- Blends of Natural Rubber and Butadiene Rubber-Distribution Quantification of Carbon Black Filler
Technical Consultancy Division, Rubber Research Institute of India, Kottayam, Kerala-686009, India
- 천연고무와 부타디엔 고무의 혼합-카본블랙 충전제의 분포 정량화
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
The present study deals with the distribution of N330 carbon black in cured 50/50 blend of natural rubber (NR) and butadiene rubber (BR). The results were reported in terms of tan δ and glass transition temperature (Tg). The reduced tan δ peaks in the BR phase with the increased filler loading and the corresponding shift in Tg of BR towards the positive side have shown that there was a preferential migration of carbon black towards the BR phase. Carbon black dispergrader was used to determine the dispersion of the filler on a series of blends with varying BR content. A theoretical evaluation on determining the preference of N330 has also been performed using Wu’s equation. The evaluation was well correlated with the result obtained from analytical data. It has been inferred that the N330 has migrated more towards the BR phase.
This study deals with the distribution of N-330 carbon black in cured 50/50 blend of natural rubber (NR) and butadiene rubber (BR). Dynamic mechanical analysis, dispergrader, filler distribution, Wu¡¯s equation inferred that the N330 have migrated more towards the BR phase.
Keywords: rubber blends, dynamic mechanical analysis, dispergrader, filler distribution, Wu’s equation.
The authors are grateful to Rubber Research Institute of India, Kottayam and Cochin University of Science and technology for the technical supports of this work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The blending of natural rubber (NR) with butadiene rubber (BR) is a common practice to achieve the desired profile characteristics for tire tread. In order to achieve the desired mechanical and physical properties, the blends is mixed with reinforcing fillers like carbon black (CB) or silica. The process of incorporation of the filler and the distribution of the filler plays a crucial role in determining the final properties of the blend.1-4 There are many reviews addressing the filler distribution issues in the literature.5-7
The methods were either by direct microscopy or any other means using a particular property of the rubber that depends upon the filler. Transmission detection microscopy and pyrolysis gas chromatography analysis of filled NR/BR blend showed that the N330 black was located preferably in the BR phase.8 Hess9,10 have performed microscopy studies on a series of carbon black concentration and concluded that the filler was concentrated in the BR phase.Phase contrast microscopy evaluation have also inferred that the CB preferred the BR phase in NR/BR blend.11 The location of carbon black was also performed using Atomic force spectroscopy.12,13 The results were demonstrated on the basis of differences in contrasts of the images. Thermal analysis was also gained considerable interest in finding the localisation of CB in NR/BR blend. Julien Portal and his collegues quantified the distribution of carbon black in NR/BR blend using differential scanning calorimetry (DSC). Varying crystallisation temperature with carbon black loading was the basis of finding.14 Crystallisation temperature of the elastomers will rise on addition of CB filler.15,16 Dynamic mechanical analysis can also be utilised to study the localisation of filler. The size of the damping peak obtained through dynamic analysis can be used to evaluate the affinity of filler in the heterogeneous blend.17 It was reported that carbon black has more affinity towards BR phase in a NR/BR blend.8 Through microscopic observations, they have established the sequence for the affinity of carbon black in the order: SBR, BR, chloroprene rubber, nitrile-butadiene rubber, NR, EPDM. Maiti and Kluppel developed a method for the calculation of the phase specific CB distribution in different rubber blends using dynamic mechanical analysis (DMA).18-20 Wang and his team have measured the macro-dispersions of silica in the vulcanizates before and after post-cure using a dispergrader.21
In the present work, phase specific distribution of N330 carbon black in NR/BR blend has been focused. The investigation was carried out using a DMA along with carbon black dispergrader. Tan δ and glass transition temperature (Tg) were used to locate the preferential absorption of the filler in the blend. The results were well supported by dispergrader analysis. Also, a theoretical evaluation has been performed to trace the thermodynamically favored phase for the accommodation of CB filler.
Materials. The BR used in this study was a linear polymer (Sabic 4610) manufactured by solution polymerization process using stereo specific nickel catalyst and NR (ISNR 5) was obtained from Rubber Research Institute of India, Kottayam.
Laboratory grade zinc oxide was obtained from Emerk Ltd., Mumbai. The coarse white powder has a specific gravity of 5.47. Antioxidants, anti-degradants, accelerators, and pre-vulcanisation inhibitor (PVI) were provided by NOCIL Ltd., Mumbai.
Staining type Antioxidant Pilnox TDQ was in the form of light brown pastille with a specific gravity of 1.1. Antidegradant Pilflex 13 is an alkyl-aryl-PPD with a specific gravity of 0.986-1.00 at 60 oC . Delayed action sulphenamide accelerator Pilcure MOR was used as accelerator in this study. The off white to light tan crystalline powdered PVI has a specific gravity of 1.33 at 25 oC. PG chemicals, Kerala supplied paraffin wax with a melting point of 58-60 oC and density of 0.82. N-grade wood rosin and stearic acid (melting point: 70 oC) was supplied by Sameera chemical Pvt. Ltd., Kottayam. Orient black 330 was supplied by Phillips Carbon Black Limited, India. High aromatic heavy bodied rubber process oil (aromatic oil). was supplied by Indian Oil Corporation.
Preparation of Compounds. The compounds (Table 1) were prepared in two stages- initially a master batch consisting of NR, BR and other additives except curing systems were mixed in an intermix (Francis Shaw, KO intermix MK3) of capacity of 1 litre for 6 min. The temperature was maintained at 50 oC with a rotor speed of 50 rpm. Then the mixes were prepared in a laboratory size two-roll mixing mill (150×325 mm) at a friction ratio of 1:1.25 according to ASTM 3182-07. The temperature of the roll was maintained at 60 oC. The samples were then cured at a temperature of 150 oC using a Monsanto Rheometer (Rheometer MDR 2000).
Characterisation. Dynamic Mechanical Analysis (DMA):DMA was carried out in DMA machine (DMA Q800) of TA instrument. For DMA analysis tension mode of deformation was used under -100 oC to 60 oC temperature range at a heating rate of 2 oC/min. The parameters were recorded at a fixed frequency of 12 Hz. A sinusoidal stress was applied and the corresponding strain in the material was measured. The temperature sweep leads to changes in the modulus which will provide information regarding the molecular motions in the matrix.
Carbon Black Dispergrader: The macro level dispersion of each surface was analyzed using the dispergrader according to the method prescribed in ISO 11345. The test piece of approximate cross-section of 8 mm thickness and 10 mm width was freshly cut from the sample using a single edge razor blade cutter.
Hardness and Mooney Viscosity: Hardness was measured using Durometer Hardness Tester (Shore A) and Mooney viscosity using Mooney Viscometer MV 2000.
|
Table 1 Mixes for Carbon Black Distribution Studies |
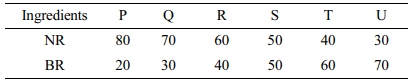
Additives in phr: ZnO-4, Stearic acid-2, N 330-50, Aromatic oil-8, TDQ-1, 6-PPD-1, Paraffin wax-1, Wood rosin-2, Sulphur-2, MOR-1, Retarder-0.20 |
Localization of N330 in the Blend. Thermodynamic Factor: A theoretical evaluation was performed to find out the thermodynamically favored localization of N330 carbon black in the blend. It has been reported that the localization of the filler in a heterogeneous blend is guided by the minimization of interfacial energy.22,23 There are two main equations to find the interfacial energies in a polymer blend. They are harmonic-mean equation (Wu’s equation) and geometric-mean equation (Owens-Wendt-Rabel-Kaelble equation). Wu’s eq. (1) is used for non-polar/non polar blend system whereas OWRK eq. (2) is for polar/non polar system
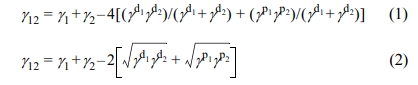
γ1 and γ2 are the surface free energy of polymer components 1 and 2, γ d1 and γ d2 are the dispersion parts of the surface energies of blend components 1 and 2 respectively, γ p1 and γ p2 are the polar parts of the surface energies of blend components 1 and 2 respectively. The values of surface free energies of dispersive and polar parts for a particular temperature were taken from the reported values (Table 2). Using these values, the surface energy at processing temperature (150 oC) can be calculated by the Katayama-Guggenheim formula (3) and (4):

K stands for the temperature correction factor, γo is the surface free energy at 0 oC. T is the processing temperature (150 oC) of the component and Tc is the critical temperature for polymers. The critical temperature for polymers is 1000 K.24,25 The thermodynamic preferential location of carbon black can be evaluated by calculating the wetting coefficient values by the eq. (5): 26

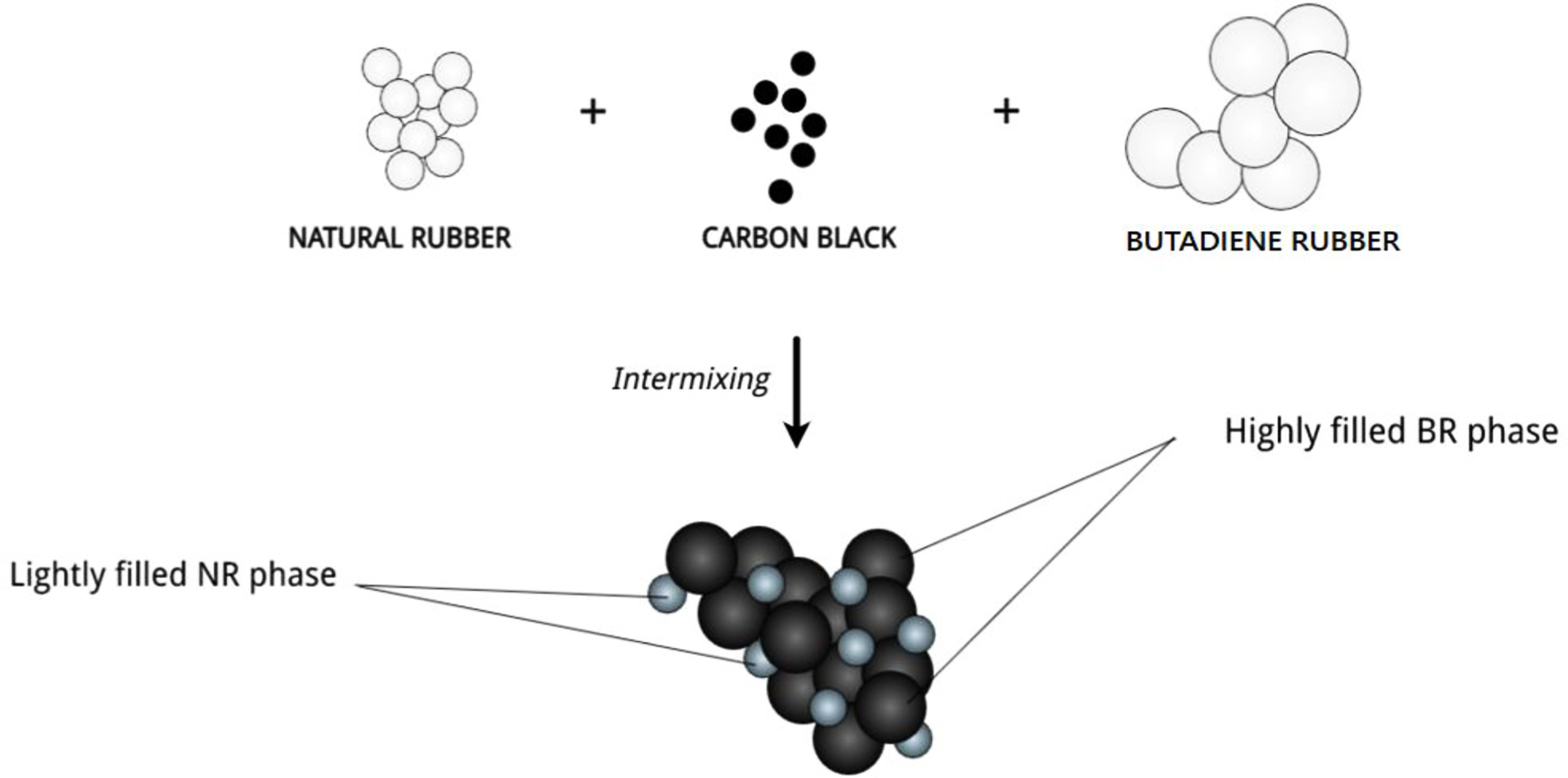
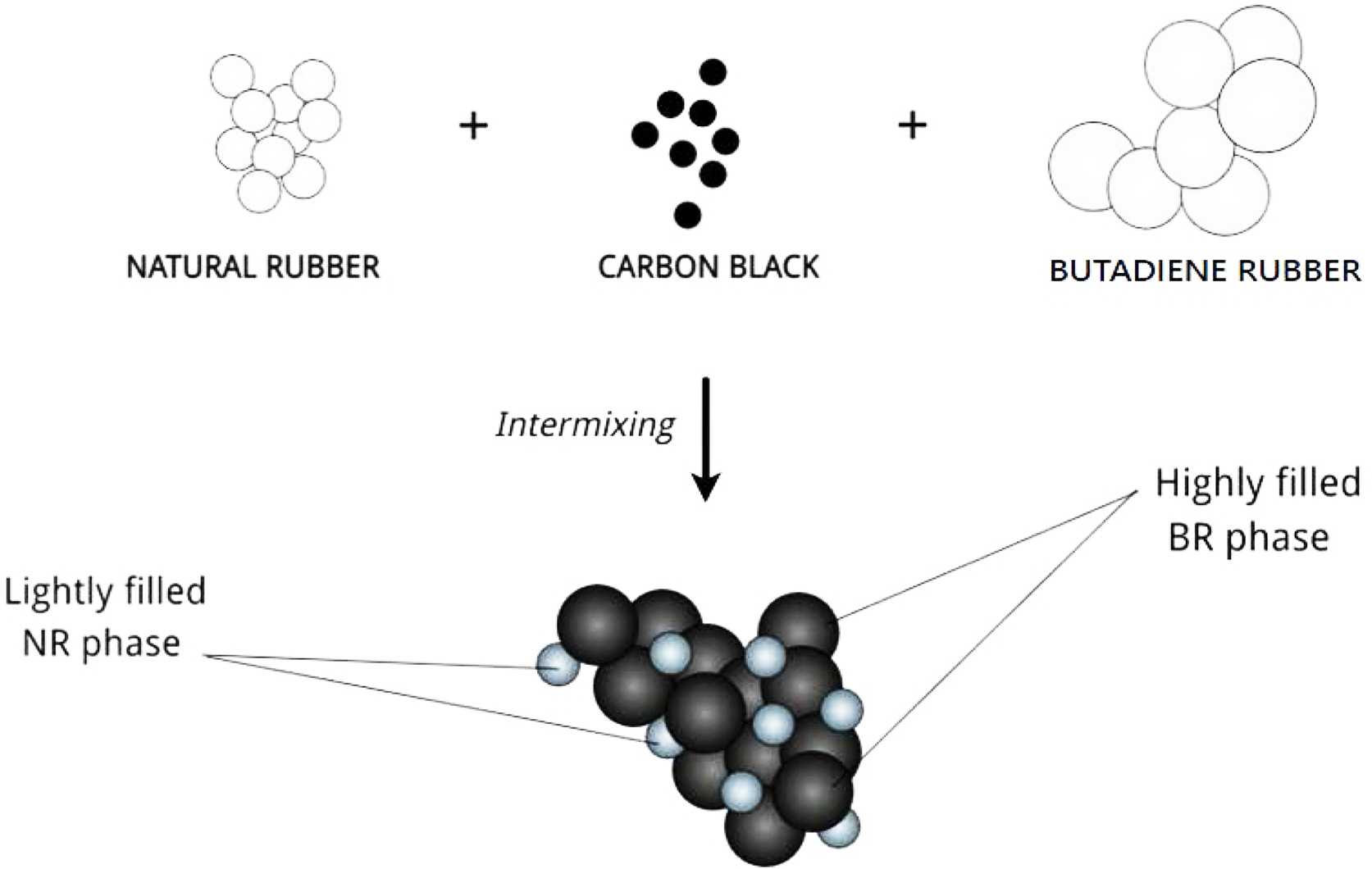
ωa > 1, then the N330 will be localised preferentially in NR phase, if ωa < -1, it will be dispersed in BR phase, but if -1 < ωa< 1, it will be located at the interface between two phases. The wetting coefficient calculated was -1.1. Thus, it can be concluded that the filler is located more in the BR phase (Figure 1).
Dynamic Mechanical Analysis. DMA was used to determine the distribution of carbon black in NR/BR blend. In order to get a co-continuous phase of the compounds, a 50/50 blend of NR/BR was selected for the study. The preferential location of N330 black in the blend was analyzed and described on the basis of damping peaks.27-29 The tan δ indicates the chain mobility and Tg.30
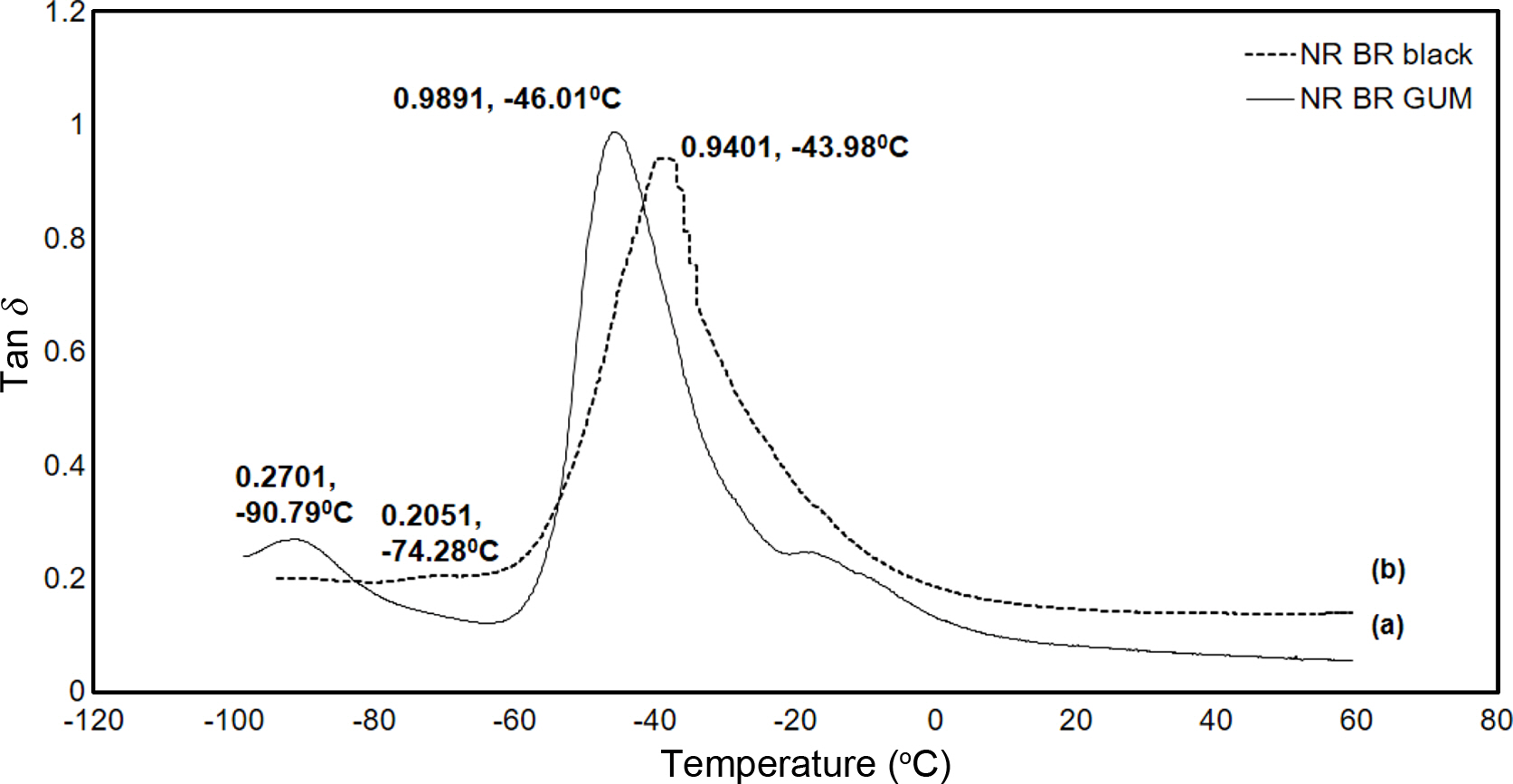
From Figure 2, it was found that the carbon black contribution in NR/BR blend is not uniform as black showed preferential migration towards the BR phase. High values of tan δ indicate that the material has high, non-elastic strain regions whereas low tan δ indicates highly elastic components. The effect of N330 loading on the loss tangent of filled and unfilled blends is shown in Figure 2. It can be seen that the peak of tan δ of filled NR/BR blend has shifted towards higher temperature compared to NR/BR gum. This is because on addition of N330, the restriction on the mobility of polymer chains has been increased. The distribution of filler can be explained on the basis of Tg since the maximum value of tan δ corresponds to the Tg. On addition of N330, there is shift on Tg value towards higher temperatures. The values are tabulated in Table 3.
The preferential migration of N330 in BR phase can be understood from the lower tan δ peak values (0.2701 to 0.2051)31 and also due to the shifting of Tg to more positive values (-90.79 to -74.28 oC). It is to be noted that in filled NR/BR blend, the reduction in tan δ peak of NR phase is 4.95 whereas it is 24% in the BR phase compared to unfilled blend (Table 4).32
On addition of the filler, the chains will constrain and lack of chain mobility will reduce the damping peaks.33,34 Eq. (6) can be used to quantify the amount of filler distribution in each phase of blend. The magnitude of reduction in the tan δ peak is calculated by:
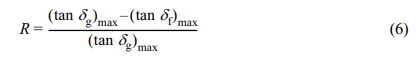
where “g” and “f ” represents the gum and filled systems respectively. The tan δmax is obtained from DMA. The calculated values (RNR=0.0495, RBR=0.2406) have shown that the degree of reduction in RBR is high compared to RNR. The greater reduction of tan δmax corresponds to the higher polymer-filler interaction as a result of preferential migration.
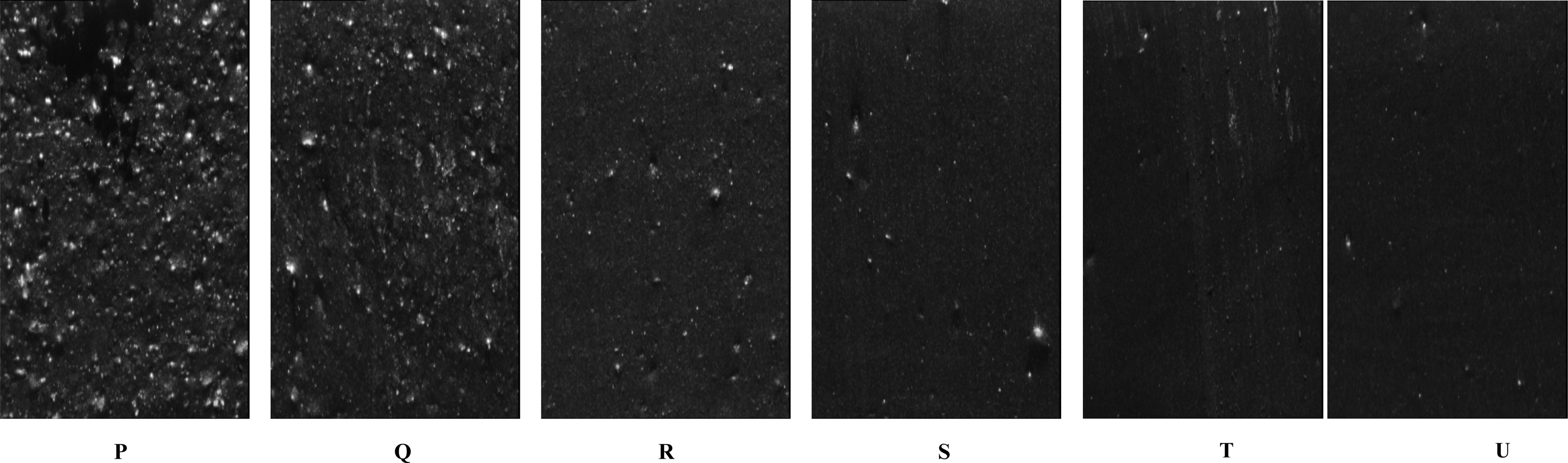
Carbon Black Dispergrader. The dispersion of carbon black in the blend was analysed using a dispergrader. The measure of the dispersion and amount of black is generally expressed by two parameters say X and Y. X-value is the measure of the dispersion of carbon black filler in the polymer matrix. The Y-value only takes into account the bigger agglomerates.35 It was found that there is a homogenous distribution of carbon black in the blend and the homogeneity increased with the BR content (Table 5). The Figure 3 (P to U) shows the dispersion details of the freshly cut surface of the samples. Lower the white areas in the field, higher will be the quality of the dispersion. The trend was found to be decreasing with the increased BR content.
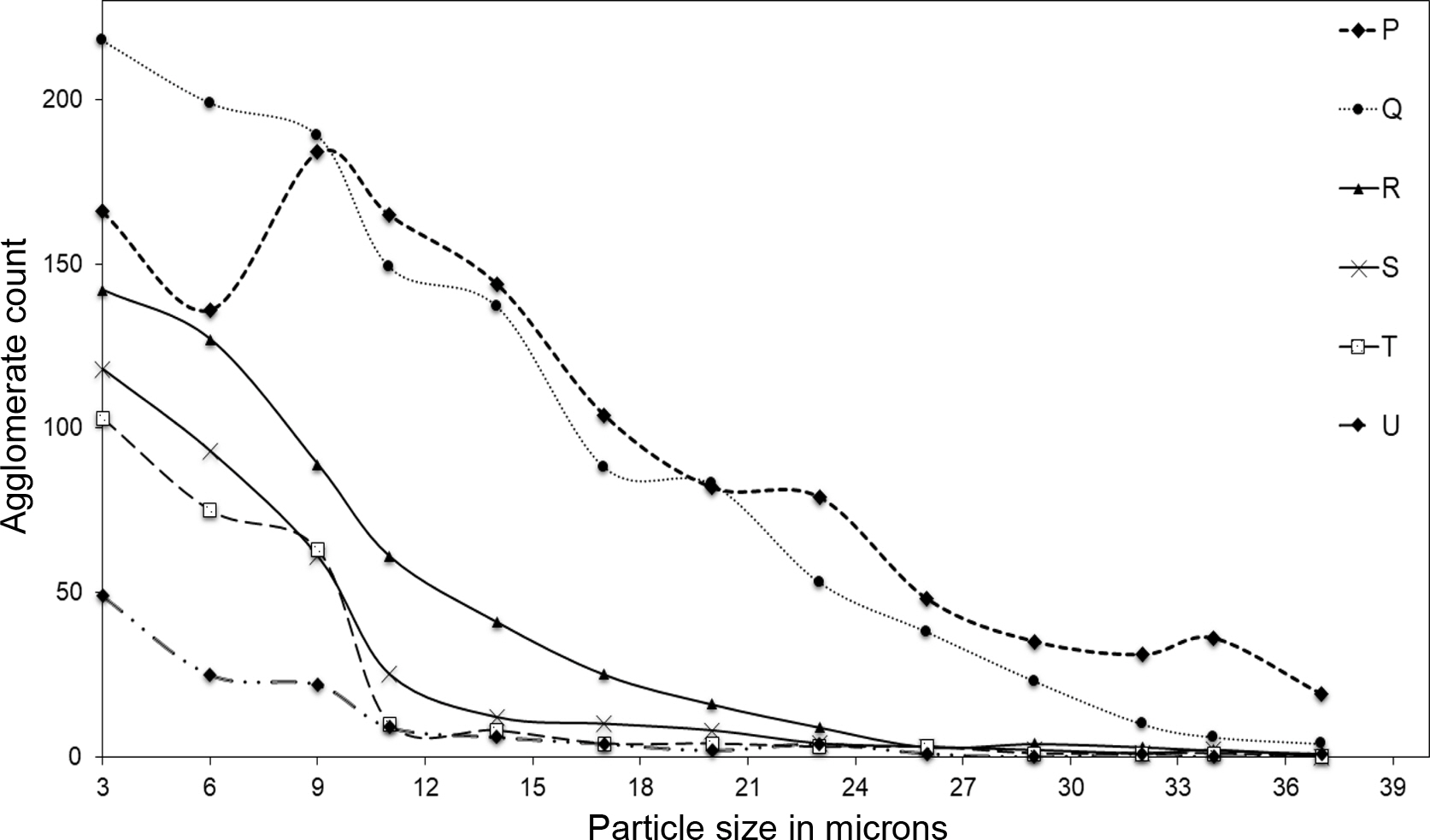
Dispergrader have analyzed the sample area of 2.1 mm×1.6 mm=3.36×106 μm2. The generated histograms have provided a distribution of average particle frequency for 3 scanning processes (Figure 4).
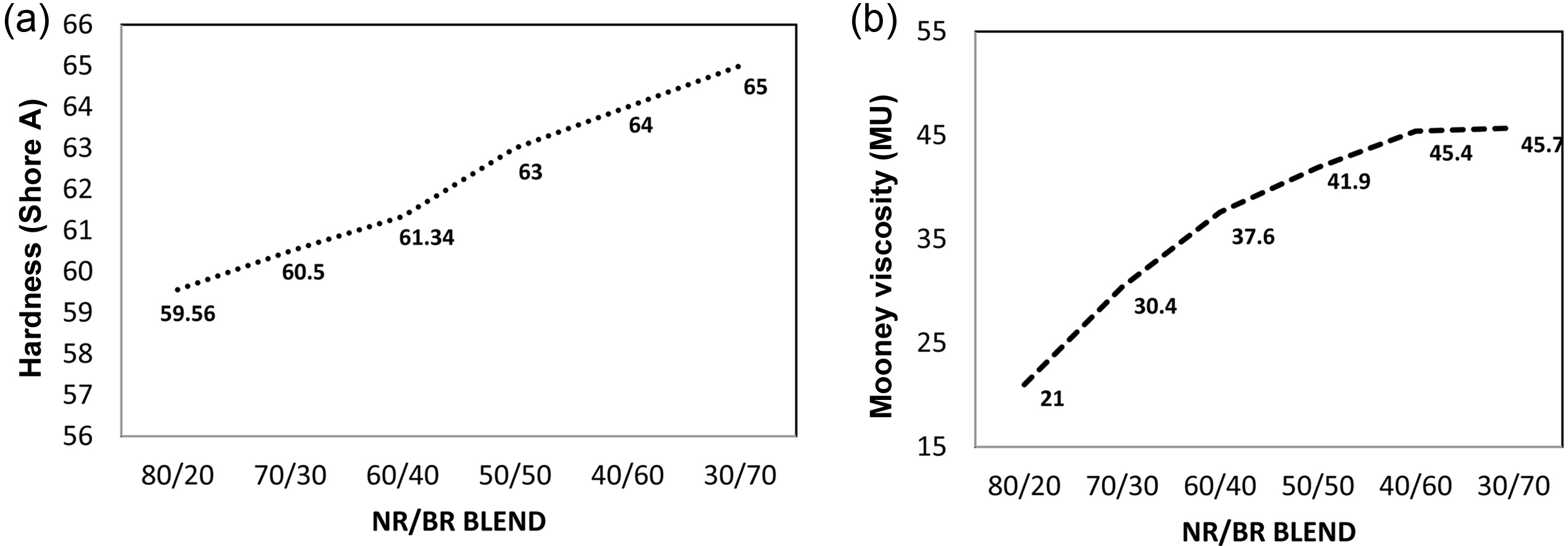
Most of the agglomerate particle size was below 32 μm and as usual the count of agglomerates was found to be decreasing with the higher content of BR. This can be due to the increased dispersion due to filler-polymer affinity. This is clearly substantiated by the increasing mooney viscosity and hardness with the increase in the BR ratio. The increased dispersion in the BR rich blend might have restricted the macromolecular movement of the chains resulting in increased mooney viscosity and hardness (Figure 5(a) and 5(b)).36,37
Apart from the X- and Y-values, the Z-values can also be considered for summarizing the filler dispersion. It is the measure of macro-dispersion. The advantage of using Z-value is that it does not rely upon the reference scales. As it is calculated directly from the size distribution histogram, the errors caused by the dispergrader in selecting the reference scales can be avoided and more reliable result can be made. From the studies (Table 5), it was found that the mixes with higher content of BR have higher Z values which indicate good dispersion and affinity.
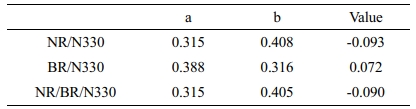
The Cunneen and Russel Plot. The interaction of the filler was assessed with the help of Cunneen and Russel equation38 in 3 systems: N330 filled NR (NR/N330), N330 filled BR (BR/N330), and NR/BR filled with N330 (NR/BR/N330). The equation uses the ratio of the volume fractions of rubbers in unfilled and filled systems instead of amount of solvent absorbed per unit weight of the rubber. The equation used for studying the dispersion of fillers:

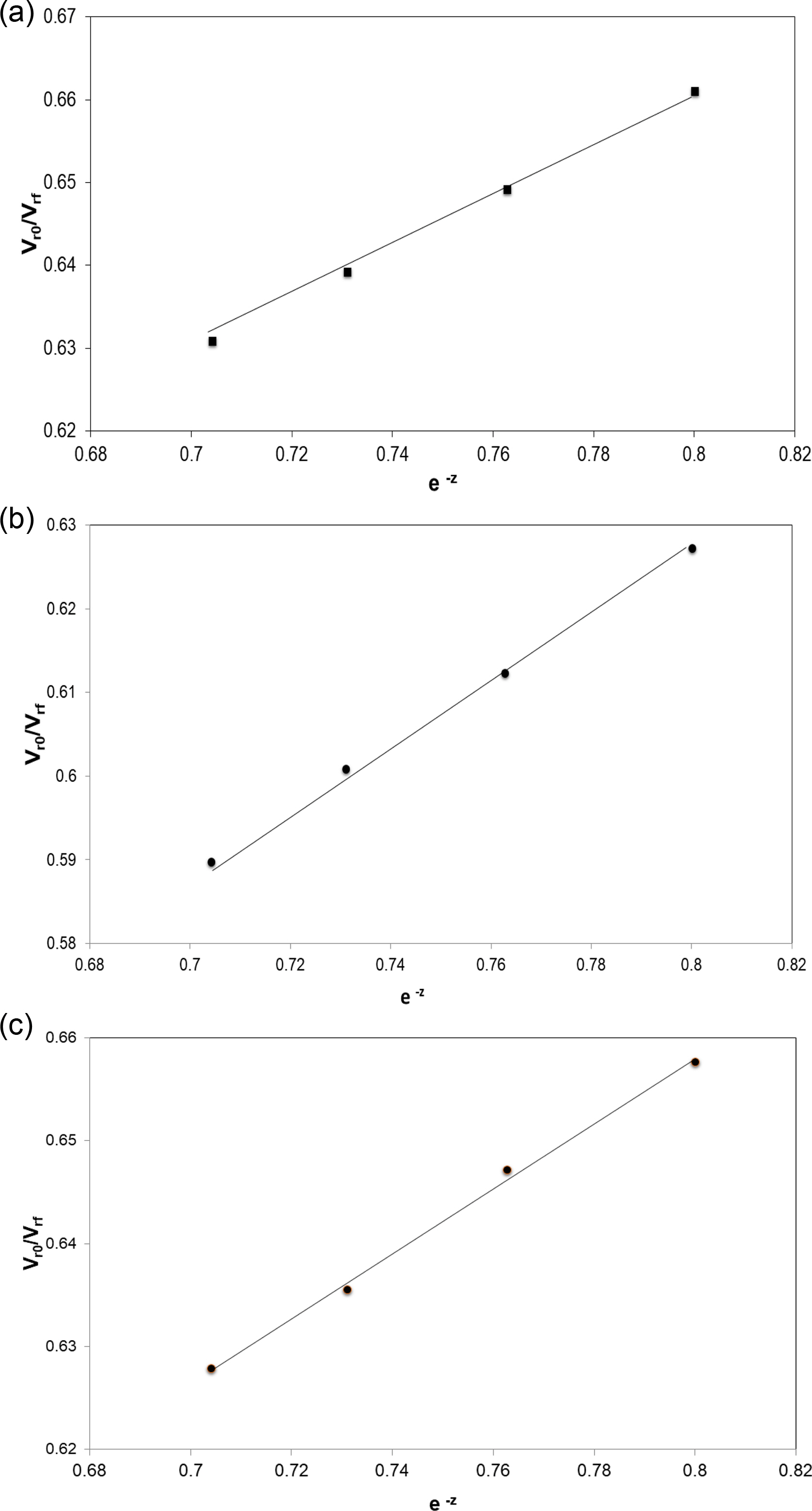
Vr0 and Vrf are the volume fraction of the swollen polymer in a fully swollen unfilled and filled sample respectively, z is the weight fraction of N 330 used (45, 50, 55, 60 phr). A plot of Vr0/Vrf vs. e-z should give a straight line. “a” and “b” represents the slope and intercept which is obtained from the straight line graph (Figure 6). The reinforcing ability and solvent resistance can be assessed using the difference between these two factors. The values obtained from Cunneen-Russel plot is given in Table 6. A simultaneous increase in the “a” value and decrease in the “b” value indicates the extent of interaction with the filler. If the difference between the two values is higher, the interaction of polymer with the filler will be higher. From the table, it is understood that BR/N330 have higher value compared to other two.39
|
Figure 1 Schematic representation of preferential migration of N330 in NR/BR (50:50) blend. |
|
Figure 2 Plot of Tan δ vs. temperature of 50/50 NR/BR blend: (a) unfilled; (b) filled. |
|
Figure 3 Dispergrader images of N-330 filled NR/BR blends. |
|
Figure 4 Plot of particle size distribution plot of N330 filled NR/ BR blend. |
|
Figure 5 Plot showing the variation of (a) hardness; (b) mooney viscosity with N330 |
|
Figure 6 Plot of Vr0/Vrf vs. e-z for (a) NR/N330; (b) BR/N330; (c) NR/BR/N330. |
Addition of filler had resulted in the reduction of tan δ as a result of dilution effect. The non-viscous behaviour of filler has overcome the viscous nature of elastomer. The damping and shift in the Tg had shown that the N 330 has presided more in the BR phase. Hardness and Mooney viscosity have also accompanied with the result. The macromolecular movement of the chains were restricted with the increase in BR content which in turn affected the viscosity and hardness. The statement has been well substantiated by the dispersion analysis using a dispergrader. Percentage of white area and average agglomerate size has been decreased with the increase in the BR content. The parameter X-value which represents the dispersion quality was increased with the increase in BR content.
- 1. Thielen, G. Hydroxy and Nitrile Modified Emulsion SBR’s in Silica Compounds. Kautsch. Gummi Kunstst.2008, 61, 377-382.
- 2. Hai Le, H.; Tiwari, M.; Ilisch, S.; Radusch, H. J. Effect of Molecular Structure on Carbon Black Dispersion in Rubber Compounds - Characterization Using the Online Measured Electrical Conductivity. Kautsch. Gummi Kunstst.2005, 58, 575-580.
- 3. Fouche, P. M.; McGill, W. J. The Effect of the Distribution of Crosslink Densities and Carbon Black Between Phases on Physical Properties of Polyisoprene/polybutadiene Blends. Plast. Rubber Compos. Process. Appl. 1992, 18, 317-321.
- 4. Supova, M.; Martynkova, G. S.; Barabaszova, K. C. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2011, 3, 1-25.
-

- 5. Le, H. H.; Ilisch, S.; Kasaliwal, G. R.; Radusch, H. J. Filler Phase Distribution in Rubber Blends Characterized by Thermo Gravimetric Analysis of the Rubber-Filler Gel. Rubber Chem. Technol. 2008, 81, 767-781.
-

- 6. Le, H. H.; Qamer, Z.; Ilisch, S.; Radusch, H. J. Carbon Black Distribution in the Components of Rubber Blends Monitored by Online Measured Electrical Conductance. Rubber Chem. Technol. 2006, 79, 621-630.
-

- 7. Sirisinha, C.; Prayoonchatphan, N. Study of carBon Black Distribution in BR/NBR Blends Based on Damping Properties: Influences of Carbon Black Particle Size, Filler, and Rubber Polarity. J. Appl. Polym. Sci. 2001, 81, 3198-3203.
-

- 8. Callan, J. E.; Hess, W. E.; Scott, C. E. Elastomer Blends, Compatibility and Relative Response to Fillers. Rubber Chem. Technol. 1971, 44, 814-837.
-

- 9. Hess, W. M.; Chirico, V. E. Elastomer Blend Properties-Influence of Carbon Black Type and Location. Rubber Chem. Technol. 1977, 50, 301-326.
-

- 10. Hess, W. M.; Swor, R. A.; Micek, E. J. The Influence of Carbon Black, Mixing, and Compounding Variables on Dispersion. Rubber Chem. Technol. 1984, 57, 959-1000.
-

- 11. Walter, M. H.; Keyte, D. N. Heterogeneous Structure in Blends of Rubber Polymers. Rubber Chem. Technol. 1965, 38, 62-75.
-

- 12. Jeon, I. H.; Kim, H.; Kim, S. G. Characterization of Rubber Micro-Morphology by Atomic Force Microscopy (AFM). Rubber Chem. Technol. 2003, 76, 1-11.
-

- 13. Galuska, A. A.; Poulter, R. R.; McElrath, K. O. Force Modulation Afm of Elastomer Blends: Morphology, Fillers and Crosslinking. Surf. Interface Anal. 1997, 25, 418-429.
-

- 14. Portal, J.; Carrot, C.; Majeste, J. C.; Cocard, S.; Pellisier, V.; Bertrand, I. A. Quantification of the Distribution of Carbon Black in Natural Rubber/Polybutadiene Blends by Differential Scanning Calorimetry. Polym. Eng. Sci. 2009, 49, 1544-1542.
-

- 15. Li, Y.; Wang, S.; Zhang, Y.; Zhang,Y. Crystallization Behavior of Carbon Black‐filled Polypropylene and Polypropylene/epoxy Composites. J. Appl. Polym. Sci. 2006, 102, 104-118.
-

- 16. Mucha, M.; Marszalek, J.; Fidrych, A. Crystallization of Isotactic Polypropelene Containing Carbon Black as a Filler. Polymer 2000, 41, 4137-4142.
-

- 17. Das, A.; Costa, F. R.; Wagenknecht, U.; Heinrich, G. Nano- composites Based on Chloroprene Rubber: Effect of Chemical Nature and Organic Modification of Nano Clay on the Vulcanisate Properties. Eur. Polym. J. 2008, 44, 3456-3465.
-

- 18. Maiti, S.; De, S. K.; Bhowmick, A. K. Quantitative Estimation of Filler Distribution in Immiscible Rubber Blends by Mechanical Damping Studies. Rubber Chem. Technol. 1992, 65, 293-302.
-

- 19. Kluppel, M.; Schuster, R. H.; Schapper J. Carbon Black Distribution in Rubber Blends: A Dynamic-Mechanical Analysis. Rubber Chem. Technol. 1999, 72, 91-108.
-

- 20. Kluppel, M.; Schuster, R. H.; Heinrich, G. Carbon Black Distribution in Rubber Blends: A Dynamic-Mechanical Analysis. Rubber Chem. Technol. 1997, 70, 243-255.
- 21. Wang, M. J.; Morris, M. D.; Kutsovsky, Y. Effect of Fumed Silica Surface Area on Silicone Rubber Reinforcement. Kautsch. Gummi Kunstst. 2008, 61, 107-117.
- 22. Das, D.; Satapathy, B. K. Microstructure-Rheological Per Location Mechanical Properties Correlation of Melt-processed Polypropylene-multiwall Carbon Nanotube Nano Composites: Influence of Matrix Tacticity Combination. Mater. Chem. Phys. 2014, 147, 127-140.
-

- 23. Schonhorn, H. Extrapolation of the Critical Temperature of n‐hydrocarbons Application to Polymers. Macromol. Chem. Phys. 1966, 93, 262-267.
-

- 24. Mural, P. S.; Madras, G.; Bose, S. Positive Temperature Coefficient and Structural Relaxations in Selectively Localized MWNTs in PE/PEO Blends. RSC Adv. 2014, 4, 4943-4954.
-

- 25. Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of Fillers and the Electrical Conductivity of Polymer Blends Filled with Carbon Black. Polym. Bulletin 1991, 25, 265-271.
-

- 26. Stickney, P. B.; Mueller, W. J. Influence of Carbon Black on Swelling Rate of Polymer in Filled Vulcanizates. Rubber Chem. Technol. 1969, 42, 604-612.
-

- 27. Lee, B. L. Progress in Multiphase Rubber Processing: Controlled Ingredient-distribution Mixing. Polym. Eng. Sci. 1985, 25, 729-740.
-

- 28. Gubbels, F.; Blacher, S.; Vanlanthem, E.; Jerome, R.; Deltour, R.; Brouers, F.; Teyssie, P. H.; Design of Electrical Composites: Determining the Role of the Morphology on the Electrical properties on the Carbon Filled Polymer Blend. Macromolecules 1995, 28, 1559-1566.
-

- 29. Raiati, M.; Kalaee, M.; Mazinani, S. Effect of Filler Type and Content on Physical and Mechanical Properties of NR/SBR Nanocomposite Blend. Rubber Chem. Technol. 2017, 90, 751-764.
-

- 30. Wang, K.; Liang, S.; Deng, J. N.; Yang, H.; Zhang, Q.; Fu, Q.; Dong, Q.; Wang, D.; Han, C. C. The Role of Clay Network on Macromolecular Chain Mobility and Relaxation in Isotactic Polypropylene/organoclay Nanocomposites. Polymer 2006, 47, 7131-7144.
-

- 31. Warasitthinon, N.; Robertson, C. G. Interpretation of the tan δ Peak Height for Particle Filled Rubber and Polymer Nano- composites with Relevance to tire Tread Performance Balance. Rubber Chem. Technol. 2018, 91,577-594.
-

- 32. Bandyopadhyay, A.; Thakur, V.; Pradhan, S.; Bhowmick, A. K. Nano Clay Distribution and its Influence on the Mechanical Properties of Rubber Blend. J. Appl. Polym. Sci. 2010, 115, 1237-1246.
-

- 33. Bose, S.; Bhattacharyya, A. R.; Kulkarni, A. R.; Potschke, P. Electrical, Rheological and Morphological Studies in Co-continuous Blends of Polyamide 6 and Acrylonitrile-butadiene-styrene with Multiwall Carbon Nanotubes Prepared by Melt Blending. Compos. Sci. Technol. 2009, 69, 365-372.
-

- 34. Hiep, N. Q.; Huy, T. H.; Nguyen, P. C.; Sy, D. T.; Khang, D. Q. Preparation and Properties of Rubber Nanocomposites Based on NR/NBR Blend Reinforced with Nanosilica and Carbon Black. Vietnam J. Chem. 2019,57, 213-217.
-

- 35. Otto, S.; Randl, O.; Goncalveset, O.; Cantaloube, B. New Reference Value for the Description of Filler Dispersion with the Dispergrader 1000 NT. Kautsch. Gummi Kunstst. 2005, 58, 390-393.
- 36. Dong, A. N.; Zhiyi, Z.; Haixiang, J.; Jinquan, S.; Huan, Z.; Yaqing, L. Effect of Carbon Black Nature on Dynamic Mechanical Properties and Reinforcement of NR Vulcanization by Latex Techniques. Rubber Chem. Technol. 2017, 90, 611-620.
-

- 37. Narongthong, J.; Sae-Oui, P.; Sirisinha, C. Effect of Mixing Parameters and Their Interaction on Properties of Carbon Black Filled Styrene-butadiene Rubber.Rubber Chem. Technol. 2018, 91, 521-536.
-

- 38. Rooj, S.; Das, A.; Stockelhuber, K. W.; Wang, D. Y.; Galiatsatos, V.; Heinrich, G.; Understanding the Reinforcing Behavior of Expanded Clay Particles in Natural Rubber Compounds. Soft Matter 2013, 9, 3798-3808.
-

- 39. Das, D.; Satapathy, B. K. Microstructure-rheological Per Location-mechanical Properties Correlations of Melt-processed Poly- propylene-multiwall Carbon Nanotube Nano Composites: Influence of Matrix Tacticity Combination. Mater. Chem. Phys. 2014, 147, 127-140.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(6): 865-871
Published online Nov 25, 2021
- 10.7317/pk.2021.45.6.865
- Received on Jun 9, 2021
- Revised on Jul 23, 2021
- Accepted on Jul 26, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Result and Discussions
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Vaishak Nambiathodi
-
Technical Consultancy Division, Rubber Research Institute of India, Kottayam, Kerala-686009, India
- E-mail: siby@rubberboard.org.in
- ORCID:
0000-0002-5803-0195












 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.