang, H.; Liu, K.; Kong, Z.; Dong, Y.; Jin, G.; Tian, J.; Qin, Z. Fabrication of Composite Membrane with Adsorption Property and Its Application to the Removal of Endocrine Disrupting Compounds During Filtration Process. Chem. Eng. J. 2018, 352, 53-63.
-

- 16. Nasef, M. M.; Güven, O. Radiation-grafted Copolymers for Separation and Purification Purposes: Status, Challenges and Future Directions. Prog. Polym. Sci. 2012, 37, 1597-1656.
-

- 17. Kavakli, P. A.; Seko, N.; Tamada, M.; Guven, O. Radiation-induced Graft Polymerization of Glycidyl Methacrylate onto PE/PP Nonwoven Fabric and Its Modification toward Enhanced Amidoximation. J. Appl. Polym. Sci. 2007, 150, 1551-1558.
-

- 18. Chen, K. S.; Tsai, J. C.; Chou, C. W.; Yang, M. R.; Tang, J. M. Effects of Additives on the Photo-induced Grafting Polymeri- zation of N-isopropylacrylamide Gel onto PET Film and PP Nonwoven Fabric Surface. Mater. Sci. Eng. C 2002, 20, 203-208.
-

- 19. Chen, J. P.; Chiang, Y. P. Surface Modification of Non-woven Fabric by DC Pulsed Plasma Treatment and Graft Polymerization with Acrylic Acid. J. Membr. Sci. 2006, 270, 212-220.
-

- 20. Chern, C. S. Emulsion Polymerization Mechanisms and Kinetics. Prog. Polym. Sci. 2006, 31, 443-486.
-

- 21. Omichi, M.; Ueki, Y.; Seko, N.; Maekawa, Y. Development of a Simplified Radiation-Induced Emulsion Graft Polymerization Method and Its Application to the Fabrication of a Heavy Metal Adsorbent. Polymers 2019, 11, 1373.
-

- 22. Madrid, J. F.; Ueki, Y.; Seko, N. Abaca/polyester Nonwoven Fabric Functionalization for Metal Ion Adsorbent Synthesis Via Electron Beam-induced Emulsion Grafting. Radiat. Phys. Chem. 2013, 90, 104-110.
-

- 23. Madrid, J. F.; Lopez, G. E. P.; Abad, L. V. Application of Full-factorial Design in the Synthesis of Polypropylene-g-polyglycidyl Methacrylate Functional Material for Metal Ion Adsorption. Radiat. Phys. Chem. 2017, 136, 54-63.
-

- 24. Sekine, A.; Seko, N.; Tamada, M.; Suzuki, Y. Biodegradable Metal Adsorbent Synthesized by Graft Polymerization onto Nonwoven Cotton Fabric. Radiat. Phys. Chem. 2010, 79, 16-21.
-

- 25. Liu, X.; Liu, H.; Ma, H.; Cao, C.; Yu, M.; Wang, Z.; Deng, B.; Wang, M.; Li, J. Adsorption of the Uranyl Ions on an Amidoxime-based Polyethylene Nonwoven Fabric Prepared by Preirradiation-induced Emulsion Graft Polymerization. Ind. Eng. Chem. Res. 2012, 51, 15089-15095.
-

- 26. Mohamed, N. H.; Tamada, M.; Ueki, Y.; Seko, N. Effect of Partial Delignification of Kenaf bast Fibers for Radiation Graft Copolymerization. J. Appl. Polym. Sci. 2013, 127, 2891-2895.
-

- 27. Madrid, J. F.; Abad, L. V.; Yamanobe, T.; Seko, N. Effects of Chain Transfer Agent on the Electron Beam-induced Graft Polymerization of Glycidyl Methacrylate in Emulsion Phase. Colloid. Polym. Sci. 2017, 295, 1007-1016.
-

- 28. Yu, M.; Zhang, B.; Deng, B.; Yang, X.; Sheng, K.; Xie, L. Preirradiation-induced Emulsion Graft Polymerization of Glycidyl Methacrylate onto Polyvinylidene Fluoride Powder. J. Appl. Polym. Sci. 2010, 117, 3575-3581.
-

- 29. Nasef, M. M.; Hegazy, E. S. A. Preparation and Applications of Ion Exchange Membranes by Radiation-induced Graft Copoly- merization of Polar Monomers onto Non-polar Films. Prog. Polym. Sci. 2004, 29, 499-561.
-

- 30. Seko, N.; Bang, L. T.; Tamada, M. Syntheses of Amine-type Adsorbents with Emulsion Graft Polymerization of Glycidyl Methacrylate. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms. 2007, 265, 146-149.
-

- 31. Wang, L.; Li, F.; Yao, M.; Qiu, T.; Jiang, W.; Fan, L. J. Atom Transfer Radical Polymerization of Glycidyl Methacrylate Followed by Amination on the Surface of Monodispersed Highly Crosslinked Polymer Microspheres and the Study of Cation Adsorption. React. Funct. Polym. 2014, 82, 66-71.
-

- 32. Zhang, B.; Hu, N.; Wang, Y.; Wang, Z.; Wang, Y.; Kong, E. S.; Zhang, Y. Polyglycidyl methacrylates-grafted Zinc Oxide Nano- wire by Surface-initiated Atom Transfer Radical Polymerization. Nano-Micro Lett. 2010, 2, 285-289.
-

- 33. Jeun, J. P.; Hua, Z. J.; Kang, P. H.; Nho, Y. C. Electron Beam Radiation-induced Grafting of Scrylonitrile onto Polypropylene Fibers: Influence of the Synthesis Conditions. J. Appl. Polym. Sci. 2010, 115, 222-228.
-

- 34. Nasef, M. M. Effect of Solvents on Radiation-induced Grafting of Styrene onto Fluorinated Polymer Films. Polym. Int. 2011, 50, 338-346.
-

- 35. Uddin, M. D. Z.; Watanabe, M.; Shirai, H.; Hirai, T. Effects of Plasticizers on Novel Electromechanical Actuations with Different Polyvinyl Chloride Gels. J. Polym. Sci. Pt. B-Polym. Phys. 2003, 41, 2119-2127.
-

- 36. Sun, J.; Chen, Z.; Ge, M.; Xu, L.; Zhai, M. Selective Adsorption of Hg(II) by γ-radiation Synthesized Silica-graft-vinyl Imidazole Adsorbent. J. Hazard. Mater. 2013, 244-245, 94-101.
-

- 37. Zhang, S.; Wang, W.; Wang, H.; Qi, W.; Yu, L.; Ye, Q. Synthesis and Characterisation of Starch Grafted Superabsorbent Via10 MeV Electron-beam Irradiation. Carbohydr. Polym. 2014, 101, 798-803.
-

- 38. Sherazi, T. A.; Sohn, J. Y.; Lee, Y. M.; Guiver, M. D. Polyethylene-based Radiation Grafted Anion-exchange Membranes for Alkaline Fuel Cells. J. Membr. Sci. 2013, 441, 148-157.
-

- 39. Lv, X.; Song, W.; Ti, Y.; Qu, L.; Zhao, Z.; Zheng, H. Gamma Radiation-induced Grafting of Acrylamide and Dimethyl Diallyl Ammonium Chloride onto Starch. Carbohydr. Polym. 2013, 92, 388-393.
-

- 40. Mu, L.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C.; Zhao, Q. Effect of Ultrasonic Treatment on the Graft Reaction between Soy Protein Isolate and Gum Acacia and on the Physicochemical Properties of Conjugates. J. Agric. Food Chem. 2010, 58, 4494-4499.
-

- 41. Yang, Y.; Li, H.; Chen, S.; Zhao, Y.; Li, Q. Preparation and Characterization of a Solid Amine Adsorbent for Capturing CO2 by Grafting Allylamine onto PAN Fiber. Langmuir 2010, 26, 13897-13902.
-

- 42. Thakur, V. K.; Thakur, M. K.; Gupta, R. K. Development of Functionalized Cellulosic Biopolymers by Graftcopolymerization. Int. J. Biol. Macromol. 2013, 62, 44-51.
-

- 43. Tseng, C. H.; Wang, C. C.; Chen, C. Y. Polypropylene Fibers Modified by Plasma Treatment for Preparation of Ag Nanoparticles. J. Phys. Chem. B 2006, 110, 4020-4029.
-

- 44. Ni, Q. L.; Fan, J. Q.; Niu, H.; Dong, J. Y. Enhancement of Graft Yield and Control of Degradation During Polypropylene Maleation in the Presence of Polyfunctional Monomer. J. Appl. Polym. Sci. 2011, 121, 2512-2517.
-

- 45. Madrid, J. F.; Ueki, Y.; Abad, L. V.; Yamanobe, T.; Seko, N. RAFT-mediated Graft Polymerization of Glycidyl Methacrylate in Emulsion from Polyethylene/Polypropylene Initiated with c-radiation. J. Appl. Polym. Sci. 2017, 134, 45270.
-

- 46. Xu, T.; Wu, Q.; Chen, S.; Deng, M. Preparation of Polypropylene Based Hyperbranched Absorbent Fibers and the Study of Their Adsorption of CO2. RSC Adv. 2015, 5, 32902-32908.
-

- 47. Zheng, Y. Q.; Deng, S.; Niu, L.; Xu, F. J.; Chai, M. Y.; Yu, G. Functionalized Cotton Via Surface-initiated Atom Transfer Radical Polymerization for Enhanced Sorption of Cu(Ⅱ) and Pb(Ⅱ). J. Hazard. Mater. 2011, 192, 1401-1408.
-

- 48. Moawia, R. M.; Nasef, M. M.; Mohamed, N. H.; Ripin, A. Modification of Flax Fibres by Radiation Induced Emulsion Graft Copolymerization of Glycidyl Methacrylate. Radiat. Phys. Chem. 2016, 122, 35-42.
-

- 49. Gao, Q.; Hu, J.; Li, R.; Xing, Z.; Xu, L.; Wang, M.; Guo, X.; Wu, G. Radiation Synthesis of a New Amidoximated UHMWPE Fibrous Adsorbent with High Adsorption Selectivity for Uranium over Vanadium in Simulated Seawater. Radiat. Phys. Chem. 2016, 122, 1-8.
-

- 50. Liu, C.; Lei, X.; Liang, X.; Jia, J.; Wang, L. Visible Sequestration of Cu2+ Ions Using Amino-Functionalized Cotton Fiber. RSC Adv. 2017, 7, 9744-9753.
-

- 51. Zhao, K.; Kong, L.; Yang, W.; Huang, Y.; Li, H.; Ma, S.; Lv, W.; Hu, J.; Wang, H.; Liu, H. Hooped Amino-Group Chains in Porous Organic Polymers for Enhancing Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2019, 11, 44751-44757.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(5): 764-774
Published online Sep 25, 2021
- 10.7317/pk.2021.45.5.764
- Received on May 10, 2021
- Revised on Jun 9, 2021
- Accepted on Jun 9, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Chan-Hee Jung
-
Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup, Jeollabuk-do 56212, Korea
- E-mail: jch@kaeri.re.kr
- ORCID:
0000-0001-7482-9426









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.