- Potential of 3D Bioprinting Techniques in Tissue Engineering
Department of Polymer Science and Engineering, Kumoh National Institute of Technology, Gumi, Gyeongbuk 39177, Korea
*Laboratory of Tissue Engineering, Korea Institute of Radiological and Medical Sciences, Nowon, Seoul 01812, Korea- 조직공학에서의 3D 바이오프린팅 기술의 잠재력
금오공과대학교 고분자공학과, *한국원자력의학원 조직공학연구실
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Three-dimensional (3D) bioprinting is an additive manufacturing technique used for the 3D modeling of biological constructs containing cells. In particular, customized artificial tissues (bone/cartilage, skin/muscle, blood vessels, organs system) with precise and uniform structures can be created using computer-aided design, 3-axis plotting instrument, and optimum physicochemical cell-laden bioinks. The main bioprinting procedures are three stages of modeling, solid freeform fabrication, and post-maturation. This review introduced the basic principles and components of 3D bioprinting and potential research cases to summarize the challenging agenda in practical tissue engineering and medical applications.
3차원(3D) 바이오프린팅은 세포를 함유한 생물학적 구조물의 입체적 조형에 사용되는 적층제작술이다. 특히, CAD(computer-aided design)와 3축 출력기구 및 세포를 함유한 최적의 물리화학적 바이오잉크를 사용하여 정확하고 균일한 구조의 맞춤형 인공조직(뼈/연골, 피부/근육, 혈관, 장기 시스템)을 제작할 수 있다. 바이오프린팅의 주요절차는 모델링, 고체 자유형상 제작 및 후성숙의 세 단계로 진행된다. 본 리뷰에서는 3D 바이오프린팅의 기본원리, 구성요소 및 잠재력 있는 연구사례들을 소개하고 실제적인 조직공학 및 의료적 응용분야에서의 도전과제를 요약하였다.
This review introduced the basic principles and components of 3D bioprinting and potential research cases to summarize the future challenges in practical tissue engineering and medical applications.

Keywords: additive manufacturing, 3D bioprinting, bioink, tissue engineering, medical.
This paper was supported by Research Fund, Kumoh National Institute of Technology (2018104106).
The authors declare that there is no conflict of interest.
Additive manufacturing (AM) or three-dimensional (3D) printing are fabrication techniques of 3D objects by layering materials using computer-aided design (CAD) or digital computed tomography (CT) scanned image models.1 In AM or 3D printing, a 3D object is built up using numerous computer-controlled processes that deposit, fuse, and solidify materials. Materials such as ceramics, metals, thermoplastics, gels in liquids, and powder grains can be used in AM. Charles W. Hull first introduced the concept of 3D printing technology in 1986.2 Since then, 3D printing techniques have gained prominence over the past few decades because of their beneficial attributes such as high precision, complex part construction, and customized object fabrication, relatively fast processing, and use of cost-effective materials and simple instruments.1-8 The essential features of 3D printing are 3D modeling software, mechanical plotting equipment, and layering materials.6 After the 3D modeling design, the 3D printing equipment reads the data from the 3D stereolithography (STL) file and fabricates a 3D object.5
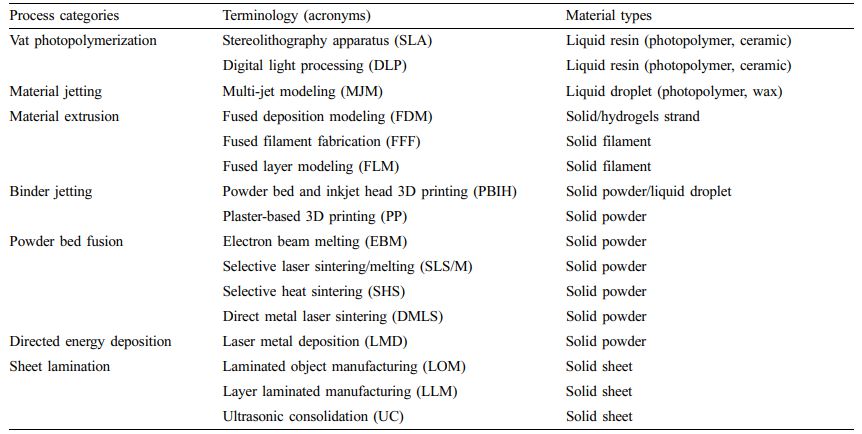
The fabrication approaches referred to as 3D printing technology based on the ISO/ASTM52900-15 standard can be classified into seven process categories (Table 1).2 In brief, vat photopolymerizable 3D printing has a container of photocurable resin, which is cured with ultraviolet (UV) light or other similar power sources. The most used techniques for this process are stereolithography apparatus (SLA) and digital light processing (DLP). This method passes a UV laser through a liquid photopolymer resin to fabricate 3D structures. The structure is then fabricated layer-by-layer and later cured using UV light.
Meanwhile, material jetting feeds the material through a small-diameter nozzle during the material injection process, which works like a general inkjet printer. Multi-jet modeling (MJM) is an inkjet printing process that uses print head technology to deposit photocurable plastic resin or cast wax material layer by layer. The most used technique in material extrusion processes is fused deposition modeling (FDM). During the material injection process, the material is fed through a small-diameter nozzle, and the operation behavior is similar to that of a typical inkjet printer. A representative method of FDM is fused filament fabrication (FFF), which is the most common and simple 3D printing method. In FFF, a thermoplastic filament is used as a printing material. The 3D print head melts the filament by applying heat and then depositing its layers over each other to create a 3D structure. The binder jetting process uses a liquid binder and a powder material. The print head drops a liquid binder selectively onto a powdered material to obtain a 3D structure. And, this method could print a various materials such as polymers metals and ceramics. Selective laser sintering/melting (SLS/M) is a commonly used technique in powder bed fusion processes.8 During the SLS process, small particles (powders) of polymers, glass, or ceramics are fused by a high-power laser to fabricate a 3D structure. Direct energy deposition has a printing unit consisting of a multi-axis robotic arm with nozzles, an energy source and a substrate for depositing molten material. During SLS, a powdered material deposited on a substrate through a nozzle is melted by an energy source and then cured to create a 3D structure. Sheet lamination technology uses an external force to incorporate other materials into a sheet. Sheets can be made of metals, polymers, hydrogels, ceramics, paper, and fabrics. In the sheet lamination process, sheets are laminated together by heat and pressure and cut into desired shapes using a laser or blade. Inductive energy deposition processes are mainly used in the advanced metal industry and rapid manufacturing applications.
As described above, most 3D printing methods have been recently applied in tissue engineering.9-11 The goal of tissue engineering is the regeneration, restoration, or replacement of defective or damaged functional living organs and tissues. To achieve this purpose, biomedical scaffolds have been used in tissue engineering applications.9 The primary focus of this approach is to functionally and structurally replace or regenerate defected tissues.10 The scaffolds generally used as tissues and organs must provide internal pathways for cell adhesion and migration. Scaffolds must also deliver various growth factors and waste products and perform several essential functions that maintain their shape during cell growth and appropriate mechanical properties.10 To secure these functions, scaffolds for tissue engineering require porous 3D structures that allow cell affinities such as proliferation, migration, adhesion, and differentiation, as well as nutrient and oxygen transport.11 Therefore, 3D printing technology is one of the most suitable methods to fabricate 3D structures for use as medical scaffolds, tissues, and organs. Scaffold 3D printing is a technology that controls cell patterns to maintain cell function and viability within the printed 3D structures. Many researchers have already studied the development of an appropriate scaffold using 3D printing in tissue engineering.9-21 Recent advances introduced by 3D printing have significantly improved the ability to control the pore dimension distribution, volume, and interconnectivity of scaffolds.9-11 In addition, 3D printing has been recognized for significant advances in tissue engineering, especially in studying biomaterials. The development of biomaterials for 3D printing is an essential prerequisite that directly affects cell growth.
3D printing studies for tissue reconstruction using biomaterials are classified into cell-free solid and cell-laden hydrogel bioink scaffolds.22 Research related to solid scaffold printing has established a technique for producing a precise and reproducible porous scaffold by controlling the characteristics of the materials and parameters of the manufacturing process. Among the various 3D printing methods for tissue engineering, 3D bioprinting processes involving living cells and bioactive molecules in biomaterials (hydrogel state bioink) have successfully created 3D structures at room temperature without significantly affecting cell viability.22,23 In particular, 3D bioprinting uses CT data to fabricate precise and uniform biological cell-laden constructs compared to general solid objects with optimum physicochemical bioinks as patient-customized artificial tissues (bone/cartilage, skin/muscle, vascular, organ systems).22-25 However, studies related to bioprinting using a cell-containing bioink have problems that require solutions, such as a bioink must have a soft and biocompatible nature for the included cells. For applications using 3D bioprinting technology in tissue engineering, researchers must consider not only the 3D structure (design) but also the biomaterial (bioink) as well (Tables 2 and 3).26,27 The ideal bioink formulation should satisfy the biological requirements of printability, mechanical properties, biodegradation, modifiable functional groups on the surface, and post-printing maturation. 3D bioprinting involves fabricating a 3D structure with the desired shape by combining living cells and biological materials (Figure 1). Researchers are developing various methods to fabricate 3D native structures with biological and mechanical properties suitable for native tissue regeneration. Hence, this review describes several types of 3D bioprinting techniques using bioinks to fabricate 3D structures and their applications in tissue engineering and regenerative medicine.
As the demand for regenerative medicine increases, tissue engineering has become a forward-looking approach to repairing tissues and organs with severe defects that cannot be repaired by natural healing and routine treatment. The human body is comprised complex tissues, such as skin, fat, muscle, blood vessels, endothelium, cartilage, ligaments, and bones. However, the form and function of regenerated tissue after severe damage cannot fully restore the organizational continuity of the original tissue. Therefore, advanced scaffolds that can serve as morphological guides for connecting and growing cells are essential. 3D scaffolds for tissue engineering are porous, allowing for the increased mass exchange efficiency of seeded cells to proliferate and metabolize structures. The combination of scaffold-based tissue engineering and 3D bioprinting technology could enable new possibilities for intact tissue restoration by printing patient-specific biological constructs. Here, we review the basic principles, components, and potential research cases of 3D bioprinting. Prospective bioinks for practical tissue engineering and medical applications are also summarized.
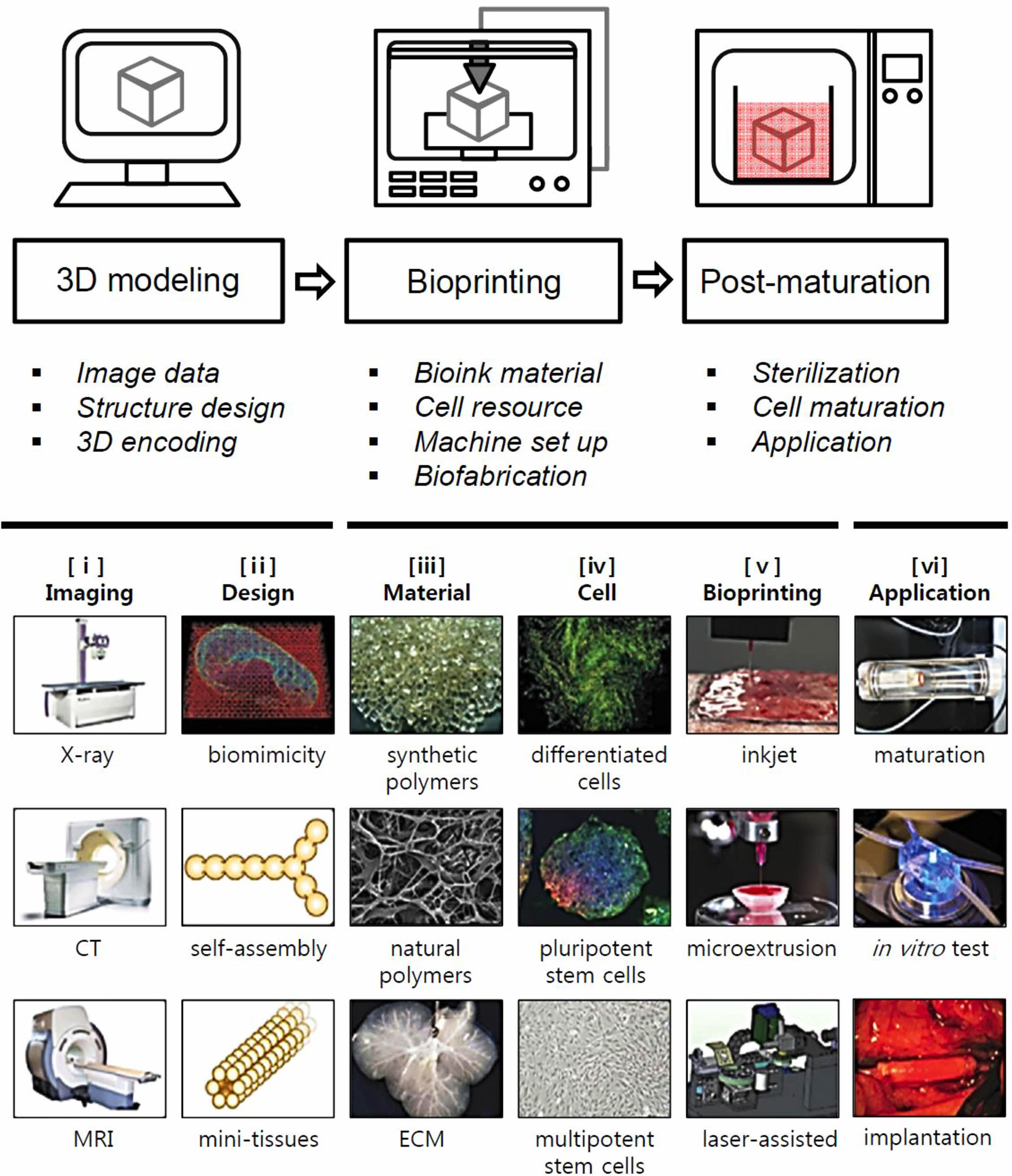
|
Figure 1 The schematic of 3D bioprinting stages with subcategories and typical process on six steps: (i) imaging the injured site/tissue using scanning instruments; (ii) image converted to 3D models; (iii) materials based on intended applications; (iv) cell selection based on intended applications; (v) bioprinting using bioprinters; (vi) post-processing application in a bioreactor for maturation, in vivo test, implantation at injury sites. Reproduced with permission from Ref. 50, S. V. Murphy et al., Nat. Biotechnol., 2014, 32, 773-785.© 2014, Nature America, Inc. |
|
Table 1 Terminology of Additive Manufacturing Technologies with Material Types (ISO/ASTM52900-15)2 |
|
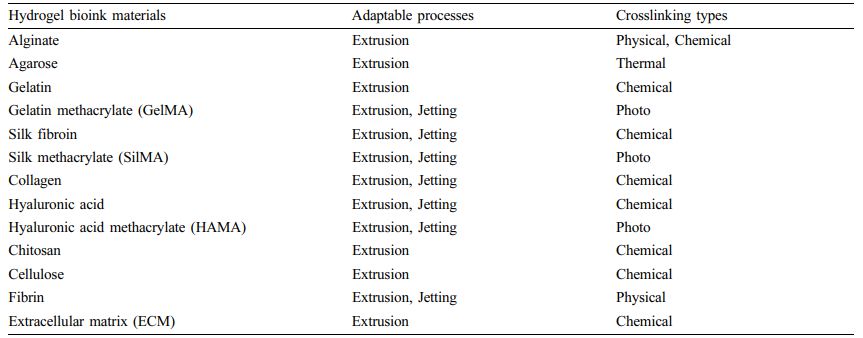
Table 2 Natural Polymer Based Bioinks with 3D Bioprinting Process and Crosslinking Types26,27 |
3D bioprinting consists of three stages: 3D modeling (pre-bioprinting), bioprinting, and postmaturation (post-bioprinting) (Figure 1).22-25 The three major types of 3D bioprinting are inkjet, laser-assisted, and extrusion processes. Inkjet bioprinters are primarily used for fast and large-scale products. The drop-on-demand inkjet allows materials to be deposited in exact amounts, minimizing cost and waste. Laser-assisted bioprinting provides high-resolution printing. Extrusion bioprinters build cells with hydrogels layer by layer. Among these, the extrusion bioprinter process is prominent for fabricating cell-laden 3D constructs.
3D Modeling and Toolpath Programming. The first stage of 3D bioprinting is the digital modeling of a 3D object. This section describes 3D modeling for printing objects from medical images of the organs or tissues to be bioprinted: CT data collected from magnetic resonance imaging (MRI), X-ray, and ultrasound. Most 3D image data are obtained from medical scans of a patient. The quality and usability of 3D models depend on the software and designer proficiency. The first step is segmenting the raw image data using image processing software. Then, image segmentation creates an STL format using a 3D CAD surface model. This surface model approximates the outer shape of the construct by using a triangular mesh. The surface model is then filled with repeating unit cells to generate a complete construct with an internal structure. Finally, the software generates a toolpath plan as a G-code file from a 3D model object. This software provides the motion path for the bioprinter to build up a bioink at the set time with the XYZ axis location.
Materials Used for Bioprinting. Bioinks are a liquid mixture of cells, substrates, and nutrients, used to produce engineered tissues via 3D printing. These inks are primarily composed of the cells that are used. The combination of cells and hydrogels is defined as a bioink.26,27 They must meet specific rheological, mechanical, biofunctional, and biocompatibility properties that ensure reproducibility and precise control over the fabricated constructs in automated 3D bioprinting.Biomaterials constituting bioink are divided into two types: natural and synthetic. Some natural biomaterials include substances present in the extracellular matrix (ECM) such as collagen, gelatin, fibrin, and hyaluronic acid and other natural biomaterials such as acellular dermal matrix, agarose, alginate, chitosan, cellulose, and silk fibroin (Tables 2 and 3). Natural materials have excellent biocompatibility. However, they have disadvantages, such as poor mechanical properties and slow gelation time. Unlike natural biomaterials, synthetic biomaterials have more controllable mechanical and chemical properties. Polyethylene glycol (PEG) and pluronics (poloxamers) are widely used as synthetic bioinks in 3D bioprinting. However, these materials lack adequate biocompatibility and biodegradability, limiting their application in 3D bioprinting. In light of this, modified natural bioinks such as gelatin methacrylate (GelMA), silk methacrylate (SilMA), and hyaluronic acid methacrylate (HAMA) have been developed to be a compromise between natural and synthetic bioinks. The physicochemical properties of hydrogel bioinks are more conducive for printability than those of general 3D printing materials. Considerations in the printability of bioinks include gelation, uniformity in filament diameter, maintenance of shape fidelity after printing but before crosslinking, printing pressure, nozzle diameter, and printing viscosity.
Bioprinting Parameters. This section describes the second stage of 3D bioprinting.22 When a toolpath plan is created from a 3D model object as a G-code file, specific cells are isolated and proliferated. The cells are then mixed with a particular liquid substance that provides oxygen and other nutrients for survival. In some processes, cells are encapsulated into cell spheroids with a diameter of 500 μm. Such cell aggregation does not require a scaffold and is required for placement in tubular tissue fusions for processes such as extrusion. In the second step of bioprinting, known as bioinks, is placed in a printer cartridge and 3D bioprinted on the motion path of the G-code file. When bioprinted pre-tissues are transferred to an incubator, cell-laden pre-tissues mature into tissues. Artificial organs, such as the liver and kidneys made with 3D bioprinting, have been shown to lack the important factors that affect the body: working blood vessels, tubules to collect urine, and the growth of billions of cells needed for these organs. Without these components, the body cannot obtain essential nutrients and oxygen from deep inside. All tissues in the body are naturally composed of different cell types. Many techniques for printing these cells differ in their ability to ensure the stability and viability of cells during manufacturing. Some methods used for the 3D bioprinting of cells are photolithography, magnetic 3D bioprinting, stereolithography, and direct cell extrusion.22
Postmaturation Process. 3D bioprinting requires a postmaturation stage to create a stable structure from biological tissues.28 If this process is not well maintained, the mechanical integrity and function of the 3D bioprinted object are at risk. Both mechanical and chemical stimuli are required to hold the object. These stimuli send signals to the cells to control tissue remodeling and growth. Recent developments in bioreactor technology have enabled rapid tissue maturation, tissue vascularization, and graft viability. Bioreactors provide convective nutrient transport, create a microgravity environment, change the pressure that forces a solution to flow through cells, and add compression for dynamic or static loading. Each type of bioreactor is ideal for different tissue types. For example, compression bioreactors are ideal for cartilage tissues.
Bone and Cartilage Tissue. This section describes the bone tissue engineering sector based on various 3D bioprinting approaches. Bone has highly specialized organic-inorganic structures that can be classified into microscopic and nanocomposite structures that are held adjacent to complex cellular components.29-34 Researchers have investigated efficient approaches to replace lost or defective bone and develop reliable bone substitutes.35-42 In general, porous scaffolds are used for bone regeneration in surgical operations. The bone exhibited an excellent self-healing ability when the defect was minor. However, massive bone loss or critical bone defects cannot be fully healed by the innate regenerative system of the body. In these cases, surgery for guided bone regeneration is necessary to replace defected bones. Researchers have attempted to manufacture bone substitutes and develop restoration methods using 3D bioprinting techniques.35 Bones can be classified into two types of structures: the cancellous and cortical bones. Cancellous bone (the inner bone) has a spongy structure with 50-90% porosity. The cortical bone formed a dense outer layer with less than 10% porosity. Owing to the differences in the internal and external structures of bone, an accurate scaffold design is necessary for bone regeneration. Scaffolds can be used to promote bone regeneration by delivering biomolecules, such as transforming growth factor-β (TGF-β), bone morphogenetic protein (BMP), insulin-like growth factor (IGF), fibroblast growth factor (FGF), or vascular endothelial growth factor (VEGF).
Recently, researchers have become interested in 3D bioprinting, which can generate complex structures using various biomaterials and cell-laden bioinks. Kang et al. demonstrated the capabilities of an integrated tissue-organ printer (ITOP) by fabricating the mandible and calvarial bone, cartilage, and skeletal muscle (Figure 2).38 Mandibular bone reconstruction is a 3D defect model obtained from the craniofacial CT image data, followed by the design of the dispensing paths of cells, poly(ε-caprolactone) (PCL), and Pluronic F-127 using self-developed software. Multiple cartridges used to deliver and pattern the ink materials were connected to a microscale nozzle, which dispensed the materials according to the design during the 3D printing process. PCL was printed as the framework, and the cell-laden hydrogel was dispensed to fill the pores, while Pluronic F-127 was used as a sacrificial material. The osteogenic potential of the scaffold was confirmed by Alizarin Red S staining after culturing in an osteogenic medium for 28 days. Dong et al. incorporated PCL and chitosan gels to fabricate a hybrid scaffold for bone regeneration.20 The 3D PCL scaffold was fabricated by the FDM bioprinting method. In vitro studies have shown that hybrid 3D scaffolds can enhance cell proliferation and improve the osteogenicity of rabbit bone marrow mesenchymal stem cells (MSCs). The hypothesis is that PCL/chitosan 3D scaffolds could improve osteoinductivity and cell seeding efficacy and provide superior mechanical properties than PCL or chitosan-thermogel 3D scaffolds alone. To realize custom scaffolds for bone tissue engineering, Corcione et al. developed a solvent-free process to produce hydroxyapatite and poly(lactic acid) (PLA) composites suitable for 3D printing processes using the FDM method.17 In their study, they successfully converted clinical images of the maxillary sinus obtained by cone-beam CT into an appropriate format. They used 3D bioprinting of the composite material to fabricate 3D maxillary sinus models. Wang et al. explained that cell-laden collagen/alginic acid scaffolds could be supplemented with bioglass particles to fabricate bone replacement scaffolds, increasing material stiffness and stimulating cell growth and mineralization.40
Only a few millimeters thick cartilages prevent friction between joints and withstand extreme load stress during limb movement. Cartilage defects due to trauma, aging, degenerative diseases and other factors inevitably lead to joint pain and chronic diseases. Despite numerous attempts, artificial cartilage that can completely mimic the composition, ECM, and mechanical properties of tissues has yet to be developed. 3D bioprinting, which can fabricate products with the desired shape using various materials and cells, offers excellent opportunities for cartilage tissue engineering. Kundu et al. fabricated cell-laden 3D scaffolds using PCL and chondrocyte-encapsulated alginate hydrogels.18 Cell-laden biochemical in vitro assays were performed to determine the DNA, total collagen content, and glycosaminoglycans (GAG) in various alginate/PCL gel configurations. Alginate/PCL gels containing TGF-β showed more significant ECM formation. Cell-printed 3D alginate/PCL gel scaffolds were implanted into the dorsal subcutaneous space of female nude mice. Immunohistochemical analysis showed improved cartilage tissue with collagen type II fibril formation in the alginate/PCL gel hybrid scaffolds after 4 weeks. The Kesti et al. has developed a cartilage-specific bioink for 3D bioprinting applications based on a mixture of alginate and gellan mixed with cartilage particles.39 They used MRI to observe bioprinted scaffolds, compare their 3D shape to the original model and evaluate the utility of MRI to detect changes in water relaxation time associated with ECM generation in tissue-engineered grafts. Bioink/BioCartilage discs containing cells were cultured in vitro for 8 weeks, with or without TGF-β3 supplementation to evaluate chondrogenesis. All properties of the 3D bioprinted scaffold were superior to those of native articular cartilage. Ren et al. used collagen hydrogels as bioinks and 3D cartilage structures.42 Chondrocyte density gradients revealed a regional distribution throughout the ECM. They evaluated the effect of chondrocyte density gradients on the formation of regional ECM distributions in bioprinted 3D structures. ECM generation was positively correlated with cell density in the early stages of culture; the biosynthetic capacity of chondrocytes was affected by both the cell density and distribution in the bioprinted 3D constructs.
Skin and Muscle Tissues. This section introduces the study of human skin models using 3D bioprinting technology. The skin is the largest organ in the human body that protects other tissues from external stimuli. Skin damage that leads to infection or other genetic or physical diseases can cause chronic ulcers.43,44 Skin damage can expose other tissues to bacteria, viruses, and the external environment, interfering with body temperature regulation.45 The pathological components of the normal skin flora can multiply if there is a damaged skin barrier. Thus, skin damage is a major problem with many effects on other tissues and can be fatal in severe cases. Autologous grafts obtained directly from patients are often used to avoid immune rejection, restore skin function and heal wounds after skin damage. Unfortunately, wounds with large areas and significant depths of skin damage do not adequately heal using autologous transplantation. Therefore, there is a need to prepare artificial dermal substitutes using novel approaches for skin regeneration. Many researchers have developed precise skin substitutes that interact with human tissue after in vitro maturation and transplantation.46-60 It has been challenging to imitate human skin while accommodating numerous derivative structures, such as nerve endings, capillaries, multilayered 3D structures, sebaceous glands, sweat glands, and hair follicles. Complex skin structures require an accurate signaling system. For this reason, 3D bioprinting is an attractive method that can mimic the structure of the human skin using various bioinks and precise patterning of cells.
Merceron et al. fabricated complex muscle-tendon unit (MTU) with polyurethane, PCL and C2C12 and NIH3T3 cell-laden hydrogel bioinks using a 3D integrated organ printing system (Figure 3).57 The MTU was comprised of a heterogeneous polymeric scaffold of elastic on the muscle side and stiff on the tendon side. The construct could be repeatedly prepared with precise shape fidelity. Cells were printed fair viability with increased gene expression.
The Cubo et al. printed bilayer skin using fibrin bioink containing human primary keratinocytes, fibroblasts, and human plasma (Figure 4).58 The function and structure of the 3D bioprinted skin were analyzed using immunohistochemical methods after in vitro 3D culture and long-term implantation into immunodeficient mice. In above cases, the regenerated skin was similar to human skin and was indistinguishable from a lab-made bilayer dermal-epidermal equivalent. Meanwhile, Koch et al. used a 3D laser-based bioprinting system to create a skin model including the dermal and epidermal layers.53,54 They used alginate hydrogels as bioinks and printed fibroblasts, keratinocytes, and hMSCs. They evaluated the effect of the laser-based bioprinting system on cell proliferation, viability, and apoptotic activity. Alterations in cell surface markers and DNA damage were statistically assessed over several days. The cells survived the previous procedure with >98% viability. All tested cell types retained their proliferative capacity, even after 3D bioprinting. A simple skin structure was formed by printing collagen bioinks, keratinocytes, and fibroblasts. The 3D bioprinted cellular structures were evaluated at various incubation times using immunohistological methods. The presence of cell-cell channels indicative of tissue formation has been investigated in important 3D structures. The Michael et al. produced a skin substitute by 3D laser-based bioprinting.55 The skin replacement was achieved using fibroblasts and keratinocytes. Subsequently, the 3D structures were tested in vivo. The bioprinted keratinocytes prepared a multilayered epidermis with initial differentiation and stratum corneum after 11 days of culturing. Proliferation was mainly observed in the basal layer. E-cadherin, an indicator of adherent junctions and tissue formation, was found in the epidermis both ex vivo and in vitro. In mice, some blood vessels have been found to grow with bioprinted orientations of cells at the wound edges and bottom of the wound.
Lee et al. demonstrated a potential applications of 3D bioprinting in tissue engineering using a human prototype skin model.56 They printed collagen with bioinks, fibroblasts, and keratinocytes as constituent cells to fabricate the dermis and epidermis. Immunohistological analysis revealed that the 3D bioprinted skin tissue was biologically and morphologically representative of the human skin tissue in vivo. These highlighted studies showed that 3D bioprinting offers several advantages in shape retention and shape, reproducibility, flexibility, and high culture throughput compared to conventional skin tissue engineering methods.
Nanoparticles have recently emerged as transdermal delivery systems. Their surface properties and size determine their efficacy and effectiveness in penetrating skin tissues. Hou et al. generated a simplified artificial skin model for quick screening the transdermal penetration ability of nanoparticles using 3D bioprinting.60 Collagen hydrogels were used as bioinks to print fibroblasts on the structures. The effectiveness of this platform was evaluated using a 3D scaffold with one layer of fibroblasts sandwiched between two layers of collagen hydrogel to screen silica nanoparticles with different surface charges for their penetrating ability. Positively charged nanoparticles showed deeper penetration, consistent with the observations of previous studies involving living skin tissues.
Vascular and Neural Tissue. This section describes the study of blood vessel formation in 3D tissues using bioprinting. Vascular network tissues play a critical role in all 3D tissues.61-63 Vascular tissues supply nutrients and oxygen to the adjacent tissues and remove waste products. Blood vessel formation is an essential consideration for fabricating highly vascularized artificial organs (the liver, kidney, lung, spleen, heart, pancreas, and thyroid). Therefore, it is generally agreed that reconstructing complex vascular networks is vital in 3D tissue engineering. However, this problem remains a significant obstacle in generating 3D-engineered constructs with the volume and complexity of human organs. 3D bioprinting technology has emerged as an attractive approach for designing small-diameter containers. An advantage of the 3D bioprinting method is that it allows researchers to produce cellular tissue constructs with easy control of the cell density. This feature provides researchers with a more sophisticated tool to solve angiogenesis problems in 3D tissue engineering. These techniques can create biomimetic microenvironments in 3D tissues and generate blood vessels with ideal functions and structures.
Using the coaxial cell printing technique and vascular tissue-specific bioink, Ge Gao et al. developed freestanding in vitro vascular models (VMs) (Figure 5).64 This technique has various advantages in plotting tubular cell-laden vessels with designed patterns. Consequently, the study enhances pump-driven circulating perfusion, generates the endothelium without EC-seeding, and implements further expansions to study vascular pathophysiology. Following the maturation of the endothelium, the VMs exhibit representative vascular functions (i.e., selective permeability, antiplatelets/leukocyte adhesion, and vessel remodeling under shear stress). Moreover, with the expansion of VMs, directional angiogenesis and inflammatory responses are demonstrated by asymmetric distributions of proangiogenic factors and an airway inflammatory ambiance, respectively.
Cui et al. fabricated fibrin microchannels using an inkjet-based bioprinting method.65 When bioprinting fibrin hydrogels containing human microvascular endothelial cells (HMVECs), they confirmed that the cells self-aligned within the fibrin channels and proliferated to fabricate confluent linings. A 3D tubular structure was fabricated from the printed pattern. They concluded that the simultaneous bioprinting of cells and scaffolds promoted HMVEC proliferation and microvascularization. Additionally, the Norotte et al. printed small-diameter multilayered tubular vascular grafts that easily perfuse for further maturation.66 Agarose was used as a bioink for printing smooth muscle cells and fibroblasts, which were aggregated into individual units such as multicellular spheroids or cylinders of controlled diameter (300-500 μm). The bioprinting fusion of individual units resulted in single- and bilayer small-diameter vessels. Wu et al. used an omnidirectional bioprinting method to fabricate a 3D microvascular network embedded within a Pluronic F127 hydrogel scaffold.67 They fabricated a 3D microvessel network using a hierarchical third-generation branching topology to form microchannels of 200-600 μm diameter, where the two large parental channels were subdivided into many smaller microchannels.
Kolesky et al. reported a novel 3D bioprinting method for fabricating 3D tissue structures filled with vasculature, ECM, and multiple types of cells.68 They confirmed the printability of these structures using silicone elastomer and Pluronic F127 and cell viability using GelMA hydrogel-containing cells. In addition, they found that human umbilical vein endothelial cells (HUVECs) and human neonatal dermal fibroblasts proliferate over time. Jia et al. developed perfusable vasculature with a highly ordered arrangement in a single-step process.69 Direct 3D bioprinting was achieved using a GelMA, 4-arm poly(ethylene glycol)-tetra acrylate (PEGTA) and alginate with a multilayer coaxial extrusion printing system. The rheological properties of the bioink and mechanical strength of the constructs were adjusted by PEGTA, which facilitated the accurate deposition of complex multilayer 3D perfusable hollow tubes. This blended bioink also exhibits beneficial biological properties that support the proliferation of encapsulated endothelial and stem cells within 3D bioprinted structures, leading to highly organized, biologically relevant, and perfusable blood vessels. Other approaches have integrated perfusionable systems and 3D bioprinting to achieve 3D tissue vascularization.
The Rodrigo Pimentel C. et al. fabricated thick (1 cm) and dense tissue structures using a 3D four-arm branch network with stiffness comparable to soft tissue.70 This construct can be perfused directly onto a fluid platform for extended periods (>14 days). They used poly(vinyl alcohol) (PVA) as the water-soluble material and PLA as the support structure. They generated artificial 3D vascular networks that mimicked the stiffness of the liver and encapsulated hepatocellular carcinoma (HepG2) cells within the ECM by selectively removing PLA and using PVA constructs. These hybrid constructs were directly perfused into the medium to induce proliferation and formation of HepG2 spheroids. In their study, the highest spheroid density was observed with perfusion, but the overall tissue composition showed two distinct regions. Therefore, this model simulated the tissue gradient within the necrotic tumor area.
Organs for Drug Screening and Cancer Models. Applying 3D bioprinting techniques to produce artificial organs such as the liver, heart, kidney, pancreas, and lungs is one of the ultimate objectives of tissue engineering and regenerative medicine. More specifically, we review the use of various 3D bioprinting approaches, as well as the performance of current 3D printed organ constructs in terms of tissue-specific functions, metabolic drug potential, and drug dose-response.71-85 Liver-related diseases are the leading cause of morbidity and mortality in the United States. These abnormalities can lead to the formation of excessive fibrous tissue. Such formations decrease liver-specific and systemic functions, leading to irreversible end-stage liver failure. The liver plays an important role in xenobiotic metabolism, detoxification, and hepatotoxicity. Therefore, the investigation of liver function is an essential component of preclinical drug research. Existing animal models are expensive and unreliable to translate into human studies, often because of changes in hepatocyte function in other species.
Moreover, the progression of liver disease and drug responses vary among individuals. Failure to predict hepatotoxicity often leads to post-market drug withdrawal. Therefore, effective in vitro human liver models based on personalized cell types are a promising approach to better understand disease mechanisms, serve as drug screening platforms, and potentially treat diseases in regenerative medicine.
In the past decades, liver tissue engineering has made significant progress in fundamental pathophysiological studies and establishing in vitro liver models for drug screening. The cell sources used in these in vitro liver models include primary hepatocytes, liver cell lines isolated from tumors or liver sections, and stem cell-derived liver cells. Monolayer culture, organoid culture, and co-culture platforms include culture plates, commercially available wells, microfluidic perfusion chips, dielectrophoretic micropatterning, and additional photopatterning based on physical masks. However, the liver-specific function of hepatocytes cultured on these platforms decreased over several weeks of in vitro culture. Therefore, liver constructs that better mimic their natural environment and help maintain liver function in vitro are in great demand. 3D bioprinting technology provides an excellent tool to achieve novel biomimetic in vitro liver models with the potential to pattern cells and biomaterials accurately.
Various 3D bioprinting approaches have been used to create liver tissue structures. Using rapid prototyping, Wang et al. prepared a hybrid hierarchical polyurethane-cell/hydrogel for regenerative medicine (Figure 6).85 This approach constructed a 3D vascular template with synthetic scaffold polymers and cell/hydrogel systems. The synthetic PU was used as an external scaffold material for mechanical support. And the gelatin/alginate/fibrinogen hydrogel was used as an internal scaffold material for adipose-derived stem cell (ADSC) accommodation (Figure 6).80 The Faulkner-Jones et al. reported using an inkjet-based bioprinter to encapsulate human iPSCs and ESC-derived hepatocyte-like cells (HLCs) in alginate hydrogels to generate 3D ring structures.81 Alginate was selected based on good biocompatibility, low immunogenicity, low toxicity, and hydrophilicity. Alginate droplets containing cells were exposed to calcium chloride solution before incubation in the culture medium and barium chloride. The viability and albumin-secreting function of HLCs were maintained after valve-based bioprinting.
Kang et al. used extrusion-based bioprinting to generate 3D liver structures.77 They constructed a five-layer alginate scaffold containing mouse-derived HLCs, each measuring 25 mm ×25 mm. The expression of albumin, ASGR1, and HNF4a gradually increased during in vitro culture. The construct was also transplanted in vivo and increased proliferation and albumin expression. This study showed that 3D bioprinted liver scaffolds are an effective option for liver treatment. Kizawa et al. also demonstrated that scaffold-free 3D bioprinting technology allows the construction of liver tissue capable of stably maintaining bile acid secretion and drug, glucose, and lipid metabolisms for several weeks.78 This was achieved using a 3D printer to connect the spheroids of human primary hepatocytes. Their work provides insights into the long-term culture of 3D bioprinted liver structures in vitro. In particular, the expression and activity of the CYP3A4 enzyme were both found to be maintained for about 2 months.
To mimic the complex microarchitecture of the liver. Ma et al. reported the construction of biomimetic liver tissue at a microscale resolution using DLP-based bioprinting technology.79 The 3D bioprinted liver construct consisted of a hexagonal array of human iPSC-derived liver cells and support cells. Hepatocytes cultured in this 3D bioprinted triplicate culture model showed better liver-specific function and metabolic drug potential after CYP induction than those cultured in the conventional 2D monolayer and 3D monoculture platforms. Direct printing into a microfluidic chamber to build a liver-on-chip platform has also been demonstrated by Bhise et al.80 Drops of a HepG2 spheroid-GelMA mixture were printed on glass slides in the cell culture chamber of the bioreactor, followed by immediate UV crosslinking. The engineered liver constructs maintained their function during a 30-day culture period and showed drug responses similar to published data.
As shown in the examples above, the application of 3D printing technology to build in vitro liver models has shown great advantages in providing long-term cultures with well-maintained liver-specific functions and metabolic drug potential. Nevertheless, maintaining liver cell function for more than 30 days and achieving a drug response profile comparable to a native liver remains a major challenge in the field. Conventional 2D cancer models cultured in vitro have provided many essential insights into cancer and have led to several major therapeutic successes. However, tomographic models cannot replicate the unique features of 3D tumor tissues. For example, tumor-stromal interaction is increasingly recognized as a factor that influences the therapeutic response of tumors to various drugs. These effects on tumor drug response include substrate-induced drug resistance and synthetic lethality. Drug development for cancer has been ongoing for decades, with more than 95% of drugs failing during clinical trials. This represents an urgent requirement for preclinical predictive models. Advances in 3D bioprinting technology have resulted in several in vitro cancer models that better replicate the tumor microenvironment (TME) critical for tumor proliferation, metastasis, and drug response.83,84 The following sections focus on several aspects of tumor progression, including cancer cell migration, proliferation, and function, tumor-stromal interactions, and 3D-printed models built to study these behaviors. TMEs are highly complex and heterogeneous. Their functions, including mechanical stimuli, biochemical gradients, geometric cues, tissue structures, and cell-cell/matrix interactions, influence metastatic events through numerous interactions with cancer cells. Metastatic progression has been found to cause 90% of cancer-related deaths and is associated with a significant decrease in 5-year survival, an important indicator of cancer prognosis.
Therefore, a focus of 3D bioprinting has been elucidating diverse mechanisms of cancer metastasis. Huang et al. used DLP-based bioprinting to create biomimetic chips with integrated vasculature to study the effect of geometric cues on the migration rates of tumor cells (HeLa cells) and normal cells (10T1/2).86 Poly(ethylene glycol) diacrylate was used to construct structures owing to their tunable mechanical properties and biocompatibility. Embedded vessels had three different chamber widths (25, 45, and 120 µm), mimicking vessels of different sizes in vivo. The results showed that HeLa cells migrated at an increased rate in narrower channels, whereas the fibroblast migration rate was not affected by the channel width. This study introduced a method for modeling the different responses of cancerous and non-cancerous cells to different geometric cues, which could potentially be used to screen anti-migration molecules. TME function affects migration events and cancer cell proliferation, and tumor properties. They constructed a 10×10×2 mm3 gridded cervical tumor model using HeLa cells from a hydrogel mixture of gelatin, alginate, and fibrinogen. By better replicating heterogeneity and mimicking the native microenvironment, the 3D printed tumor model exhibited higher proliferation rates than the 2D control model, including matrix metalloproteinase protein (MMP) expression and chemical resistance to paclitaxel chemotherapy. It exhibited highly simulated tumor characteristics. More biomimetic cell-cell interactions and cell-matrix interactions within the 3D model may cause cell behavior and function differences.
In studying tumor-stromal interactions, Xu et al. patterned human ovarian cancer cells and fibroblasts onto a Matrigel stroma using a droplet printing technique.87 The printing system consisted of a micron-resolution XYZ stage and a nanoscale dispensing valve. A 150 μm diameter nozzle was used to dispense droplets. The printed OVCAR-5 human cancer cell line with a co-culture of fibroblasts proliferated and formed 3D acinar structures similar to ovarian cancer micromodules. The results showed that patterning cancer cells with normal stromal cells could generate more physiologically relevant tumor models to better understand cancer mechanisms.87
3D printing technology can also build a specialized tumor models to evaluate novel formulations in vivo. For example, Tang et al. used a 3D printing technique to construct subcutaneous glioblastoma xenografts that mimicked the resected tumor cavity.88 They used this tumor model to evaluate the effectiveness of a tailored drug-releasing implant in preventing postoperative glioblastoma recurrence.
Current 3D printed cancer models are still limited in model design to represent cell types and TME in vitro. Future studies using patient-specific and primary cancer cells from patients will provide more insight into patient-and disease stage-specific cellular behaviors and cell-cell interactions. Additional work is required to develop biomaterials and printing approaches for building scaffolds that mimic dynamic biochemical and mechanical environments.89,90
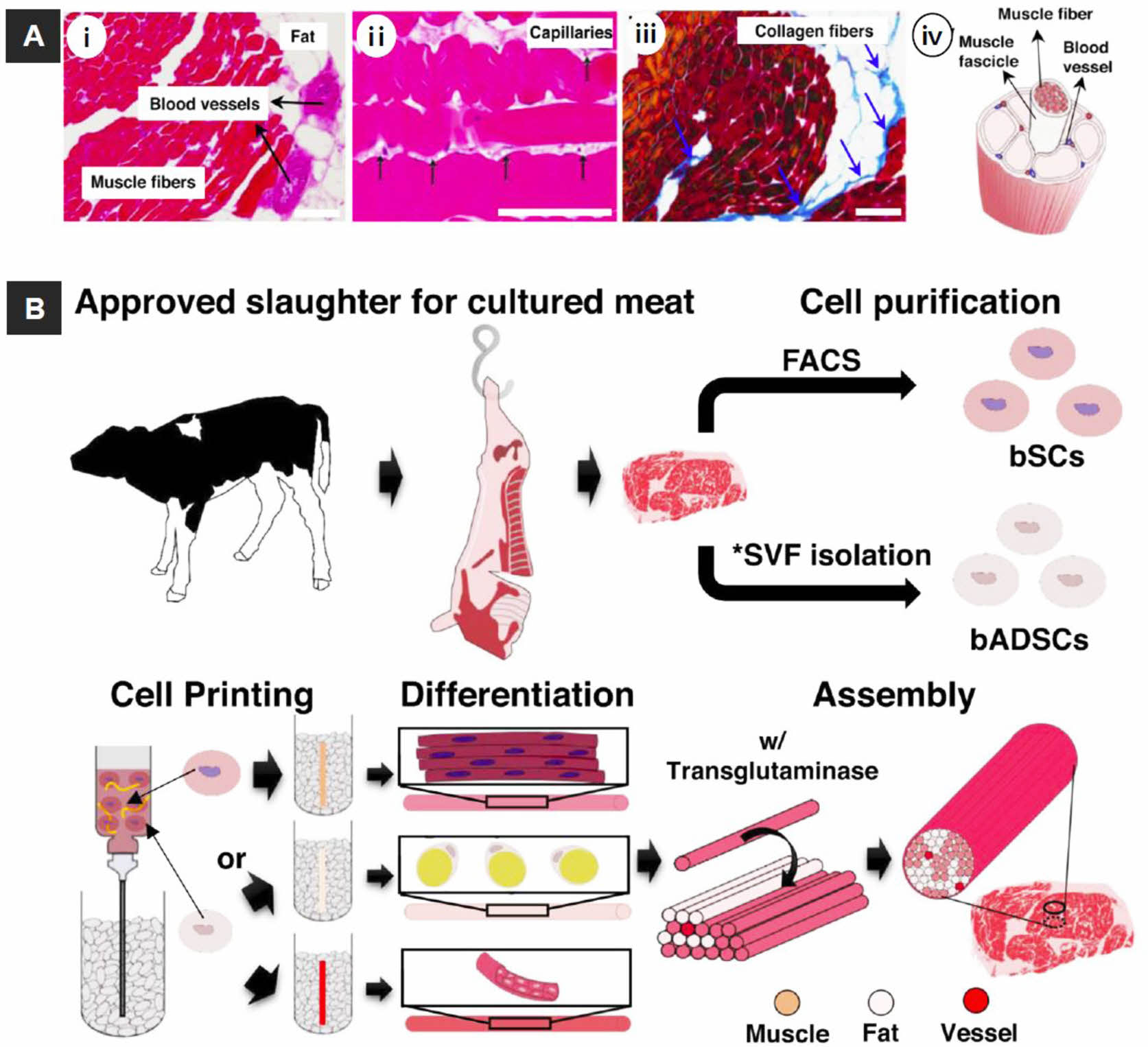
Cosmetic and Food Production. 3D bioprinting techniques can be utilized in cosmetic and food production. Kang et al. prepared engineered a whole-cut meat-like tissue by assembling cell fibers using tendon-gel integrated bioprinting (Figure 7).91 They demonstrated the in vitro construction of engineered steak-like tissue assembled from three types of bovine cell fibers (muscle, fat, and vessel). A total of 72 fibers comprising 42 muscles, 28 adipose tissues, and 2 blood capillaries were constructed by tendon-gel integrated bioprinting and manually assembled to fabricate steak-like meat with a diameter of 5 mm and length of 10 mm, inspired by a meat cut.
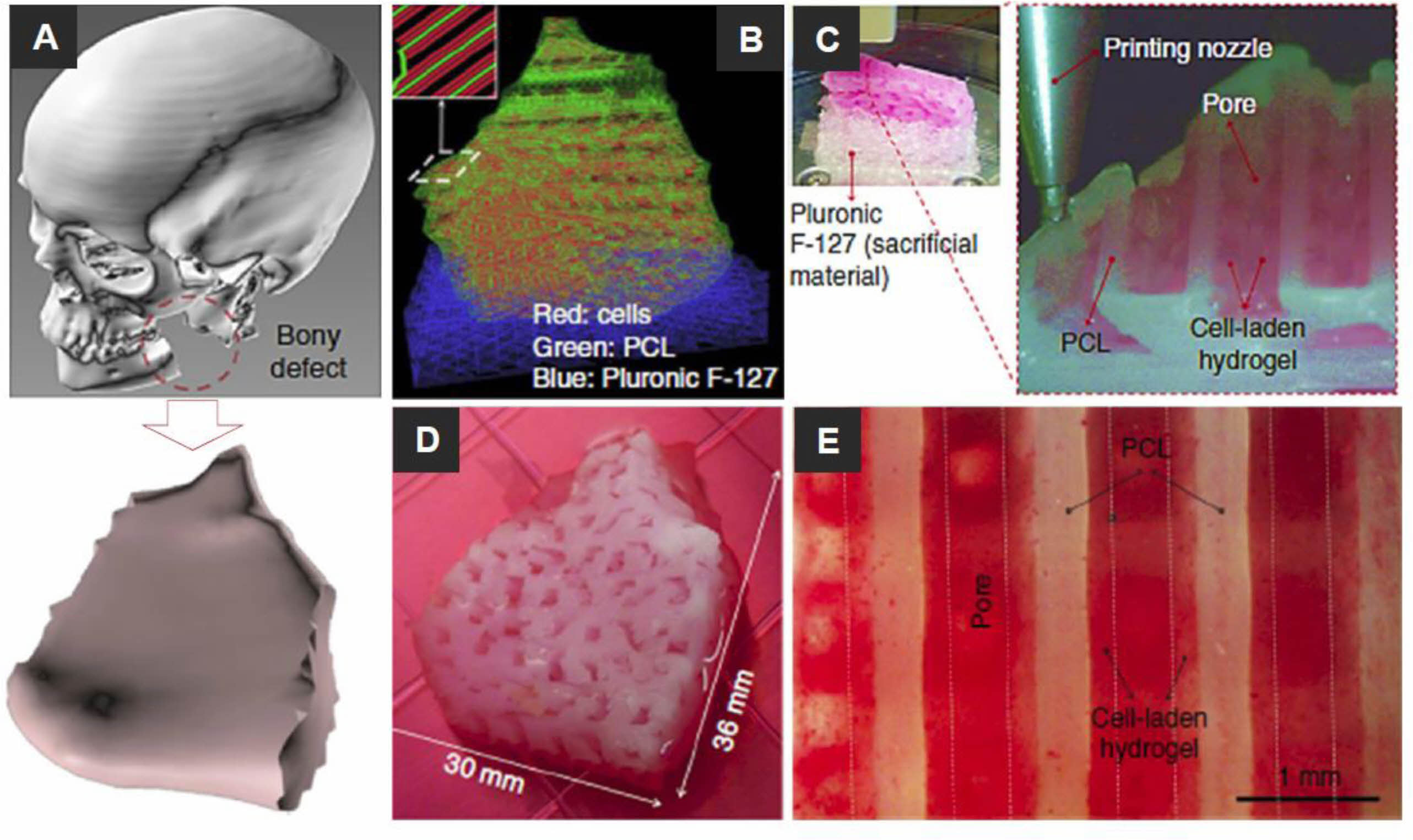
|
Figure 2 Reconstruction of a human mandible graft with the bioprinted construct: (A) 3D CAD model obtained from CT image data of the defected bone; (B) construction of the bone defect 3D architecture using CAM (computer-aided manufacturing) software: the red, blue, and green lines indicate the pathways to dispense various bioinks of cell-laden hydrogel, Pluronic F-127, and PCL, respectively; (C) 3D bioprinting: the enlarged view shows the patterning of the construct layer; (D) image of 3D bioprinted constructs cultured in osteogenic medium for 28 days; (E) human amniotic fluid stem cells (hAFSCs) osteogenic differentiation in the bioprinted construct was confirmed by Alizarin Red S staining (indicating calcium deposition). Reproduced with permission from Ref. 38, H.-W. Kang et al., Nat. Biotechnol., 2016, 34, 312- 319.© 2016, Nature America, Inc. |
|
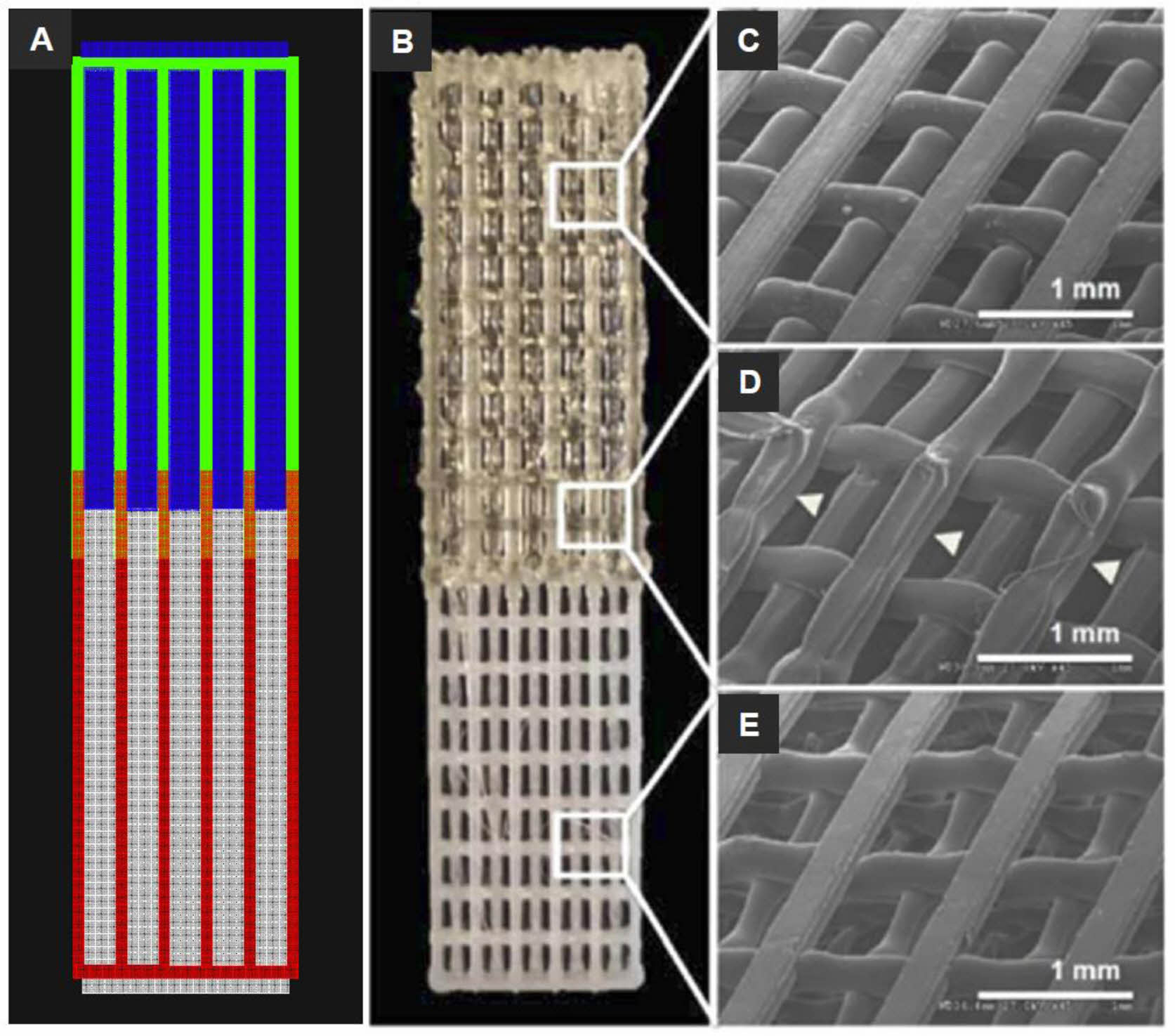
Figure 3 3D bioprinted muscle-tendon unit (MTU) construct: (A) CAD and tool path of the MTU construct with cells (green, PU; red, PCL; blue, C2C12 cells; white, NIH3T3 cells); (B) The MTU construct with PU side on top, PCL side on the bottom, and 10% overlap area at the interface in the center. SEM images of the (C) PU side; (D) interface region; (E) PCL side. Arrowheads indicate the welding areas between the two polymers. Reproduced with permission from Ref. 57, T. K. Merceron et al., Biofabrication., 2015, 7, 035003.© 2015, IOP Publishing Ltd. |
|
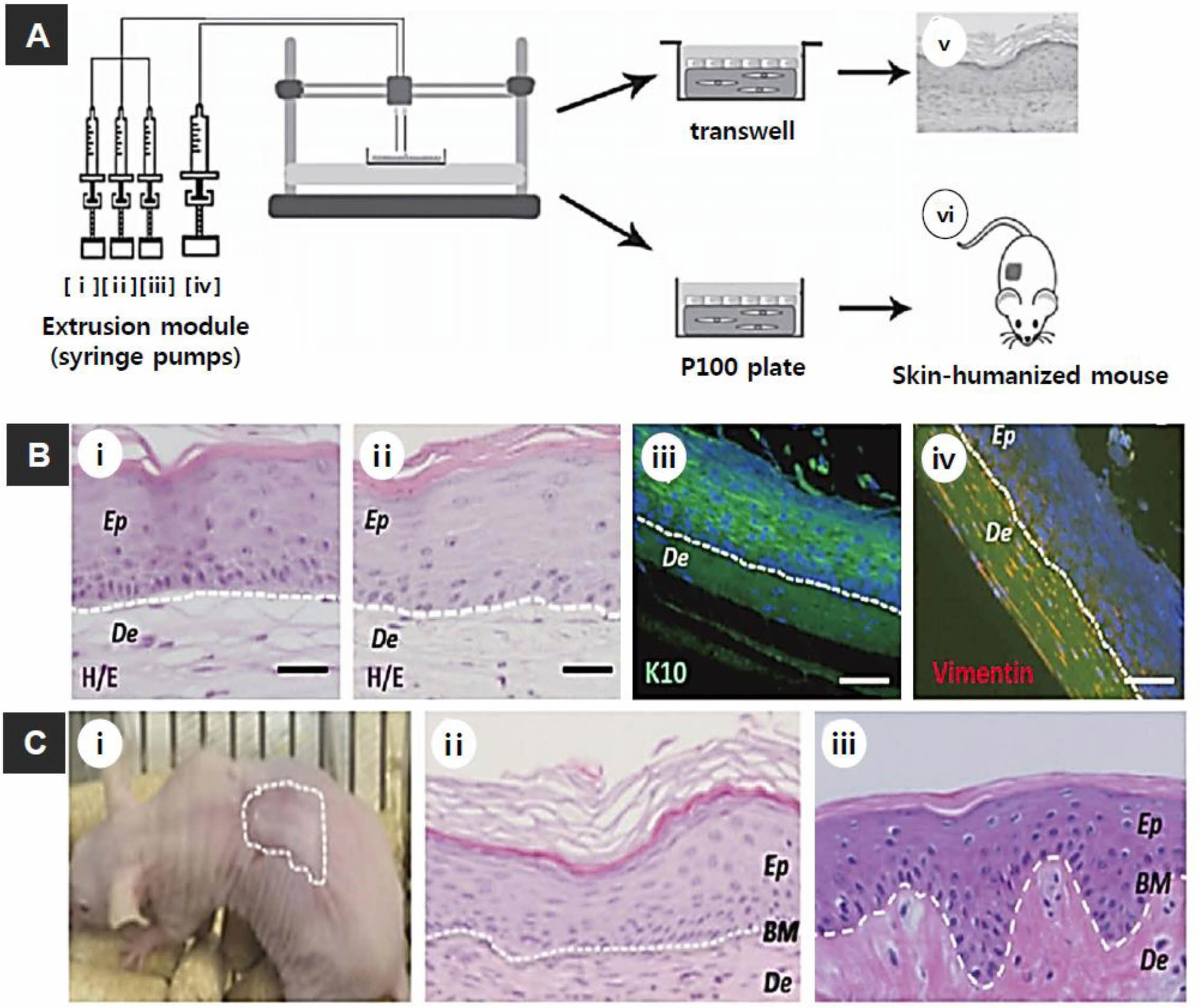
Figure 4 (A) Scheme of skin bioprinting process: The extrusion modules contained four syringes, loaded with (i) hFbs; (ii) plasma; (iii) CaCl2; (iv) hKCs; (v) equivalents printed on Transwell inserts that could differentiate at the air-liquid surface for 17 days; (vi) equivalents printed on P100 plates that were grafted onto the backs of immunodeficient mice for 8 weeks; (B) in vitro 3D human skin constructs obtained after 17 days of differentiation at the air-liquid interface: (i) “handmade” skin equivalent; (ii-iv) bioprinted skin equivalents; (i and ii) HE staining; (iii and iv) immunostaining using (iii) an anti-K10 antibody; (iv) an anti-human vimentin antibody. Ep and De in (i-iv) denote the epidermal and dermal compartments, respectively; (C) Histological analysis (8 weeks post-grafting) of bioprinted human skin grafted onto immunodeficient mice: (i) visual appearance of the grafted human skin; (ii) HE staining of the regenerated human skin; (iii) HE staining of the normal human skin. The white dotted lines in (ii) and (iii) indicate the dermo-epidermal junction (basal membrane, BM). Scale bar: 100 μm. Reproduced with permission from Ref. 58, N. Cubo et al., Biofabrication., 2017, 9, 015006.© 2017, IOP Publishing Ltd. |
|
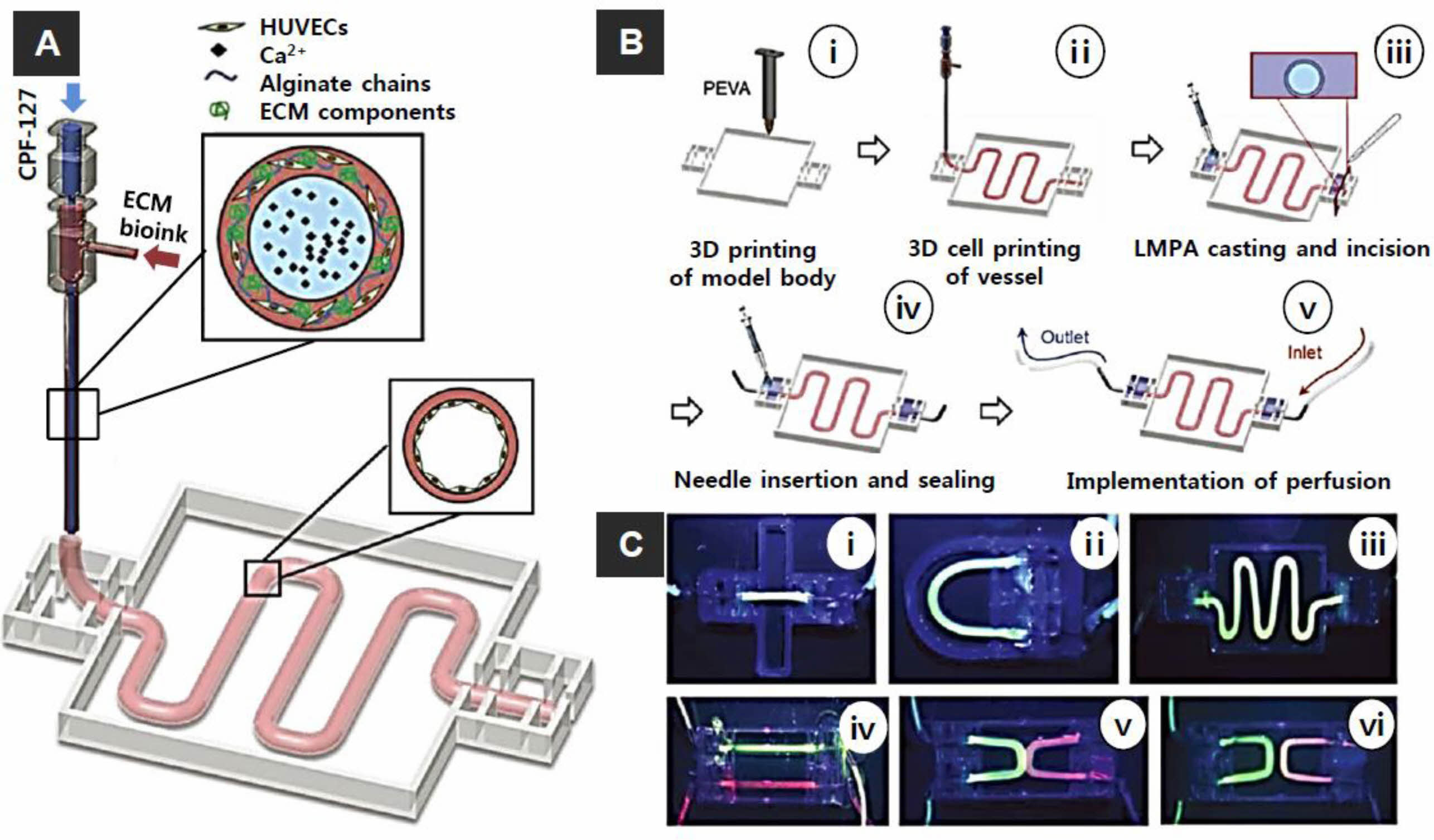
Figure 5 (A) Bioprinting system for ECM-based bioink with a core of Pluronic and calcium ions and a shell of cell (HUVECs)-laden in alginate chains with ECM components; (B) steps in the fabrication of the vascular model: (i) 3D printing of the poly ethylene-vinyl acetate (PEVA) model body; (ii) 3D cell printing; (iii) casting and incision of low melting point agarose; (iv) needle insertion and sealing; and (v) perfusion; (C) Perfusion of vessels construct with different designs: (i) straight; (ii) curved; (iii) serpentine; (iv) dual parallel; (v) attached dualcurves; (vi) discrete dual-curves. Reproduced with permission from Ref. 64, G. Gao et al., Adv. Healthcare. Mater, 2018, 7, 1801102.© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. |
|
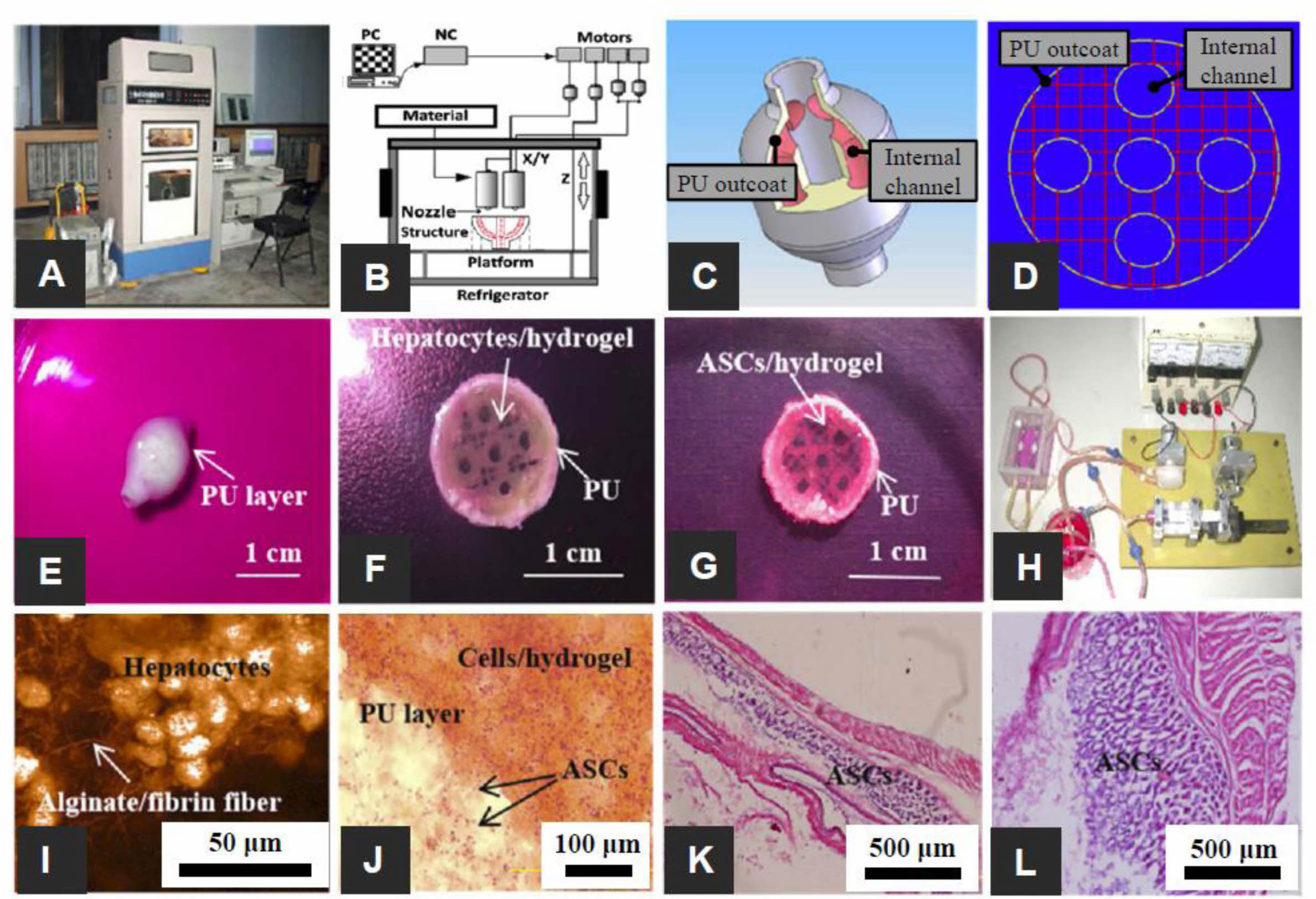
Figure 6 The 3D-printed complex organs with vascularized liver constructed via a dual nozzle low temperature 3D bioprinter: (A) dual nozzle low temperature 3D bioprinter; (B) the principle of an elliptical hybrid hierarchical polyurethane cell/hydrogel structure constructed via a dual nozzle low temperature 3D bioprinter; (C) CAD model comprising a branched vascular network; (D) cross-section of the CAD model including five sub-branching channels; (E) an oval structure containing both the cell-containing hydrogel and the PU envelope; (F) oval structure of the middle section containing a gelatin-based hydrogel containing hepatocytes and a PU envelope; (G) the oval structure of the middle section containing the ASCs containing gelatin-based hydrogel and PU outcoat; (H) two composite constructs undergoing pulsatile culture in vitro; (I) Hepatocytes encapsulated in a gelatin-based hydrogel; (J) PU and cell/hydrogel layers 2 weeks after transplantation; (K) HE staining micrographs of constructs containing ADSCs after 3 days of static culture; (L) HE staining micrographs of gelatin/alginate/fibrinogen hydrogels after 12 days of pulsatile culture. Reproduced with permission from Ref. 85, X. Wang et al., Micromachines, 2019, 10, 814.© 2019 MDPI. |
|
Figure 7 Overview of the cultured steak by 3D bioprinting technique. (A) structure of steak: (i, ii) H&E-; (iii) Azan-stained images of a piece of steak. All scale bars denote 100 μm; (iv) Schematic of a hierarchical structure in muscle; (B) Schematic of the construction process for a cultured steak. The first step is cell purification of tissue from cattle to obtain bovine satellite cells (bSCs) and bovine adipose-derived stem cells (bADSCs). *SVF; stromal vascular fraction. The second supports bath-assisted printing (SBP) of bSCs and bADSCs to fabricate the muscle, fat, and vascular tissues with a fibrous structure. The third step is assembly of cell fibers to mimic the commercial steak structure. Reproduced with permission from Ref. 91, D.-H. Kang et al., Nat. Commun., 2021, 12, 5059.© 2021 Springer Nature Limited. |
Innovative research in tissue engineering using various 3D bioprinting techniques has been consistently challenging. The use of 3D bioprinting in tissue engineering to mimic various tissues could extend the limitations of traditional research concepts and provide versatile applications. 3D bioprinting allows the precise manipulation of biomaterials and living cells to reconstruct complex tissue substitutes for disease modeling and drug screening of organ-specific functions. In addition, bioprinted skin tissues are used in the cosmetic industry instead of in animal model experiments. Despite recent remarkable achievements in this field, challenges remain for printing instrument platforms, cells, and bioink materials used to build tissue models that have the cellular organization and structural complexity comparable to original tissues.
The technical challenges associated with 3D printing instrument platforms include the need for resolution, printing speed, biocompatibility, and scalability. Currently, only light-assisted bioprinters can achieve microscale resolution, which depends on the type of material used and the cellular concentration of the printing mixture. Higher print resolutions are still required to fabricate complex cell structures, such as capillaries and neural networks. A higher print speed remains a fundamental challenge for cell viability, especially for metabolically active cell types, such as liver and muscle cells. Also, the viability of these cells in the printing solution decreases with delayed printing time. The biocompatibility of the 3D printing instrument platform has been reported to be satisfactory in terms of cell viability. However, its impact on gene expression and functional aspects has rarely been studied. Depending on the type of bioprinter used, various mechanical and optical disturbances in the cells are involved. Further studies on the mechanical and optical effects of the bioprinting process will provide more insights into the biocompatibility of the 3D printing process.
The biomaterials used in 3D bioprinting also have significant limitations. The requirement for biomaterials to possess certain qualities reduces the number of materials viable for 3D bioprinting. Efforts have been made to develop composite bioinks for extrusion-based printing. Recently, decellularized ECMs have been studied to create printable biomaterials for extrusion-based and light-assisted bioprinting platforms. The dECM bioinks, which contain heterogeneous components of the native ECM, allow researchers to fabricate specific cell-laden constructs using a customized microenvironment, unlike commonly used highly purified forms of ECM components, such as gelatin and collagen bioinks. The native cellular ECM plays a vital role in regulating biological activities. This challenging approach has become more critical as we investigate the development of bioprinted biomimetic tissues.
Finally, scaling up bioprinted tissue constructs remains a challenge as the current applications are primarily based on small sample sizes.92 The continued fabrication of actual volume tissue models for clinical and commercial applications requires further studies to standardize printers, cells, bioink materials, and the printing process.
- 1. Wong, K. V.; Hernandez, A. A Review of Additive Manufacturing. Int. Sch. Res. Notices 2012, 2012, 208760.
-

- 2. Ahn, D.; Kweon, J. H.; Lee, S. Quantification of Surface Roughness of Parts Processed by Laminated Object Manufacturing. J. Mater. Proc. Technol. 2012, 212, 339-346.
-

- 3. Kruth, J. P.; Leu, M. C.; Nakagawa, T. Progress in Additive Manufacturing and Rapid Prototyping. CIRP Annals 1998, 47, 525-540.
-

- 4. Bikas, H.; Stavropoulos, P.; Chryssolouris G. Additive Manu- facturing Methods and Modeling Approaches. Int. J. Adv. Manuf. Technol. 2016, 83, 389-405.
-

- 5. Turner, B. N.; Strong, R.; Gold, S. A. A Review of Melt Extrusion Additive Manufacturing Processes: I. Process Design and Modeling. Rapid Prototyp. J. 2014, 20, 192-204.
-

- 6. Holmers, L. R.; Riddick, J. C. Research Summary of an Additive Manufacturing Technology for the Fabrication of 3D Composites with Tailored Internal Structure. JOM 2014, 66, 270-274.
-

- 7. Quan, Z.; Wu, A.; Keefe, M.; Qin, X.; Yu, J.; Suhr, J.; Byun, J. H.; Kim, B. S.; Chou, T. W. Additive Manufacturing of Multi-directional Perfoms for Composites: Opportunities and Challenges. Mater. Today 2015, 18, 503-512.
-

- 8. Land, W. S. II.; Zhang, B.; Ziegert, J.; Davies, A. In-situ Metrology System for Laser Powder Bed Fusion Additive Process. Procedia Manuf. 2015, 1, 393-403.
-

- 9. Chan, B. P.; Leong, K. W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467-479.
-

- 10. O’Brien, F. J. Biomaterials and Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88-95.
-

- 11. Howard, D.; Buttery, L. D.; Shakesheff, K. M.; Roberts, S. J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66-72.
-

- 12. Henkel, J.; Woodruff, M. A.; Epari, D. R.; Steck, R.; Glatt, B.; Dickinson, I. C.; Choong, P. F. M.; Schuets, M. A.; Hutmacher, D. W. Bone Regeneration Based on Tissue Engineering Conceptions – a 21st Century Perspective. Bone Res. 2013, 1, 216-248.
-

- 13. Shivalkar, S.; Singh, S. Solid Freeform Techniques Application in Bone Tissue Engineering for Scaffold Fabrication. Tissue Eng. Regen. Med. 2017, 14, 187-200.
-

- 14. Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496-504.
-

- 15. Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 10, 546-554.
-

- 16. Amini, A. R.; Laurencin, C. T.; Nukavarapu, S. P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363-408.
-

- 17. Corcione, C. E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D Printing of Hydroxyapatite Polymer-Based Composites for Bone Tissue Engineering. J. Polym. Eng. 2017, 37, 741-746.
-

- 18. Kundu, J.; Shim, J. H.; Jang, J.; Kim, S. W.; Cho, D. W. An Additive Manufacturing-Based PCL-Alginate-Chondrocyte Bioprinted Scaffold for Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286-1297.
-

- 19. Kim, J. E.; Kim, S. H.; Jung, Y. Current Status of Three-Dimensional Printing Inks for Soft Tissue Regeneration. Tissue Eng. Regen. Med. 2016, 13, 636-646.
-

- 20. Dong, L.; Wang, S. J.; Zhao, X. R.; Zhu, Y. F.; Yu, J. K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-Laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412.
-

- 21. Rengier, F.; Mehndiratta, A.; Tengg-Kobligk, H.; Zechmann, C. M.; Unterhinninghofen, R.; Kauczor, H. U.; Giesel, F. L. 3D Printing Based on Imaging Data: Review of Medical Applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335-341.
-

- 22. Essentials of 3D Biofabrication and Translation; Atala, A., Yoo, J. J., Eds.; Academic Press: San Diego, 2015.
-

- 23. Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H. J., Park, C. H., Kwon, I. K., Khang, G., Eds.;Springer: Singapore, 2018.
-

- 24. Gu, B. K.; Choi, D. J.; Park, S. J.; Kim, M. S.; Kang, C. M.; Kim, C. H. 3-Dimensional Bioprinting for Tissue Engineering Applications. Biomater. 2016, 20, 1-12.
-

- 25. Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials. 2016, 9, 802.
-

- 26. Kim, J. S.; Hong, S. Y.; Hwoang, C. M. Bio-ink Materials for 3D Bio-Printing. J. Int. Soc. Simul. Surg. 2016, 3, 49-59.
-

- 27. Katja, Hölzl.; Shengmao L.; Liesbeth, T.; Sandra V. V.; Linxia, G.; Aleksandr O. Bioink Properties Before, During and After 3D Bioprinting, Biofabrication 2016, 8, 032002.
-

- 28. Knight, E.; Przyborski, S. Advances in 3D Cell Culture Technologies Enabling Tissue-Like Structures to be Created in Vitro. J. Anat. 2015, 227, 746-756.
-

- 29. Hunziker, E. B. Articular Cartilage Repair: Basic Science and Clinical Progress. A Review of the Current Status and Prospects. Osteoarthr. Cartil. 2001, 10, 4732-463.
-

- 30. Hutmacher, D. W. Scaffold in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529-2543.
-

- 31. Wu, T.; Yu, S.; Chen, D.; Wang, Y. Bionic Design, Materials and Performance of Bone Tissue Scaffolds. Materials 2017, 10, 1187
-

- 32. Mota, R. C. A. G.; Silva, E. O.; Lima, F. F.; Menezes, L. R.; Thiele, A. C. S. 3D Printed Scaffolds as a New Perspective for Bone Tissue Regeneration: Literature Review. Mater. Sci. App. 2016, 7, 430-452.
-

- 33. Mravic, M.; Péault, B.; James, A. W. Current Trends in Bone Tissue Engineering. BioMed. Res. Int. 2014, 865270:1.
-

- 34. Stevens, M. M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18-25.
-

- 35. Santos, M. I.; Reis, R. L. Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges. Macromol. Biosci. 2010, 10, 12-27.
-

- 36. Shin, M.; Yoshimoto, H.; Vacanti, J. P. In Vivo Bone Tissue Engineering Using Mesenchymal Stem Cells on a Novel Electrospun Nanofibrous Scaffold. Tissue Eng. 2004, 10, 33-41.
-

- 37. Tian, X. F.; Heng, B. C.; Ge, Z.; Lu, K.; Rufaihah, A. J.; Fan, V. T. W.; Yeo, J. F.; Cao, T. Comparison of Osteogenesis of Human Embryonic Stem Cells Within 2D and 3D Culture Systems. Scand. J. Clin. Lab. Invest. 2008, 68, 58-67.
-

- 38. Kang, H.-W.; Lee, S. J.; Ko, I. K.; Kengla, C.; Yoo, J. J.; Atala, A. A 3D Bioprinting System To Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312-319.
-

- 39. Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Wong, M. Z. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406-7417.
-

- 40. Wang, X.; Tolba, E.; Schröder, H. C.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Müller, W. E. G. Effect of Bioglass on Growth and Biomineralization of SaOS-2 Cells in Hydrogel after 3D Cell Bioprinting. PLoS ONE 2014, 9, e11497.
-

- 41. Wu, H.; Lei, P.; Liu, G.; Zhang, Y. S.; Yang, J.; Zhang, L.; Xie, J.; Niu, W.; Liu.; Ruan, J.; Hu, Y.; Zhang, C. Reconstruction of Large-scale Defects with a Novelhybrid Scaffold Made from Poly(L-lactic acid)/Nanohydroxyapatite/Alendronate-loaded Chitosan Microsphere: In Vitro and In Vivo Studies. Sci. Rep. 2017, 7, 359.
-

- 42. Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering Zonal Cartilage Through Bioprinting Collagen Type II Hydrogel Constructs with Biomimetic Chondrocyte Density Gradient. BMC Musculoskelet. Disord. 2016, 17, 301.
-

- 43. Andreassi, A.; Bilenchi, R.; Biagioli, M.; D’Aniello, C. Classification and Pathophysiology of Skin Grafts. Clin. Dermatol. 2015, 23, 332-337.
-

- 44. Sheridan, R. Closure of the Excised Burn Wound: Autografts, Semipermanent Skin Substitutes, and Permanent Skin Substitutes. Clin. Plastic. Surg. 2009, 36, 643-651.
-

- 45. Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS Collection: Using Human Adipose-Derived Stem/Stromal Cells for the Production of New Skin Substitutes. Stem Cells 2008, 26, 2713-2723.
-

- 46. Loss, M.; Wedler, V.; Künzi, W.; Meuli-Simmen, C.; Meyer, V. E. Artifcial Skin, Split-Thickness Autograft and Cultured Autologous Keratinocytes Combined to Treat a Severe Burn Injury of 93% of TBSA. Burns 2000, 26, 644-652.
-

- 47. MacNeil, S. Progress and Opportunities for Tissue-Engineered Skin. Nature 2007, 445, 874-880.
-

- 48. Ng, W. L.; Wang, S.; Yeoung, W. Y.; Naing, M. W. Skin Bioprinting: Impending Reality or Fantasy? Trends Biotechnol. 2016, 34, 689-699.
-

- 49. Shevchenko, R. V.; James, S. L.; James, S. E. A Review of Tissue-Engineered Skin Bioconstructs Available for Skin Reconstruction. J. R. Soc. Interface 2010, 7, 229-258.
-

- 50. Murphy, S. V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773-785.
-

- 51. Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V. A.; Singh, S. R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789.
-

- 52. Jean, J.; Garcia-Perez, M. E.; Pouliot, R. Bioengineered Skin: The Self-Assembly Approach. J. Tissue Sci. Eng. 2011, S5, 001.
-

- 53. Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogy, P. M.; Chichkov, B. Skin Tissue Generation by Laser Cell Printing. Biotechnol. Bioeng. 2012, 109, 1855-1863.
-

- 54. Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; Vogt, P. M.; Steinhoff, G.; Chichkov, B. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C Methods 2010, 16, 847-185.
-

- 55. Michael, S.; Sorg, H.; Peck, C. T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P. M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741.
-

- 56. Lee, V.; Singh, G.; Trasatti, J. P.; Bjornsson, C.; Xu, X.; Tran, T. N.; Yoo, S. S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473-484.
-

- 57. Merceron, T. K.; Burt, M.; Seol, Y.-J.; Kang, H.-W.; Lee, S. J.; Yoo, J. J.; Atala, A. A 3D Bioprinted Complex Structure for Engineering the Muscle-Tendon Unit, Biofabrication 2015, 7, 035003.
-

- 58. Cubo, N.; Garcia, M.; del Cañizo, J. F.; Velasco, D.; Jorcano, J. L. 3D Biopriting of Functional Human Skin: Production and In Vivo Analysis. Biofabrication 2017, 9, 015006.
-

- 59. Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; Wang, X. 3D Bioprinting for Skin Tissue Engineering: Current Status and Perspectives. J. Tissue Eng. 2021, 12, 1-28.
-

- 60. Hou, X.; Liu, S.; Wang, M.; Wiraja, C.; Huang, W.; Chan, P.; Tan, T.; Xu, C. Layer-By-Layer 3D Constructs of Fibroblasts in Hydrogel for Examining Transdermal Penetration Capability of Nanoparticles. SLAS Technol. 2017, 22, 447-453.
-

- 61. Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D Bioprinting for Vascularize Tissue Fabrication. Ann. Biomed. Eng. 2016, 45, 132-147.
-

- 62. Lee, V. K.; Lanzi, A. M.; Ngo, H.; Yoo, S. S.; Vincent, P. A.; Dai, G. Generation of Multi-Scale Vascular Network System within 3D Hydrogel Using 3D Bio-Printing Technology. Cell Mol. Bioeng. 2014, 7, 460-472.
-

- 63. Leong, M. F.; Toh, J. K. C.; Du, C.; Narayanan, K.; Lu, H. K.; Lim, T. C.; Wan, A. C. A.; Ying, J. Y. Patterned Prevascularised Tissue Constructs by Assembly of Polyelectrolyte Hydrogel Fibers. Nat. Commun. 2013, 4, 2353.
-

- 64. Gao, G.; Park, J. Y.; Kim, B. S.; Jang, J.; Cho, D. W. Coaxial Cell Printing of Freestanding, Perfusable, and Fucntional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102.
-

- 65. Cui, X.; Boland, T. Human Microvasculature Fabrication Using Thermal Inkjet Printing Technology. Biomaterials 2009, 30, 6221-6227.
-

- 66. Norotte, C.; Marga, F. S.; Niklason, L. E.; Forgacs, G. Scaffold-Free Vascular Tissue Engineering Using Bio-Printing. Biomaterials 2009, 30, 5910-5917.
-

- 67. Wu, W.; DeConick, A.; Lewis, J. A. Omnidirectional Printing of 3D Microvascular Networks. Adv. Mater. Technol. 2011, 23, H178-H183.
-

- 68. Kolesky, D. B.; Truby, R. L.; Gladman, A. S.; Busbee, T. A.; Homan, K. A.; Lewis, J. A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructions. Adv. Mater. Technol. 2014, 26, 3124-3130.
-

- 69. Jia, W. J.; Gungor-Ozkerim, P. S.; Zhang, Y. S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M. R.; Shin, S. R.; Khademhosseini, A. Direct 3D Bioprinting of Perfusable Vasular Constructs Using a Blend Biolink. Biomaterials 2016, 106, 58-68.
-

- 70. Pimentel C., R.; Ko, S. K.; Caviglia, C.; Wolff, A.; Emnéus J.; Keller, S. S.; Dufva, M. Three-Dimensional Fabrication of Thick and Densely Populated Soft Constructs with Complex and Actively Perfused Channel Network. Acta Biomater. 2018, 65, 174-184.
-

- 71. Colton, C. K. Implantable Biohybrid Artificial Organs. Cell Transplant. 1995, 4, 415-436.
-

- 72. Jung, J. P.; Bhuiyan, D. B.; Ogle, B. M. Solid Organ Fabrication: Comparison of Decellularization to 3D Bioprinting. Biomater. Res. 2016, 20, 27.
-

- 73. Benam, K. H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K. J.; Karalis, K.; Kim, H. J.; MacQueen, L.; Mahmoodian, R.; Musah, S.; Torisawa, Y.; Meer, A. D.; Villenave, R.; Yadid, M.; Parker, K. K.; Ingber, D. E. Engineered In Vitro Disease Models. Ann. Rev. Pathol. 2015, 10, 195-262.
-

- 74. Zang, D.; Pekkanen-Mattila, M.; Shahsavani, M.; Falk, A.; Teixeria, A. I.; Herland, A. A 3D Alzheimer’s Disease Culture Model and the Induction of P21-Activated Kinase Mediated Sensing in Ipsc Derived Neeurons. Biomaterals 2014, 35, 1420-1428.
-

- 75. Zhang, X. D.; Chen, J.; Min, Y.; Park, G. B.; Shen, X.; Song, S. S.; Sun, Y. M.; Wang, H.; Long, W.; Xie, J.; Gao, K.; Zhang, L.; Fan, S.; Fan, F.; Jeong, U. Metabolizable Bi2Se3 Nanoplates: Biodistribution, Toxicity, and Uses for Cancer Radiation Therapy and Imaging. Adv. Funct. Mater. 2014, 24, 1718-1729.
-

- 76. Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-Dimesional Printing of Hela Cells for Cervical Tumor Model In Vitro. Biofabrication 2014, 6, 035002.
-

- 77. Kang, K. J.; Kim, Y. H.; Jeon, H. Y.; Lee, S. B.; Kim, J. S.; Park, S. A.; Kim, W. D.; Yang, H. M.; Kim, S. J.; Jeong, J. M.; Choi, D. H. Three-Dimensional Bioprinting of Hepatic Structures with Directly Converted Hepatocyte-Like Cells. Tissue Eng. Part A 2018, 24, 7-8.
-

- 78. Kizawa, H.; Nagao, E.; Shimamura. M.; Zhang, G.; Torii, H. Scaffold-Free 3D Bio-Printed Human Liver Tissue Stably Maintains Metabolic, Functions Useful for Drug Discovery. Biochem. Biophys. Rep. 2017, 10, 186-191.
-

- 79. Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C. S. E.; Zanella, F.; Feng, G.-S.; Sheikh, F.; Chien, S.; Chen, S. Deterministically Patterned Biomimetic Human iPSC-derived Hepatic Model Via Rafid 3D Bioprinting. PNAS. 2016, 113, 2206-2211.
-

- 80. Bhise, N. S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y. S.; Shin, S. R.; Calzone, G.; Annabi, N; Shupe, T. D.; Bishop, C. E.; Atala, A.; Dokmeci, M. R.; Khademhosseini, A. A Liver-On-a-Chip-Platform with Bioprinted Hepatic Spheroids. Biofabrication 2016, 8, 014101.
-

- 81. Kukowska-Latallo, J. F.; Candido, K. A.; Cao, Z.; Nigavekar, S. S.; Majoros, I. J.; Thomas, Y. P.; Balogh, L. P.; Khan, M. K.; Baker, J. R. Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human epithelial Cancer. Cancer Res. 2005, 12, 5317-5324.
-

- 82. Palanisamy, N.; Ateeq, B.; Sundaram, S. K.; Pflueger, D.; Ramnarayanan, K.; Shankar, S.; Han, B.; Cao, Q.; Cao, X.; Suleman, K.; Sinha, C. K.; Dhanasekaran, S. M.; Chen, Y. B.; Esgueva, R.; Banerjee, S.; LaFargue, C. J.; Siddiqui, J.; Demichelis, F.; Moeller, P.; Bismar, T. A. K. R.; Fullenn, D. R.; Johnson, T. M.; Greenson, J. K.; Rubin, M. A.; Maher, C. A.; Chinnaiyan, A. M. Rearragements of the RAF Kinase Pathway in Prostate Cancer, Gastric Cancer and Melanoma. Nat. Med. 2010, 16, 793-798.
-

- 83. Snyder, J. E.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H. Bioprinting Cell-Laden Matrigel for Radioprotection Study of Liver by Pro-Drug Conversion in a Dual-Tissue Microfluidic Chip. Biofabrication 2011, 3, 034112.
-

- 84. Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatuura, T.; Minami, H. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837-1843.
-

- 85. Wang, X. Advanced Polymers for Three-Dimensional (3D) Organ Bioprinting. Micromachines 2019, 10, 814.
-

- 86. Farokhzad, O. C.; Cheng, J.; Teply, B. A.; Sherifi, I.; Jon, S.; Kantoff, P. W.; Richie, J. P.; Langer, R. Rargeted Nanoparticle-Aptamer Bioconjugates for Cancer Chemotherapy In Vivo. PNAS. 2006, 103, 6315-6320.
-

- 87. Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A Three-Dimensional In Vitro Ovarian Cancer Coculture Model Using a High-Throughput Cell Patterning Platform. Biotechnol. J. 2011, 6, 204-212.
-

- 88. Tang, M.; Xie, Q.; Gimple, R. C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R. L.; Wu, Q.; Prager, B. C.; Qiu, Z.; Yu, A.; Zhu, Z.; Mesci, P.; Jing, H.; Schimelman, J.; Wang, P.; Lee, D.; Lorenzini, M. H.; Dixit, D.; Zhao, L.; Bhargava, S.; Miller, T. E.; Wan, X.; Tang, J.; Sun, B.; Cravatt, B. F.; Muotri, A. R.; Chen, S.; Rich, J. N. Three-Dimensional Bioprinted Glioblastoma Microenvironments Model Cellular Dependencies and Immune Interactions. Cell Res. 2020, 30, 833-853.
-

- 89. Huang, T. Q.; Qu, X.; Liu, J.; Chen, S. 3D Printing of Biomimetic Microstructures for Cancer Cell Migration. Biomed. Microdevices 2014, 16, 127-132.
-

- 90. Xie, Z.; Gao, M.; Lobo, A. O.; Thomas J.; Webster, T. J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717.
-

- 91. Kang, D.-H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; Irie, S.; Kitano, S.; Matsusaki, M. Engineered Whole Cut Meat-Like Tissue by the Assembly of Cell Fibers Using Tendon-Gel Integrated Bioprinting. Nat. Commun. 2021, 12, 5059.
-

- 92. Ozbolat, I. T. Bioprinting Scale-Up Tissue and Organ Constructs for Transplantation. Trends Biotechnol. 2015, 33, 395-400.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(3): 301-317
Published online May 25, 2022
- 10.7317/pk.2022.46.3.301
- Received on Mar 14, 2022
- Revised on Apr 1, 2022
- Accepted on Apr 4, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Basic Procedures of 3D Bioprinting
Potential Research Cases on Tissue Engineering in Utilizing Bioprinting
Future Direction and Challenge of 3D Bioprinting
- References
Shared
 Correspondence to
Correspondence to
- Oh Hyeong Kwon
-
Department of Polymer Science and Engineering, Kumoh National Institute of Technology, Gumi, Gyeongbuk 39177, Korea
- E-mail: ohkwon@kumoh.ac.kr
- ORCID:
0000-0002-7160-0105










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.