- Effect of Boehmite and Vermiculite on Flame Retardancy and Mechanical Properties of PET
Hunan Chemical Vocational Technology College, Zhuzhou 412000, China
- PET의 난연 특성 및 기계적 특성에 대한 Boehmite와 Vermiculite의 영향
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
The effects of boehmite (BM) and vermiculite (VMT) on flame retardancy, combustion behavior and thermal degradation behaviors of polyethylene terephthalate (PET), BM/PET, and VMT/BM/PET composites were investigated by methods of limited oxygen index (LOI) measurement, vertical burning test, cone calorimeter test and thermogravimetry analysis (TG). The results showed that BM can effectively flame retardancy PET, when 12% BM was added into PET, 12-BM/PET composite possessed LOI value of 32.4% and V0 rating (3.2 mm) in UL-94 test. The flame retardancy, thermal stability and combustion behavior data of 2.5-VMT/BM/PET composite were better than that of 12-BM/PET composite, indicates that appropriate amounts of VMT and BM in PET had synergistic flame-retardant effects. Replace part of BM with VMT, with the increased of VMT substitution amount, the flexural strengths and tensile strengths of VMT/BM/PET composites increased at first, and then decreased, the flexural modules was increased and the notched izod impact strength was decreased.
The results showed that boehmite (BM) can effectively flame retardant polyethylene terephthalate (PET), when 12% BM was added to PET, 12-BM/PET composite possessed limited oxygen index (LOI) value of 32.4% and V0 rating (3.2 mm) in UL-94 test. Appropriate amounts of vermiculite (VMT) and BM in PET had synergistic flame-retardant effects. With the increased of VMT substitution amount, the flexural strengths and tensile strengths of VMT/BM/PET composites increased at first, and then decreased, the flexural modules was increased and the notched izod impact strength was decreased.
Keywords: boehmite, vermiculite, synergistic flame-retardant effects, polyethylene terephthalate, cone calorimeter test.
The author would like to acknowledge the Hunan Chemical Vocational Technology College and Jinan University, which provide a test platform for us.
The authors declare that there is no conflict of interest.
Polyethylene terephthalate (PET) is a semi-crystalline thermoplastic resin, with excellent mechanical properties, electrical properties, heat resistance and good creep resistance, friction resistance, etc, and it’s the thermoplastic polyester with the largest yield and the lowest price.1,2 In recent years, PET has been used as engineering plastics with good development momentum, and widely used in the fields of electronics, automobiles and instrumentation.3,4 These industries had strict requirements on the combustion and safety performance of materials, while PET was easy to burn and generates a large number of molten droplets when burning, which limits its application to a large extent. Therefore, how to improve the flame retardancy of PET had become a research hotspot.
The main component of boehmite (BM) was AlOOH, which had good thermal stability.5 BM was one of the most frequently used precursors in the preparation of crystalline Al2O3 film, in which the Al3+ ions exist in distorted, edge-sharing octahedral arrays of oxide ions that forms a double layer, with the layers being connected by zigzag chains of H-bonds.6 Because of the large amount of -OH groups on the surface, BM was inclined to interact with foreign molecules, thus a variety of composite functional materials may be prepared.7,8 BM was often used as a flame retardant because it breaks down to produce water vapor (which absorbs a lot of heat and dilutes combustible gases) and Al2O3 (which covers the surface of the material and acts as a heat insulation and oxygen insulation) during heating.9 Layered silicates had generated a great deal of interest in recent years in both industrial and academic research.10-17 This was because the high rigidity and aspect ratio of the clay particles, when well dispersed, lead to remarkable improvements in several properties, in particular mechanical, dimensional stability, hot deformation temperature and flame retardancy.18-24 Moreover, these improved properties were obtained at low filler loadings compared with those of conventional microcomposit.25-28 Vermiculite (VMT), as well as MMT, was a mica-type silicate and possesses a layered structure. Each layer consists of octahedrally coordinated cations (typically Mg, Al, and Fe) sandwiched by tetrahedrally coordinated cations (typically Si and Al).29,30 Owing to its layered structure, the VMT had blocking performance, VMT and flame retardant were added to the polymer at the same time, which had a synergistic flame retardancy effect under certain circumstances.31,32
In this paper, PET, BM/PET, and VMT/BM/PET composite were prepared by melt blending, and the effect of boehmite and vermiculite on flame retardancy of PET were studied. It was found that BM could effectively flame retardancy PET, and a proper amount of VMT and BM had synergistic flame retardancy effect in PET.
Materials. Polyethylene terephthalate (PET, CR-8816) was purchased from China Resources Chemical Materials Technology Co., LTD (Changzhou, China). Boehmite (BM, the effective substance content was ≥ 99%) was purchased from Shanghai Yanguo Chemical Co., LTD (Shanghai, China). Vermiculite (VMT, <54 um) with cation exchange capacity of 135 mmol/100 g was purchased from Hebei Lingshou Micro-mineral Co., LTD (Hebei, China).
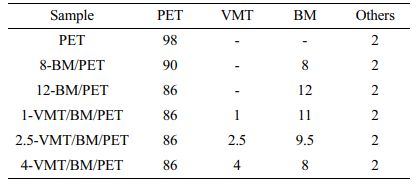
Preparations of Samples. Formulations of the mixtures and abbreviations used for the respective composites were illustrated in Table 1. The PET, BM and VMT were premixed before being fed into the first zone of the extruder. All composites were prepared by using a twin screw extruder (SHJ-20, Nanjing Jaya Extrusion Equipment Co., LTD, China), with a temperature profile of 240 ºC/250 ºC/255 ºC/260 ºC/260 ºC/265 ºC and a rotating speed of 150 rpm, and then the extruded strand was passed through a water bath and pelletized. The pellets were injected into ISO standard specimens by using an injection molding machine (HMT OENKEY) at 250-270 ºC.
Limited Oxygen Index (LOI) Measurement. The LOI was measured according to ISO 4589-2:2017 by an HC-2C oxygen index meter (Nanjing Shangyuan Analytical Instrument Co., LTD, China). The specimens used for the test had dimensions of 150 mm×12.8 mm×3 mm.
Vertical Burning Test. The Underwriter Laboratories 94 (UL-94) vertical burning test was performed using a vertical burning instrument (HVR-4type; Guangzhou Xinna Electronic Equipment Co., LTD, China), and the specimens for testing had dimensions of 128 mm×12.8 mm×3.2 mm and 128 mm×12.8 mm×2.5 mm.
Cone Calorimeter Test. Combustion behavior was studied using a cone calorimeter (UK Testing Technology Limited. UK) according to ISO 5660 at an external heat flux of 50 kW/m2, and the specimens for testing had dimensions of 100 mm×100 mm×3.0 mm.
Thermogravimetry Analysis. Thermogravimetric analysis (TG) of the samples under nitrogen atmospheres was performed on a TAQ50 apparatus (TA Instruments Inc., USA). All the samples were heated from 35 ºC to 700 ºC at the rate of 10 ºC/min.
Mechanical Properties Test. The tensile and flexural tests were carried out by using a universal testing machine (LLOYD LR100K) according to ISO standards 527-1 and ISO standards 178 respectively. The notched Izod impact strengths were conducted following ISO standards 8256 with impact type test machine (ZBC-50). Five samples of each category were tested and their average values were reported.
|
Table 1 Formulations of the Mixtures and Abbreviations for Respective Composites (unit : %) |
Flame Retardancy of PET, BM/PET and VMT/BM/PET Composites. The LOI is one of the important parameters of flame retardancy properties of polymer materials, and high LOI means that the polymer materials are not easy to burn. The underwriter laboratories 94 (UL-94) vertical burning test can characterize the direct combustion phenomenon of materials. The flame retardancy properties of the PET, BM/PET, and VMT/BM/PET composites were detected by the LOI value and UL-94 testing, the results were illustrated in Table 2.
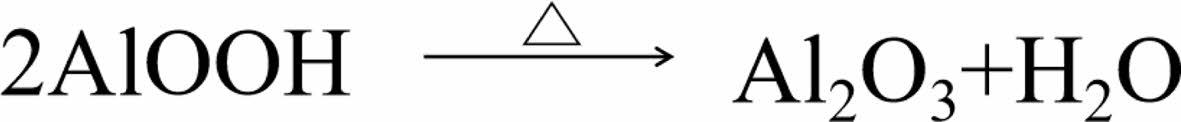
As shown in Table 2, PET presented LOI value of 21.5% with burning to the fixture in UL-94 test, indicating poor flame retardancy of PET. When 8% of BM was added into PET, 8-BM/PET composite showed LOI value of 30.2% with V1 rating (3.2 mm) in UL-94 test. BM/PET composites presented increased LOI values and flame retardancy rating with the further increase in BM loading. When BM loading was increased to 12%, 12-BM/PET composite possessed LOI value of 32.4% and V0 rating (3.2 mm) in UL-94 test. This result indicates that the flame retardancy of BM/PET composite was enhanced compared with that of PET. This enhancement might be ascribed to when BM was thermally decomposed (Figure 1): on the one hand, water vapor are generated, which could absorb a lot of heat, reducing the temperature of material surface, and diluting the combustible gases produced by matrix decomposition at the same time, increasing the gas phase flame retardancy of BM/PET composites; On the other hand, Al2O3 solid are generated, which covering the surface of the material, isolating part of oxygen and heat, and the flame retardancy of the condensate phase was improved. so the overall flame retardancy of the BM/PET composite was enhanced.
Replace part of BM with VMT, with the increased of VMT substitution amount, the LOI and the flame retardancy rating of VMT/BM/PET composites increased at first, and then decreased. The result might be attributed that: on the one hand, VMT was a mica-type silicate and possesses a layered structure with blocking effects, which covering the material surface could insulate oxygen, increasing the density of carbon layer and improving the flame retardancy effect of VMT/BM/PET composites in the condensed phase, whereas with higher content, VMT began to agglomerate and reduction of coverage, which leading the effect of improvement flame retardancy of condensed phase was reduced; on the other hand, the increased of VMT was leaded to the decreased of BM under the total amount of flame retardant added was unchanged, which leading the flame retardancy of VMT/BM/PET composites in the gas phase become worse. So, when VMT replaced BM in a small amount, the enhancement of the flame retardancy effect in the condensed phase was greater than the weakening of the flame retardancy effect in the gas phase, which leading the overall flame retardancy of the VMT/BM/PET composites become better, whereas, when VMT excess replaced BM, the enhancement of the flame retardancy effect in the condensed phase was less than the weakening of the flame retardancy effect in the gas phase, which leading the overall flame retardancy of the VMT/BM/PET composites become worse. When 2.5% BM and 9.5% BM were added into PET, 2.5-VMT/BM/PET composite showed LOI value of 35.1% with V0 rating (2.5 mm) in UL-94 test, the flame retardancy was better than that of 12-BM/PET composite, which indicated that appropriate VMT and BM had synergistic flame retardancy effect in PET.
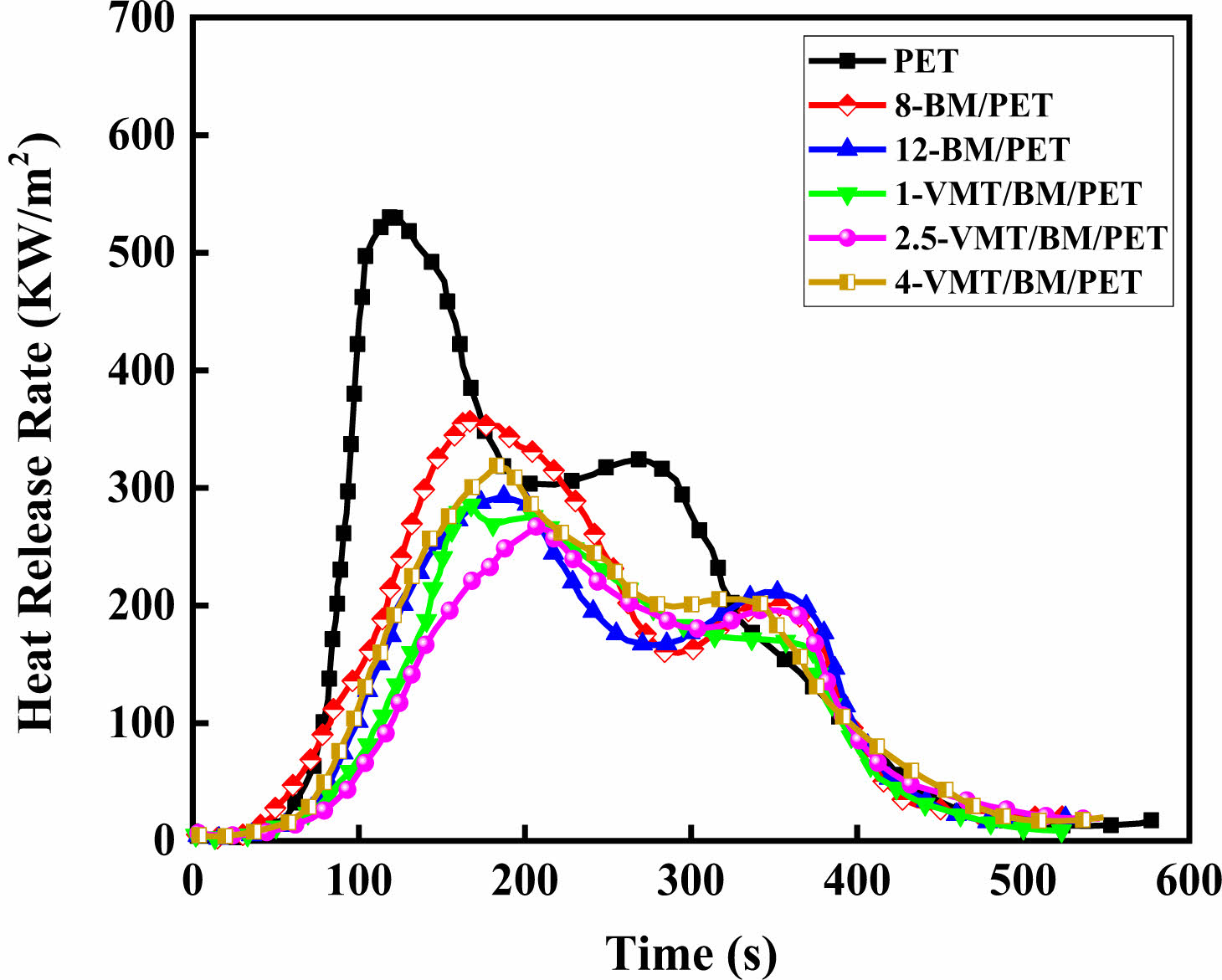
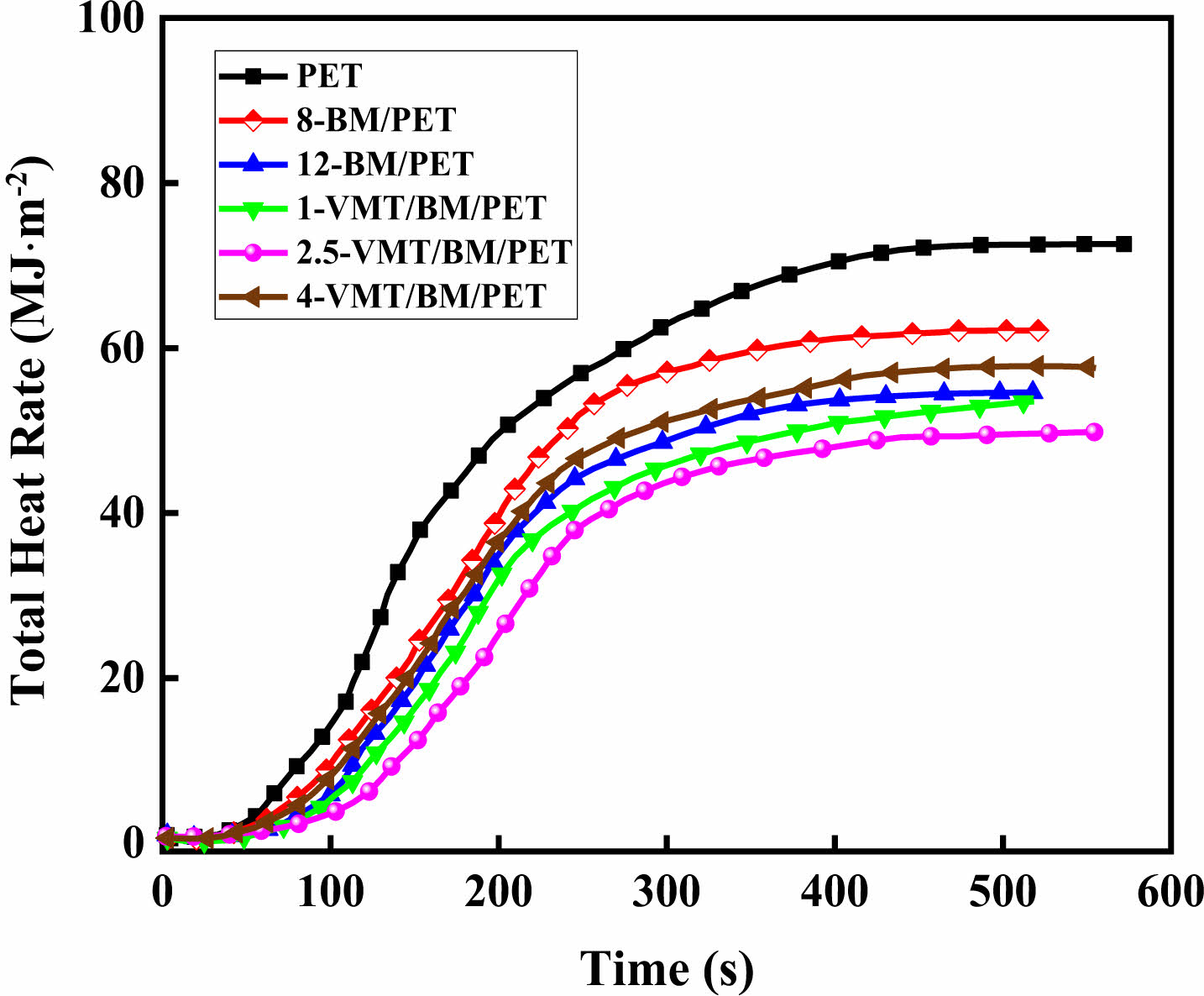
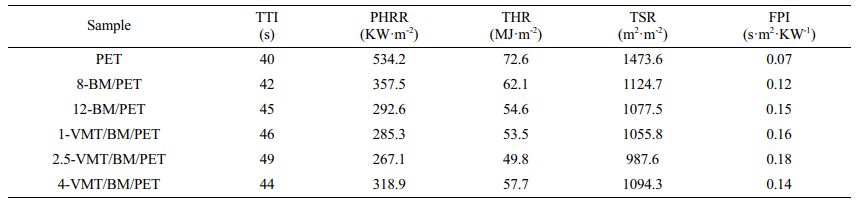
Combustion Behavior of PET, BM/PET, and VMT/BM/PET Composites. The cone calorimeter was usually used to evaluate the flammability characteristics and fire safety of polymer materials in a real fire condition. Heat release rate (HRR) and total heat rate (THR) curves of PET, BM/PET and VMT/BM/PET composites were shown in Figure 2, 3, and the related data were presented in Table 3.
As can be seen from Figure 2, 3 and Table 3, the time to ignition (TTI), peak of heat release rate (PHRR), total heat rate (THR), total smoke release (TSR) and fire performance index (FPI) of PET were 40 s, 534.2 KW·m-2, 72.6 MJ·kg-2, 1473.6 m2·m-2, and 0.07 s·m2·kw-1, respectively. When 12% BM was added into PET, the PHRR, THR, and TSR of 12-BM/PET composite were 292.6 KW·m-2, 54.6 MJ·m-2, and 1077.5 m2·m-2, which decreased by 45.2.0%, 24.8%, and 26.9% compared with PET, respectively. The TTI and FPI of 12-BM/PET composite were 45 s·m2·kw-1 and 0.15 s·m2·kw-1, which were 12.5% and 114.3% higher than that of PET, respectively. This result might be attributed the water vapor generated by the thermal decomposition of BM absorbs a lot of heat, and the generated Al2O3 solid covers the surface of the material to isolate oxygen, heat and combustible gas, which had a good inhibiting effect on the fierce combustion intensity, combustion spread and combustion smoke emission of the composite material, and improving the flame retardancy of the material.
Replace part of BM with VMT, with the increased of VMT substitution amount, the PHRR, THR and TSR of VMT/BM/PET composites decreased at first, and then increased, the TTI and FPI increased at first, and then decreased, which might be attributed when VMT replaced BM in a small amount, the low content of VMT had homogeneous dispersion and covered on Al2O3 solid (formed by thermal decomposition of BM), which improved the density of carbon layer, and increased the flame retardancy effect of condensed phase, and the enhancement of the flame retardancy effect in the condensed phase was greater than the weakening of the flame retardancy effect in the gas phase (due to the reduction of BM), so the overall flame retardancy of the VMT/BM/PET composites was improved; However, when VMT excess replaced BM, the higher content of VMT began to agglomerate, meanwhile, the content of Al2O3 solid too less (due to the content of the remaining BM was too less), so the coverage and density of carbon layer was relatively decreased, which leading the enhancement of the flame retardancy effect in the condensed phase was reduced, and the weakening of the flame retardancy effect in the gas phase (due to the reduction of BM) was greater than the enhancement of the flame retardancy effect in the condensed phase at this time, so the overall flame retardancy of the VMT/BM/PET composites was decreased. When 2.5% VMT and 9.5% BM were added into PET, 2.5-VMT/BM/PET composite showed TTI and FPI were 49 s and 0.18 s·m2·kw-1, which 8.9% and 20% higher than that of 12-BM/PET composite, respectively, the PHRR, THR and TSR of 2.5-VMT/BM/PET composite were 267.1 KW·m-2, 49.8 MJ·m-2, and 987.6 m2·m-2, which decreased by 8.7%, 8.8%, and 8.3% compared with 12-BM/PET composite.
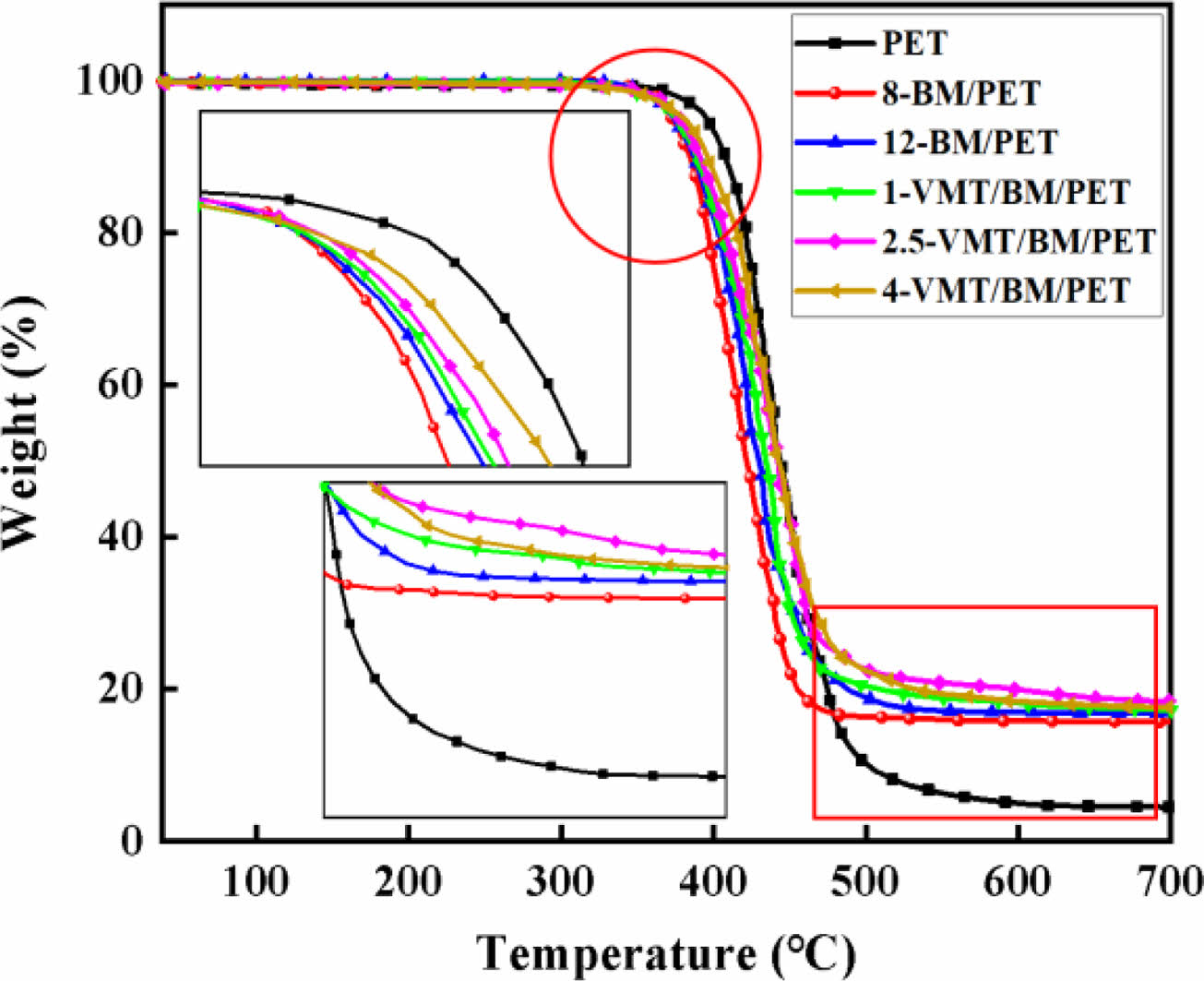
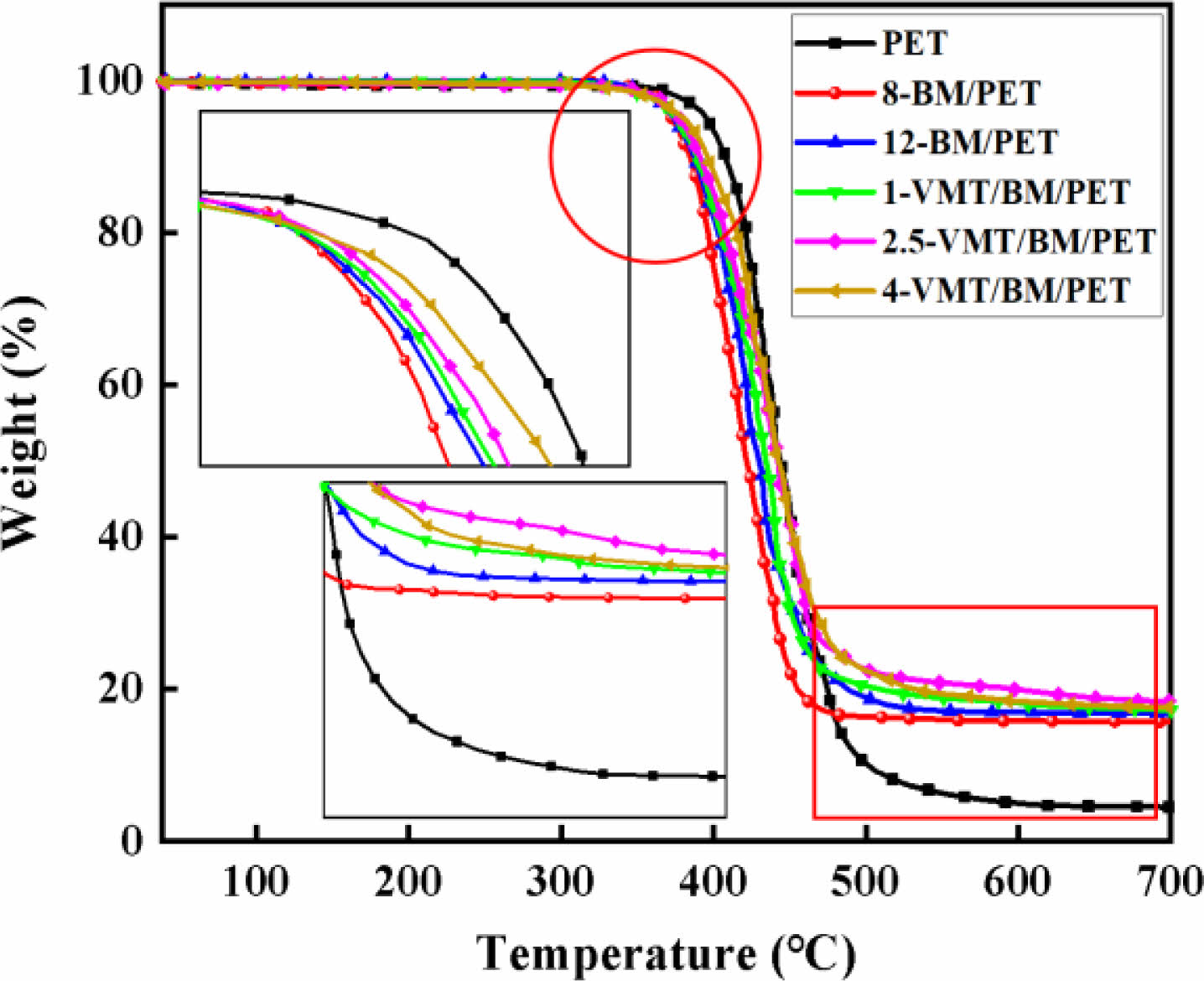
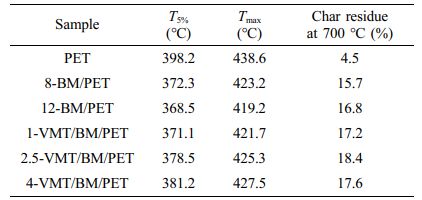
Thermal Degradation Behaviors of PET, BM/PET and VMT/BM/PET Composites. Thermogravimetric analysis (TG) as a useful tool for investigating the mechanism of action for flame-retardance of composites. The thermal degradation curves of PET, BM/PET and VMT/BM/PET composites were shown in Figure 4, and the related data were presented in Table 4.
As shown in Figure 4 and Table 4, PET presented initial decomposition temperature (T5%), maximum decomposition temperature (Tmax) and the char residue at 700 °C were 398.2 °C, 438.6 °C, and 4.5%, respectively. When 8% of BM was added into PET, 8-BM/PET showed T5%, Tmax and the char residue at 700 °C were 372.3°C, 423.2°C, and 15.7%, respectively. BM/PET composites presented decreased T5%, Tmax and increased char residue with the further increase in BM loading. When BM loading was increased to 12%, the T5% and Tmax of 12-BM/PET composite were decreased to 368.5 °C and 419.2 °C, and the char residue at 700 °C was increased to 16.8%. The results indicate that the thermal decomposition temperature of BM/PET composites was decreased, and the char residue at high temperature was increased with BM was added, which might be attributed to the thermal decomposition temperature of BM was lower than that of PET, so the BM has been gradually decomposed before the thermal degradation of PET, resulting in the thermal degradation temperature of BM/PET composites was lower than that of PET, however, the Al2O3 solid (generated by thermal decomposition of BM) covered the surface of the material, which insulating part of the heat and oxygen, and promoting the dehydration of the material into carbon, so the carbon residue at high temperature of BM/PET composites was higher than that of PET.
Replace part of BM with VMT, the T5% and Tmax of VMT/BM/PET composites were gradually increased with the increased of VMT substitution amount, the results indicate that the thermal decomposition temperature of VMT/BM/PET composites was increased with VMT was added, which might be attributed to: Firstly, the thermal decomposition temperature of VMT was higher than that of BM and PET, and the overall thermal decomposition temperature of VMT/BM/PET composites was improved by replacing BM (relatively low thermal stability of material) with VMT (relatively high thermal stability of material). Secondly, VMT was dispersed in PET, which insulating part of the heat, and reducing the thermal decomposition rate of the PET. Unlike T5% and Tmax, the carbon residue at 700 ℃ of VMT/BM/PET composites increased at first, and then decreased, which might be attributed to low content of VMT had homogeneous dispersion and large coverage area in VMT/BM/PET composites, which had good insulation from heat and oxygen, resulting in the carbon residue at 700 ℃ of VMT/BM/PET composite was increased. whereas with higher content, VMT began to agglomerate and had poor dispersion in VMT/BM/PET composites, which led to inferior insulation, and the carbon residue at 700 ℃ of VMT/BM/PET composites was decreased. When 2.5% VMT and 9.5% BM were added into PET, 2.5-VMT/BM/PET composite showed T5%, Tmax and the char residue at 700 °C were 378.5 °C, 425.3 °C, and 18.4%, respectively, which were 10 ℃, 6.1 ℃, and 1.6% higher than that of 12-BM/PET composite.
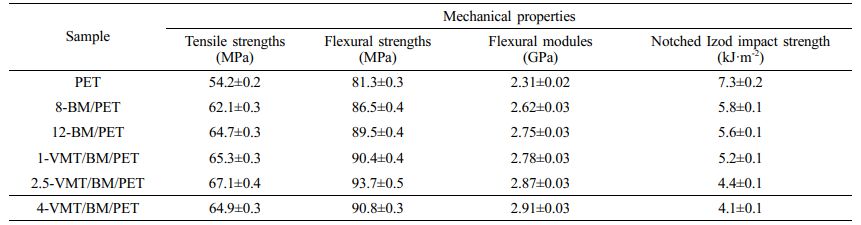
Mechanical Properties of PET, BM/PET and VMT/BM/PET Composites. The mechanical properties of PET, BM/PET and VMT/BM/PET were shown in Table 5. It could be observed that the flexural strengths and tensile strengths of 8-BM/PET and 12-BM/PET increased compare with PET. The result was attributed that the BM was a rigid material with a short rod structure, which could transfer stress from matrix to BM. It also observed that the flexural modules of 12-BM/PET come to 2.75GPa, which was 19.0% more than PET (2.31 GPa), nevertheless, the notched izod impact strength of PET was reduced when added BM. Such result was because the BM restricted motion of polymer chains, decreased the deformation capacity of the matrix in the elastic zone and reduced the ductility of composites, therefore, the BM/PET become brittler and stiffer.
Replace part of BM with VMT, with the increased of VMT substitution amount, the flexural strengths and tensile strengths of VMT/BM/PET composites increased at first, and then decreased, the result might be because the VMT was a mica-type silicate and possesses a layered structure, the effect of transfer stress was better than BM, when VMT replaced BM in a small amount, the low content of VMT had homogeneous dispersion and strong interfacial interactions in the BM/PET, which in turn increased stress transfer from matrix to VMT, leading in high flexural strength and tensile strengths, whereas VMT replaced BM with higher content, VMT began to agglomerate and had poor dispersion in BM/PET. Unlike the flexural strength and tensile strengths, the flexural modules was increased and the notched izod impact strength was decreased with the increased of VMT substitution amount, which was attributed that the effect of VMT restricted motion of polymer chains was better than BM. When 2.5% VMT and 9.5% BM were added into PET, the flexural strength, tensile strengths and flexural modules of 2.5-VMT/BM/PET composite were 93.7 MPa, 67.1 MPa, and 2.87 GPa, respectively, which were 4.7%, 3.7%, and 4.4% higher than that of 12-BM/PET composite.
|
Figure 1 The thermal decomposition equation of BM. |
|
Figure 2 Heat release rate curves of PET, BM/PET and VMT/BM/ PET composites |
|
Figure 3 Total heat rate curves of PET, BM/PET and VMT/BM/ PET composites. |
|
Figure 4 TG curves of PET, BM/PET and VMT/BM/PET composites. |
|
Table 2 LOI Value and UL-94 Rating of PET, BM/PET, and VMT/BM/PET Composites |
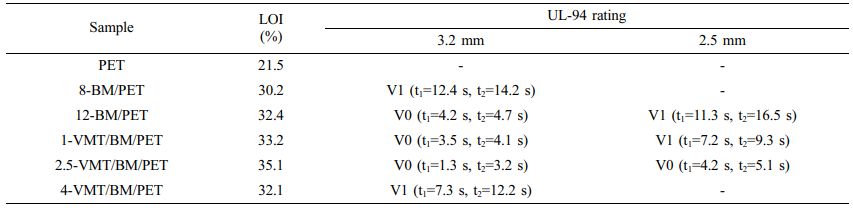
Note: “-” means burning to the fixture; “t1” represents the average time of the first combustion; “t2” represents the average time for the second combustion. |
|
Table 3 Typical Parameters of PET, BM/PET, and VMT/BM/PET Composites in the Cone Calorimetry Test |
|
Table 4 Typical Parameters of PET, BM/PET, and VMT/BM/ PET Composites in the Thermogravimetric Analysis |
In a conclusion, BM was used as the flame retardant of PET, and a series of PET composites were prepared. The results showed that BM could effectively flame retardancy PET, 12-BM/PET composites passed UL-94 V0 rating (3.2 mm) with LOI of 32.4% when BM loading was 12%. The PHRR, THR and TSR of BM/PET composite were lower than those of PET, and the carbon residue at 700 ℃, TTI, and FPI was higher than that of PET. Replace part of BM with VMT, with the increased of VMT substitution amount, the flame retardancy of VMT/BM/PET composites got better at first, and then got worse. In combination with Table 2, 3, and 4, the flame retardancy, thermal stability and combustion behavior data of composites with 2.5% VMT and 9.5% BM (2.5-VMT/BM/PET) were better than that with 12% BM (12-BM/PET) alone, this indicates that appropriate amounts of VMT and BM in PET had synergistic flame-retardant effects. The tensile strength, flexural strength and modules of composites were improved and the notched izod impact strength of composites were reduced when BM was added into PET. Replace part of BM with VMT, with the increased of VMT substitution amount, the flexural strengths and tensile strengths of VMT/BM/PET composites increased at first, and then decreased, the flexural modules was increased and the notched izod impact strength was decreased.
- 1. Lee, S.; Kang, S.; Kim, J. H. Electrical and Thermal Properties of PET/Polyetherimide/Multiwalled Carbon Nanotube Nano- composites. Polym. Korea 2017, 41, 287-294.
-

- 2. Hahm, M.; Kim, C. H.; Ryu, J. A Study on Polypropylene and Surface Modified PET Fiber Composites. Polym. Korea 2008, 32, 7-12.
- 3. Didane, N.; Giraud, S.; Devaux, E. Thermal and Fire Resistance of Fibrous Materials Made by PET Containing Flame Retardant Agents. Polym. Degrad. Stabil. 2012,97, 2545-2551.
-

- 4. Kim, S.; Hong, I. K.; Lee, S. Compatibilization of Linear PPS/PET Blends with SEBS Copolymers. Polym. Korea 2013, 37, 405-410.
-

- 5. Laachachi, A.; Ferriol, M.; Cochez, M. A Comparison of the Role of Boehmite (AlOOH) and Alumina (Al2O3) in thE Thermal Stability and Flammability of Poly(methyl methacrylate). Polym. Degrad. Stabil. 2009, 94, 1373-1378.
-

- 6. Li, M.; Sun, H. Y.; Liu, X. L. Preparation of Porous Boehmite Nanosolid and its Composite Fluorescent Materials by a Novel Hydrothermal Hot-press Method. Mater. Lett. 2006, 60, 2738-2742.
-

- 7. Camino, G.; Maffezzoli, A.; Braglia, M. Effect of Hydroxides and Hydroxycarbonate Structure on Fire Retardant Effectiveness and Mechanical Properties in Ethylene-vinyl Acetate Copolymer. Polym. Degrad. Stab. 2001, 74, 457-464.
-

- 8. Ceren, O.; Krzysztof, K.; Stephen, J. Preparation and Characterization of Titanate-modified Boehmite-polyamide-6 Nanocomposites. Polymer 2005, 46, 6025-6034.
-

- 9. Florian, T.; Bernhard, S.; Michael, W. Particle Size Related Effects of MultiComponent Flame-Retardant Systems in Poly(butadiene terephthalate). Polymer 2020, 12, 1315-1332.
-

- 10. Lee, E. J.; Yoon, Y. K.; Lim, K. H. Silanized Montmorillonite Capsule and Functional Materials Filled Hybrid Polymer-modified Waterproofing Asphalt. Polym. Korea 2020, 44, 672-683.
-

- 11. Granado, A.; Eguiazabal, J. I.; Nazabal, J. J. Effects of the Processing Temperature on the Nanostructure and Mechanical Properties of PCTG-Based Nanocomposites. Appl. Polym. Sci. 2011, 127, 136-144.
-

- 12. Huang, J. C.; Zhu, Z. K.; Yin, J. Poly(etherimide)/Montmorillonite Nanocomposites Prepared by Melt Intercalation: Morphology, Solvent Resistance Properties and Thermal Properties. Polymer 2011, 42, 873-877.
-

- 13. Azeez, A. A.; Rhee, K. Y.; Park, S. J. Epoxy Clay Nano- composites-processing, Properties and Applications: A review. Compos. Part B-Eng. 2013, 45, 308-320.
-

- 14. Kornmann, X.; Lindberg, H.; Berglund, L. A. Synthesis of Epoxy-clay Nanocomposites: Influence of the Nature of the Clay on Structure. Polymer 2001, 42, 1303-1310.
-

- 15. Azad, A. K.; Unnikrishn, L.; Mohanty, S. Nanomaterial Enhanced Polyelectrolyte Membranes for Hydrogen-Oxygen Fuel Cells. Polym. Korea 2021, 45, 101-112.
-

- 16. Gudivada, G.; Kandasubramanian, B. Polymer-phyllosilicate Nanocomposites for High-temperature Structural Application. Polym-Plast. Technol. 2020, 59, 537-591.
-

- 17. Wang, K. H.; Choi, M. H.; Koo, C. M. Synthesis and Characterization of Maleated Polyethylene/Clay Nanocomposites. Polymer 2001, 42, 9819-9826.
-

- 18. Ray, S. S.; Bousima, M. Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Materials World. Prog. Mater. Sci. 2005, 50, 962-1079.
-

- 19. Lee, P. C.; Ha, J. U.; Kim, S. Y. Effects of Temperature and Nano-filler Content on Water Uptake in Nanocomposites. Polym. Korea 2019, 43, 584-588.
-

- 20. Borralleras, P.; Segura, I.; Aranda, M. A. G. Influence of the Polymer Structure of Polycarboxylate-based Superplasticizers on the Intercalation Behaviour in Montmorillonite Clays. Constr. Build. Mater. 2019, 220, 285-296.
-

- 21. Krikorian, V.; Pochan, D. Poly(L-lactide acid)/layered Silicate nanocomposite: Fabrication, Characterization, and Properties. Chem. Mater. 2003, 15, 4317-4324.
-

- 22. Srivastava, S. K.; Pramanik, M.; Acharya, H. Poly(L-lactide acid)/Layered Silicate Nanocomposite: Fabrication, Characterization, and Properties. J. Polym. Sci. Polym. Phys. 2006, 44, 471-480.
-

- 23. Moll, J. F.; Akcora, P.; Rungta, A. Mechanical Reinforcement in Polymer Melts Filled with Polymer Grafted Nanoparticles. Macromolecules 2011, 44, 7473-7477.
-

- 24. Seo, S. D.; Kang, K. C.; Jeong, J. W. Preparation and Characteri- zation of Poly Methyl Methacrylate/Clay Nanocomposite Powders by Microwave-Assisted In-Situ Suspension Polymerization. J. Nanosci. Nanotechno. 2020, 20, 4193-4197.
-

- 25. Scalfaro, R.; Mistretta, M. C.; Lamantia, F. P. Compatibilized Polyamide 6/Polyethylene Blend-Clay Nanocomposites: Effect on the Degradation and Stabilization of the Clay Modifier. Polym. Degrad. Stab. 2008, 93, 1267-1274.
-

- 26. Karimzadeh, I.; Sabzi, M.; Safibonab, B. Improving Environ- mental Durability of Epoxy Resin Using Tetra-n-butylammonium (TBA) Salt Modified Montmorillonite Nanoplatelets. Polym. Korea 2017, 41, 98-103.
-

- 27. Cui, L.; Khramo, D. M.; Bielawski, C. W. Effect of Organoclay Purity and Degradation on Nanocomposite Performance, Part 1: Surfactant Degradation. Polymer 2008, 49, 3751-3761.
-

- 28. Shelley, J. S.; Mather, P. T.; DeVries, K. L. Reinforcement and Environmental Degradation of Nylon 6/Clay Nanocomposites. Polymer 2002, 42, 5849-5858.
-

- 29. Qian, Y.; Lindsay, C. I.; Macosko, C. Synthesis and Properties of Vermiculite-Reinforced Polyurethane Nanocomposites. ACS. Appl. Mater. Interfaces 2011, 3, 3709-3717.
-

- 30. Li, X.; Lei, B. R.; Lin, Z. D. The Utilization of Organic Vermiculite to Reinforce Wood-plastic Composites with Higher Flexural and Tensile Properties. Ind. Crop. Prod. 2013, 51, 310-316.
-

- 31. Cheong, J. Y.; Ahn, J.; Seo, M. Flame-retardant, Flexible Vermiculite-polymer Hybrid Film. RSC. Adv. 2015, 5, 61768-61774.
-

- 32. Yan, L.; Fu, L.; Chen, Y. Improved Thermal Stability and Flame Resistance of Flexible Polyimide Foams by Vermiculite Reinforcement. J. Appl. Polym. Sci. 2017, 134, 44828-44831.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(4): 463-469
Published online Jul 25, 2022
- 10.7317/pk.2022.46.4.463
- Received on Jan 13, 2022
- Revised on Apr 25, 2022
- Accepted on Apr 25, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Xiang Li
-
Hunan Chemical Vocational Technology College, Zhuzhou 412000, China
- E-mail: iverson25@126.com
- ORCID:
0000-0002-1394-3759










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.