- In situ Copolymerized Toughened Polymethyl Methacrylate (PMMA) with Highly Transparency for Support Film of Polarizers
Yingying Wang*, Bin Yang*, **,†
 , Liangyong He*, Yuqing Yang*, Nuo Zhang*, Yang Wang*, Zhiqiang Shi*, **, Yuchao Ke**, Lifen Su***, Jiasheng Qian*, Ru Xia*, and Tao Jiang****
, Liangyong He*, Yuqing Yang*, Nuo Zhang*, Yang Wang*, Zhiqiang Shi*, **, Yuchao Ke**, Lifen Su***, Jiasheng Qian*, Ru Xia*, and Tao Jiang*****College of Chemistry & Chemical Engineering, Key Laboratory of Environment-Friendly Polymeric Materials of Anhui Province, Anhui University, Hefei 230601, Anhui, P.R. China
**Anhui Zhongding Sealing Parts Co., Ltd., Ningguo 242399, Anhui, P.R. China
***School of Materials Science & Engineering, Anhui University, Hefei 230601, Anhui, P.R. China
****Longteng Security & Surveillance Technology Co, Ltd., Lu’an 237001, Anhui, P.R. China- 편광자 지지체를 위한 투명도가 높은 Insitu 공중합 강화 PMMA 고분자
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this study, a series of poly(methyl methacrylate) (PMMA) copolymer films were prepared via solution polymerization of methyl methacrylate (MMA) with butyl acrylate (BA) and lauryl methacrylate (LMA) as monomers. Mechanical properties, hydrophobic properties, and optical properties of the films were intensively investigated. The rheological results showed that the fluidity of the copolymer was considerably enhanced. When the monomer ratio of MMA:BA:LMA was 100:30:10, the copolymer film S4 showed the best overall performance with perfect optical transparency maintained. The results of the dynamic mechanical and thermal analysis suggested that the glass transition temperature (Tg) moved towards lower temperature, with enhanced ductility of the PMMA films. A large number of yield folds and crazes appeared on the cross-sectional surface of copolymer films through morphological observations, displaying the obvious characteristics of toughness fracture and obeying the energy dissipation mechanism of cracks shear band. The present study provided a facile way of preparing PMMA films with high toughness and light transmittance by appropriate selection of the monomers, which will be of practical significance for further studies on the replacement of triacetyl cellulose as a support film of polarizers.
P(MMA-BA-LMA) copolymer films were prepared by using methyl methacrylate (MMA), butyl acrylate (BA), and lauryl methacrylate (LMA) as the monomers. Due to the introduction of soft monomers and monomers with long side groups, irregular flexible molecular chains were formed, causing entanglement among molecular chains. Under external stress, yield folds and cracks occur, presenting ductile fractures.
Keywords: poly(methyl methacrylate), copolymer film, toughness, polarizer.
The authors acknowledge the support provided by National Natural Science Foundation of China (No. 51973002), University Collaborative Innovation Projects of Anhui Province (Nos. GXXT-2019-001 and GXXT-2019-017), Key Projects of Anhui Provincial Department of Education for Universities (No. KJ2020A0023) as well as “211 Project” of Anhui University. Dr. B. Yang is also indebted to the financial support (No. K160133336) from the Longteng (Lu’an) Security & Surveillance Technology Co., Ltd. for this work.
The authors declared that there is no conflict of interest.
The emergence of liquid crystal display (LCD) has exerted an impact on modern society. As a flat panel display, LCD has a wide range of applications, such as notebook computers, mobile phones, digital cameras, display panels, and other commercial consumer electronics.1 The structure of a typical multilayer polarizer included surface protective films-triacetyl cellulose (TAC) film, uniaxial polyvinyl alcohol (PVA) layer, TAC film wide-vision (WV) compensation layer, pressure-sensitive adhesive layer and release film, among which the polarizing layer is PVA film.2,3 Since TAC films had disadvantages such as poor weather resistance, dimensional instability, expensive materials and complicated preparation techniques, scientists are looking for replaceable alternatives.4 Polymeric materials such as poly(methyl methacrylate) (PMMA) film, cyclic olefin copolymer (COP) film and polyethylene terephthalate (PET) film are beginning to be introduced to replace TAC film in large quantities.5-7 Among those non-TAC films, PMMA film accounted for the highest percentage of protective film.
PMMA, which is also known as plexiglass and commonly polymerized from methyl methacrylate (MMA). It is an amorphous polymer with good optical properties, rigidity, dimensional stability, UV aging resistance and other excellent properties.8-12 PMMA has been widely used in various fields, such a optical sensors, supercapacitor electrode materials, optoelectronic devices, as well as biomolecular applications.13-15 However, due to a low crack propagation energy, PMMA always showed obvious notch sensitivity and lower notch impact toughness during the fracture process. The application of PMMA has been limited by its high brittleness. Many studies have been extensively carried out to improve the toughness of PMMA over the past several decades. Jamaluddin et al. used solution casting technology to prepare nanocomposites of PMMA and cellulose nanofibers (CNF) modified with propionic anhydride (PA), and they found that the addition of 1 wt% CNF helped to enhance the tensile properties and toughness of the composites coupled with good transparency.16 Mao et al. designed a co-continuous phase structure in PMMA/chlorinated polyethylene (CPE) to improve PMMA’s impact toughness and they found at a CPE loading of 40 wt% the notched impact strength of the blend reached 28.5 kJ/m2, which was 26 times higher than that of neat PMMA.17 Kenan et al. used nettle fibers obtained by natural methods to prepare nettle fiber-reinforced PMMA composites, the results showed that after adding 10% nettle fiber, the impact strength increased by 40% and the fracture toughness increased by 106%.18 Xia et al. used emulsion polymerization to prepare core/shell type organic-inorganic hybrid polymer nanoparticles (Si-ASA HPNs), using silicon-modified butyl acrylate copolymer (PBA) as the core and styrene-acrylonitrile copolymer (SAN), and their results showed that the addition of core-shell particles could improve PMMA’s elongation at break and impact strength.19 In recent years, copolymerization was considered as an attractive method for preparing the ductile polymeric materials,20 and had also considered as a potential method for improving the toughness of PMMA. Ye et al. designed and synthesized an organogel with a long alkyl chain structure to modify PMMA and prepare toughened PMMA composites, and their results showed that the impact strength and elongation at break of the composites were 1.6 and 9.6 times higher than those of neat PMMA, and the maximum value of impact strength and breaking elongation were achieved when 1.0 wt% gelator was added.21 Kubiak et al. prepared a hybrid particle brushes and star polymers with a core-shell structure via atom transfer radical polymerization (ATRP) and incorporate them into PMMA, and found that both strength and toughness of PMMA were improved without a significant reduction in optical transparency.22 However, the preparation of high toughness PMMA by ternary random copolymerization was rarely reported.
In this study, solution polymerization was selected to produce ternary copolymerization of MMA, butyl acrylate (BA) and lauryl methacrylate (LMA) to fabricate toughened PMMA films with high transparency. By adjusting the ratio of MMA, BA, LMA, and optimized ratios of three monomers were explored on the basis of the experimental data of molecular weight distribution (MWD), cross-sectional morphology and physio-mechanical properties of the prepared PMMA films via gel permeation chromatography (GPC), scanning electron microscope (SEM) as well as dynamically rheological measurement. Our findings showed that the toughness of PMMA film was significantly improved with a light transmittance of 93% maintained, together with considerably improved hydrophobic properties of the prepared PMMA films. The present work supplies a facile way of preparing a high toughness and high light transmission PMMA films, which will surely be important to replacement of TAC film as a supportive film for polarizers.
Materials. Methyl methacrylate (MMA) [99.0%, containing 30 ppm MEHQ stabilizer], butyl acrylate (BA) [AR, 99%], lauryl methacrylate (LMA) [96%, containing 500 ppm MEHQ stabilizer], and ethyl acetate (EAC) [AR, 99.5%] were all purchased from Aladdin (Shanghai) Co., China. Azobisisobutyronitrile (AIBN) was purified and recrystallized by using ethanol.
Sample Preparation. The main monomer used in polymerization to PMMA is MMA, which provides good rigidity for macromolecular chains. The synthesized PMMA films are required to have perfect toughness to protect PVA films in place of TAC films, and soft monomers e.g., BA and LMA, were used to toughen the PMMA films in this work. The existence of LMA and BA made them copolymerized with MMA randomly, and the selection of ratio of those monomers was the focus of this study.
The sample preparation process was illustrated in Figure 1. Firstly, weigh different masses of MMA, BA, LMA, and mix them in a beaker, add 0.56 g AIBN and stirred the mixture to dissolve the initiator completely. Weigh 200 g EAC mixed with monomers, place them in an oil bath at 70 °C under nitrogen atmosphere by magnetic stirring for 8 h to obtain the copolymer. Then, the copolymer was added dropwise on a glass plate, and the films with a thickness of 1000 μm which were obtained via being scraped using a doctor blade coating method were dried in a vacuum for 12 h. Samples with different ratio were denoted as S1-S6, with the detailed compositions summarized in Table 1.
Characterization and Measurement. GPC: 4 mg polymer was weighed and dissolved in chromatographic tetrahydrofuran (THF) to prepare a sample solution of 1 mg·mL-1. After completely dissolved, the solution was filtered using a 0.45 μm copolymerization syringe to obtain the filtrate, and polymer samples were subsequently injected into the sampling system. GPC (GelMaster-3000, POLYTECH, BEIJIN) was operated with chromatographic neat THF as the mobile phase at a flow rate of 1000 μL·min-1, and all GPC tests were carried out at 40 °C.
Fourier Transform Infrared Spectroscopy (FTIR): The vertex 80 FTIR spectrometer provided by Bruker Co. Germany was used for FTIR characterization of the functional groups in the copolymerization films. The spectrometer was equipped with a Hyperion 2000 infrared microscope, using a thin film mode with a wavelength ranging from 600 to 4000 cm-1.
Dynamically Rheological Measurement: A strain-controlled rheometer (Bohlin Gemini-200, Malvern Instruments Ltd., UK) was adopted for dynamic rheological testing, using a dynamic frequency sweep with samples of 20 mm diameter in the cone-plate mode. The cone-plate angle was 4° at a temperature of 25 °C. All rheological measurements were carried out in a scanning frequency range from 0.01 to 100 Hz.
Mechanical Performance Measurement: Electronic universal testing machine (5967, Instron, USA) was used to evaluate the mechanical properties of the films at room temperature. All film samples were fixed vertically with two pairs of clamps with an initial distance of 50.0 mm, and tested at a constant elongation rate of 10 mm/min until the samples were completely broken. At least 3 samples were measured for each sample and average values were recorded for comparison.
Water Contact Angle (WCA) Measurement: A contact angle tester (DSA 30, KRUSS, Germany) was used to evaluate the hydrophilicity of the PMMA films. All measurements were performed at the room temperature with double distilled water as the probe liquid.
Optical Performance Test: The transmittance and haze of the films were obtained using a transmittance-haze meter (color haze meter YJD-3600C, Chongqing Inspection Instruments Co., China). The square samples were cut out with dimensions of 4 cm×4 cm from the films for testing.
Dynamic Mechanical Thermal Analysis (DMTA): A DMTA device (Q-800, TA Instruments, USA) was used to study the dynamic mechanical behaviors of the prepared PMMA films. The temperature of the samples (with dimensions of 1 mm×7 mm×30 mm) rose from room temperature to 190 °C at a heating speed of 5 °C/min with the modulus curve recorded simultaneously.
SEM Observation: A SEM (S-4800, Hitachi, Japan) was used to study the morphology of the PMMA films, which were first fractured in liquid nitrogen and then sputtered with gold in vacuum. Observation of the cross-sectional morphology of the samples was done at an electronic accelerating voltage of 30 kV.

|
Figure 1 Schematic diagram of the process of preparing a copolymer film. |
GPC. Figure 2 was the GPC curves of S1-S6 copolymerization films, and it could be seen that the MWD of the synthesized ternary copolymer was unimodal without side reaction products (such as oligomer) detected. The experimental data were listed in Table 2. The MWD of S2-S6 was similar to that of S1. The Mw of the S1 was 80515 g/mol and its Mn was 40693 g/mol with a polydispersity index (PDI=Mw/Mn, an indicator of MWD) of 1.98. The molecular weights (Mw) of films S2-S6 were almost the same as that of film S1. The values of PDI of S1, S3, and S4 were 1.98, 1.95, and 1.99 respectively (all less than 2.0), indicating that the MWD of the samples was sufficiently narrow.
FTIR Analysis. Figure 3showed the infrared spectrum of S1-S6, and the peak at 1733 cm-1 was the stretching vibration characteristic of ester carbonyl group and that at 1452 cm-1 was the asymmetric stretching of PMMA's O-CH3 group.23-30 The peaks at 1149 and 1152 cm-1 were the symmetric stretching vibrations of the carbon-oxygen bond of the ester group,31 and that at 1149 cm-1 was the characteristic peak of the ester bond of MMA.32 The absorption bands peaks at 1461 cm-1 and 1068 cm-1 were closely related to the bending vibrations of the C-H and C-O bonds, respectively. The area change in the vicinity of 3000-2700 cm-1 belonged to the -CH2 of the LMA side chain in the copolymer.33 The peak at 3447 cm-1 was the vibration peak of -COOH, that at 751 cm-1 was the characteristic peak of LMA and that at 838 cm-1 was the characteristic peak of BA. The stretching vibration peak for C=C double bond was usually 1700-1600 cm-1, and this characteristic peak did not appear in the spectrum, indicating the PMMA copolymer had been successfully prepared in this work.34
Dynamically Rheological Behaviors. The rheological analysis of the copolymers was carried out, as shown in Figure 4, which displayed the frequency dependence of the composite viscosity η*. It could be seen that at a lower frequency, the viscosity of each sample varied greatly. The shear viscosity of the neat PMMA film dropped from 5.90×105 Pa·s to 1.8×102 Pa·s (suggesting a typical non-Newtonian fluid characteristics). It could be seen that the viscosity of neat PMMA was maximum at low shear frequency, which exhibited much greater decreasing rate in viscosity, because the PMMA chains were rigid and the molecular bonds were tightly stacked. This kind of behavior in viscosity had been reported to be typically encountered in inhomogeneous systems (e.g., polymer gels and liquid-crystalline polymers).35 The viscosities of S3 and S5 were lower since the copolymer structure contained a large number of alkane side groups, and the spatial site resistance increased the molecular chain spacing.36
At high shear frequencies, polymer chains untwisted and separated from each other, and intermolecular interactions were weakened, leading to a decrease in viscosity. The changes in storage modulus (G') and loss modulus (G'' ) with shear rate were presented in Figures 4(b-c), and G' clearly exceeds G'', indicating the fact that the flow deformation was subject to the elastic deformation. Combined with Figure 4(a), as the shear frequency increased, the viscosity (η* ) decreased, indicating that the fluid displayed a typical shear thinning behavior, which was in line with the characteristics of pseudoplastic fluids.37
WCA of Various Copolymer Films. WCA experiments were also carried out to explore the hydrophilicity of samples S1-S6, the results were presented in Figure 5.With different monomer ratios, the WCA of film S1 was 76.4°, and that of copolymer film was generally larger than that of S1. Therefore, copolymer film had better hydrophobicity than neat PMMA film, considering the hydrophobic groups, such as ester groups and methyl side chains.38-40 Molecular chain may form entanglements during polymerization, and accumulate more ester groups and methyl side chains, so copolymer film showed greatly improved hydrophobic properties in comparison to neat PMMA film, which made them potentially applicable for replacing the TAC as supportive film especially in the presence of pressure-sensitive adhesive.
Optical Properties of Various Copolymer Films. As is known, crystalline and semi-crystalline polymers are usually opaque and have low visible light transmittance because light is refracted and reflected at the interface of crystal domains, while amorphous polymers are usually transparent and have high visible light transmittance. Haze was defined as the fraction of transmitted light scattered at a large angle (>2.5°) and represented the milky color of the sample.41 Figure 6 showed the light transmittance data graphs of various films and PMMA film had a high light transmittance. As can be seen in Figure 6, the copolymer film also had good transparency with a light transmission ranging from 80% to 93%. As shown in Figure 7, the prepared films all had low haze and high light transmittance (the text was clearly visible under the film), suggesting that the addition of both monomers improved the mechanical properties of the films without reducing the films’ transparency.
Mechanical Performance of Various Copolymer Films. Figure 8 showed the correlation between tensile strength (st), elongation at break (eb) of various films. It could be seen from Figure 8(a) that at a tensile rate of 10 mm/min, the st for S1 was 40.8 MPa, and the respective eb was 2.09%. With an incorporation of LMA, the eb increased first and then decreased, with a maximum eb at 143.23% for S5. A small increase in toughness of films S2 and S3 indicated that the improvement in toughness was actually limited when single monomer was added. As compared with the eb at 2.09% for neat PMMA (S1), the eb of S5 increased by 67 times, however, the st of S5 was just 27.4 MPa, much lower than that of S1 (40.8 MPa). The st and eb of S4 were 40.29 MPa and 82.45%, respectively. As compared with films S1 and S5, film S4 had the best tensile strength while maintaining high toughness. This could be mainly due to the irregular arrangement of three monomers to form flexible molecular chains with long-chain side groups, which tended to produce entanglement between the molecular chains, leading to improved toughness of the copolymer films.
The DMTA curves of neat PMMA and copolymer films were shown in Figure 9. In the temperature range studied, the viscoelasticity of the samples varied considerably with the variation of the monomer ratio. As presented in Figure 9(a), the storage modulus decreased as the temperature rose, showing an inverted "S" type. The value of the lower slope in the middle of the storage modulus curve gradually increased, indicating that the temperature sensitivity of the storage modulus increased gradually. It could be seen that the addition of different ratios of monomers caused the storage modulus of copolymer films to be lower than that of neat PMMA film, indicating that the addition of these monomers reduced the rigidity of the film.
The random copolymer chains formed by BA, LMA, and MMA had higher mobility to enhance the toughness of PMMA. Figure 9(b) presented the variation of tan δ with temperature for various copolymer films, and the tan δ of the copolymer films was lower than that of neat PMMA. The peak on the tan δ curve always represented the glass transition temperature (Tg) of the polymeric materials, and it could be seen that the Tg of the copolymer films was lower than that of neat PMMA, indicating that the movement of molecular chains in the copolymer film became easier than that of neat PMMA, and the presence of other monomers helped to improve the toughness of the films.
In this work, PMMA copolymer films with high toughness and light transmittance were successfully prepared with a highest elongation at break (eb) of 82.45%, tensile strength of 40.3 MPa and a light transmittance of 90%, which were actually achieving the standard for a commercial TAC film (e.g., Model: #TAC-10%-60MPA-92%) made by Hefei Lucky film Co., Ltd. China, whose products were mainly used in various application areas (e.g., imaging, photoelectric, polarizer, etc). Last but not the least, PMMA copolymer films will potentially be applicable in replacing TAC film as support film for polarizers.
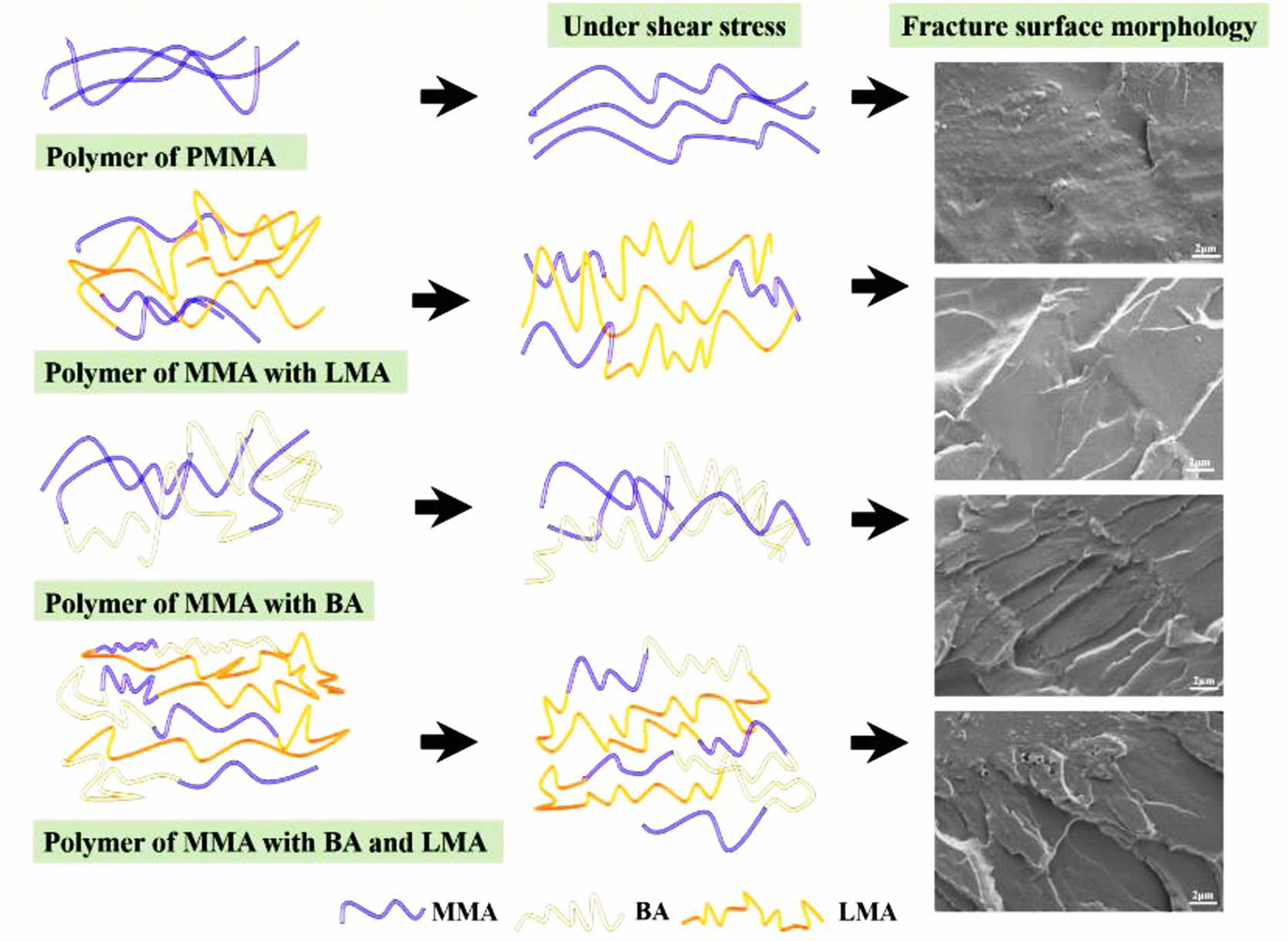
Morphological Observation of Toughened Copolymer Films. Figure 10 showed the cross-sectional morphology of the films, and it could be seen that the surface of the cross-section looked flat and smooth without cracks (cf. Figures 10(a-a')), displaying typical brittle fracture characteristics. When BA and LMA monomer were added, the cross-sectional morphology gradually became rough, suggesting an improved toughness compared with neat PMMA. As shown in Figures 10(b-f '), the fracture surfaces of S2-S6 were rough due to absorbing a lot of energy to produce deformation, showing the characteristics of toughness fracture. Films S3, S4, and S5 displayed more cracks and more obvious yield, indicating better flexibility of S3, S4, and S5 than those of S1, S2, and S6. The toughening mechanism of the present copolymer films could be inferred as the energy dissipation of the crazing shear band.42,43 It could thus be concluded that the toughness of the copolymer films was greatly improved after the addition of BA and LMA monomers. Among them, S4 had a higher tensile strength while maintaining high toughness as well as good optical transparency.
Besides, mechanical properties can be reflected by the cross-sectional morphology of films to disclose the toughening mechanism involved. As shown in Figure 11 that PMMA was a brittle unit with short molecular chain, neat PMMA without soft chain had difficulty in movement and showed no obvious deformation. Due to its hard and orderly molecular chain, the cross-sectional morphology was relatively smooth, exhibiting typically brittle fracture characteristics.44 When the LMA with flexible and longer alkyl chains was added, the cross-section of the films became rough, with a small amount of stress whiteness and no apparent yield folds. With the addition of flexible BA alone, the surface of P(MMA-BA) copolymer films became rougher with cracks in large quantities, which was due to the absorption of energy and deformation. Previous studies have reported that the addition of BA monomer was beneficial to improving the toughness of the film. However, the tensile strength was found to decrease substantially.45 After being suffered with shear stress, the molecular chains moved to restrain the propagation of cracks. The yield folds formed on the fracture surface due to the ripping effect of stress when the shear stress was big enough and the molecular chain was flexible enough. Copolymer chains with flexible elements tended to move and deform obviously under shear stress, resulting in increased toughness of copolymers. Interestingly, when LMA with long alkyl side chains was introduced, soft units BA and LMA were irregularly arranged between the hard molecular chains of PMMA, reducing the regularity of the macromolecular chains. The molecular chains of P(MMA-BA-LMA) were more prone to deformation when copolymer was subjected to external stress,46,47 leading to an enhanced toughness. As a result, the present copolymer films displayed desirable overall performance.

|
Figure 2 GPC traces of PMMA copolymer films. |

|
Figure 3 FTIR spectrum of P(MMA-BA-LMA) copolymer films. |

|
Figure 4 (a) Frequency dependence of complex viscosity; (b) frequency dependence of storage modulus; (c) frequency dependence of loss modulus for PMMA copolymer films. |

|
Figure 5 WCA of copolymer films of (a) S1; (b) S2; (c) S3; (d) S4; (e) S5; (f) S6. |

|
Figure 6 Variation of the transparency and haze test for various samples. |

|
Figure 7 Photograph of copolymer films of (a) S1; (b) S2; (c) S3; (d) S4; (e) S5; (f) S6. |

|
Figure 8 (a) Diagram of relationship between tensile strength and sample ratio (b) Elongation at break with sample ratio. |

|
Figure 9 Dynamic viscoelastic curves of PMMA copolymer film with various ratio: (a) temperature dependence of storage modulus; (b) tan δ versus temperature transition region. |

|
Figure 10 Cross-sectional morphologies of copolymer films at 20 μm and 2 μm: (a-a') S1; (b-b') S2; (c-c') S3; (d-d') S4; (e-e') S5; (ff ') S6. |

|
Figure 11 Microscopic deformation mechanism of PMMA copolymers. |
In this study, PMMA copolymer films were prepared via solution polymerization using BA, LMA, and MMA as monomers. The effect of various ratios of BA and LMA on the physical-mechanical properties of the prepared films were investigated intensively. When BA or LMA was added separately, the toughness of the film was improved slightly at the expense of a decrease in tensile strength. With the joint incorporation of both BA and LMA, the toughness of the films was found to be greatly enhanced. When the monomer ratio of MMA:BA:LMA was 100:30:10, the elongation at break (eb) for film S4 could reach 82.45% together with a relatively high tensile strength of 40.29 MPa. with acceptable optical transparency. The combination of rheological data and toughness suggested that a lower solution viscosity (better fluidity) of copolymers tended to obtain films with higher toughness. The experimental data of DMTA and mechanical tests also disclosed that copolymer films had lower Tg values than neat PMMA film, which helped to improve the film ductility considering that the motion of molecular chains of copolymer films was easier. The morphological observations showed that the fracture surface displayed yield folds, and the fracture mechanism of P(MMA-BA-LMA) copolymer gradually changed from brittle fracture to ductile fracture with varying monomer ratios, and a large number of cracks appeared in the fracture surface of the copolymer films. It was thus confirmed that the toughening mechanism for the present work belonged to the energy dissipation mechanism of cracks shear band. The present work will be of practical significance to further research work on the replacement of TAC films as a protective film for polarizer.
- 1. Huang, Y.; Zhang, X.; Li, C.; Zhao, Y.; Zhang, Y. H.; Qu, J. Atmospheric Persistence and Toxicity Evolution for Fluorinated Biphenylethyne Liquid Crystal Monomers Unveiled by In silico Methods. J. Hazard. Mater. 2022, 424, 127519.
-

- 2. Wang, Y.; Wang, R.; Zhang, C.; Wang, J. Full Components Recovery of Organic Matter and Indium from Discarded Liquid Crystal Display Panels. J. Cleaner Prod. 2021, 299, 126862.
-

- 3. Ma, J.; Ye, X.; Jin, B. Structure and Application of Polarizer Film for Thin-film-transistor Liquid Crystal Displays. Displays 2011, 32, 49-57.
-

- 4. Wu, C. L.; Yao, P. H.; Lin, C. H.; Sung, C. K.; Chen, C. H. Fabrication of Flexible Metallic Wire Grid Polarizer Using Thermal NIL and Lift-off Processes. Microelectron. Eng. 2012, 98, 117-120.
-

- 5. Park, E. J.; Kim, I. S.; Park, S. S.; Lee, H. S.; Lee, M. S. Miscibility of Melt-mixed PLLA/PMMA Blends for Optical Film Application. Polym. Korea 2013, 37, 744-752.
-

- 6. Kim, H. J.; Kim, D. W.; Kim, S. W. Improvement of Optical and Thermo-mechanical Properties of Polycarbonate-based Diffusers for LED Backlight Unit by Incorporation of Porous Silica Particles. Polym. Korea 2013, 36, 761-767.
-

- 7. Han, K. H.; Kim, W. N.; Kim, J. Effect of Immiscible Polymer in Improving the Reflectivity and Productivity of Polymer Blending Film Containing Polyethylene Terephthalate. Polym. Korea 2021, 45, 363-371.
-

- 8. Song, D. G.; Lee, J.; Choi, H. J.; Kim, J. K. Study on Contact Lens That Blocks Blue Light from LED. Polym. Korea 2022, 46, 62-67.
-

- 9. Zhang, Y.; Zhuang, S.; Xu, X.; Hu, J. Transparent and UV-shielding ZnO@PMMA Nanocomposite Films. Opt. Mater. 2013, 36, 169-172.
-

- 10. Tran, T. N.; Paul, U.; Heredia Guerrero, J. A.; Liakos, I.; Marras, S.; Scarpellini, A.; Ayadi, F.; Athanassiou, A.; Bayer, I. S. Transparent and Flexible Amorphous Cellulose-acrylic Hybrids. Chem. Eng. J. 2016, 287, 196-204.
-

- 11. Xu, J.; Li, D. Preparation and Photoluminescence of Transparent Poly(methyl methacrylate)-based Nanocomposite Films with Ultra-high-loading Pendant ZnS Quantum Dots. Polymers (Basel) 2018, 10, 1217.
-

- 12. Martín-de León, J.; Jiménez, M.; Pura, J. L.; Bernardo, V.; Rodriguez-Pérez, M. A. Easy-way Production of Highly Transparent Nanocellular Polymers Films. Polymer 2021, 236, 124298.
-

- 13. Wie, J.; Kim, J. Thermal Conductivity Enhancement Derived from Poly(Methyl Methacrylate)-grafted Carbon Nanotubes in Poly(methyl methacrylate)/polystyrene Blends. Polymers (Basel) 2019, 11, 1347.
-

- 14. Wang, Y.; Liu, P.; Wang, H.; Zeng, B.; Wang, J.; Chi, F. Flexible Organic Light-emitting Devices with Copper Nanowire Composite Transparent Conductive Electrode. J. Mater. Sci. 2018, 54, 2343-2350.
-

- 15. Hamlaoui, F. Z.; Naar, N. Improvement of the Structural and Electrical Properties of PMMA/PANI-MA Blends Synthesized by Interfacial In situ Polymerization in a Continuous Organic Phase. Polym. Bull. 2020, 79, 37-63.
-

- 16. Jamaluddin, N.; Hsu, Y. I.; Asoh, T. A.; Uyama, H. Optically Transparent and Toughened Poly(methyl methacrylate) Composite Films with Acylated Cellulose Nanofibers. ACS Omega 2021, 6, 10752-10758.
-

- 17. Mao, Z.; Jiang, T.; Zhang, X.; Jiang, G.; Zhang, J. Co-continuous Phase Structure Formed in Melt Processing Inducing Shear Bands to Prevent Crack Propagation: Significant Improvement in Impact Toughness of PMMA. Polym. Test. 2020, 85, 106425.
-

- 18. Büyükkaya, K.; Demirer, H. Examining the Mechanical and Thermomechanical Properties of Polymethylmethacrylate Composites Reinforced with Nettle Fibres. Arabian J. Sci. Eng. 2019, 45, 665-674.
-

- 19. Xia, J.; Luo, X.; Huang, J.; Ma, J.; Yang, J. Preparation of Core/Shell Organic–inorganic Hybrid Polymer Nanoparticles and Their Application to Toughening Poly(methyl methacrylate). RSC Adv. 2021, 11, 34036-34047.
-

- 20. Zheng, J.; Wang, L.; Hu, Y.; Yao, K. Toughening Effect of Comonomer on Acrylic Denture Base Resin Prepared via Suspension Copolymerization. J. Appl. Polym. Sci. 2012, 123, 2406-2413.
-

- 21. Ye, F.; Chen, S.; Tang, G.; Ma, M.; Wang, X. Self-assembled Nanofibrillar Gel Network Toughened PMMA Nanocomposite by In situ Thermal Polymerization of MMA Gel. Colloids Surf., A 2015, 480, 1-10.
-

- 22. Kubiak, J. M.; Yan, J.; Pietrasik, J.; Matyjaszewski, K. Toughening PMMA with Fillers Containing Polymer Brushes Synthesized via Atom Transfer Radical Polymerization (ATRP). Polymer 2017, 117, 48-53.
-

- 23. Monsores, K. G. d. C.; Silva, A. O. d.; Oliveira, S. d. S. A.; Rodrigues, J. G. P.; Weber, R. P. Influence of Ultraviolet Radiation on Polymethylmethacrylate (PMMA). J. Mater. Res. Technol. 2019, 8, 3713-3718.
-

- 24. Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The Effect of Copolymerisation on the Performance of Acrylate-based Hybrid Sol-gel Coating for Corrosion Protection of AA2024-T3. Prog. Org. Coat. 2020, 147, 105701.
-

- 25. Krishnan, M. R.; Aldawsari, Y. F.; Alsharaeh, E. H. Three‐ dimensionally Cross‐linked Styrene‐methyl Methacrylate‐divinyl Benzene Terpolymer Networks for Organic Solvents and Crude Oil Absorption. J. Appl. Polym. Sci. 2020 138, 49942.
-

- 26. Suresh, S. S.; Mohanty, S.; Nayak, S. K. Preparation and Characterization of Recycled Blends Using Poly(vinyl chloride) and Poly(methyl methacrylate) Recovered from Waste Electrical and Electronic Equipments. J. Cleaner Prod. 2017, 149, 863-873.
-

- 27. Fu, Q.; Lin, G.; Chen, X.; Yu, Z.; Yang, R.; Li, M.; Zeng, X.; Chen, J. Mechanically Reinforced PVDF/PMMA/SiO2 Composite Membrane and Its Electrochemical Properties as a Separator in Lithium-ion Batteries. Energy Technol. 2018, 6, 144-152.
-

- 28. El Sayed, A. M. Synthesis, Optical, Thermal, Electric Properties and Impedance Spectroscopy Studies on P(VC-MMA) of Optimized Thickness and Reinforced with MWCNTs. Results Phys. 2020, 17, 103025.
-

- 29. Joseph, J.; Deshmukh, K.; Chidambaram, K.; Faisal, M.; Selvarajan, E.; Sadasivuni, K. K.; Ahamed, M. B.; Pasha, S. K. K. Dielectric and Electromagnetic Interference Shielding Properties of Germanium Dioxide Nanoparticle Reinforced Poly(vinyl chloride) and Poly(methylmethacrylate) Blend Nanocomposites. J. Mater. Sci.: Mater. Electron. 2018, 29, 20172-20188.
-

- 30. Arunkumar, R.; Babu, R. S.; Usha Rani, M.; Kalainathan, S. Effect of PBMA on PVC-based Polymer Blend Electrolytes. J. Appl. Polym. Sci. 2017, 134, 44939.
-

- 31. Le, N. X. T.; Trinh, K. T. L.; Lee, N. Y. Poly(acrylic acid) as an Adhesion Promoter for UV-assisted Thermoplastic Bonding: Application for the In vitro Construction of Human Blood Vessels. Mater. Sci. Eng., C 2021,122, 111874.
-

- 32. He, Z.; Zhao, J.; Li, F.; Zhang, D.; Guo, F.; Guo, H.; Wang, X.; Hu, H. In situ Synthesis of Polymer-modified Boron Nitride Nanosheets via Anionic Polymerization. Appl. Surf. Sci. 2021, 537, 147966.
-

- 33. Skandalis, A.; Pispas, S. PLMA-b-POEGMA Amphiphilic Block Copolymers: Synthesis and Self-assembly in Aqueous Media. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 155-163.
-

- 34. Bagheri, S.; Kalantari, M.; Fozooni, S.; Hashemipour Rafsanjani, H. Synthesis of Organic‐inorganic Hybrid Nanocomposites Based on the Acrylate Monomers and Investigation of Scratch Resistance of These Nanocomposites. Polym. Compos. 2019, 41, 142-160.
-

- 35. Lyoo, W. S.; Seo, I. S.; Yeum, J. H.; Yoon, W. S.; Ji, B. C.; Kim, B. S.; Lee, S. S.; Kim, B. C. Effect of Degree of Saponification on the Rheological Properties of Syndiotactic Poly(vinyl alcohol)/Water Solution. J. Appl. Polym. Sci. 2002, 86, 463-467.
-

- 36. Lyoo, W. S.; Yeum, J. H.; Kwon, O. W.; Shin, D. S.; Han, S. S.; Kim, B. C.; Jeon, H. Y.; Noh, S. K. Rheological Properties of High Molecular Weight (HMW) Syndiotactic Poly(vinyl alcohol) (PVA)/HMW Atactic PVA Blend Solutions. J. Appl. Polym. Sci. 2006, 102, 3934-3939.
-

- 37. Vildanova, R. R.; Sigaeva, N. N.; Kukovinets, O. S.; Kolesov, S. V. Preparation and Rheological Properties of Hydrogels Based on N-succinyl Chitosan and Hyaluronic Acid Dialdehyde. Polym. Test. 2021, 96, 107120.
-

- 38. Ioelovich, M. Adjustment of Hydrophobic Properties of Cellulose Materials. Polymers (Basel) 2021, 13, 1241.
-

- 39. Gao, S. H.; Zhang, G. X.; Zhang, F. X. Surface Modification of Polyester Fibers by Encasing Sucrose Ester and Grafting Soybean Protein. Adv. Mater. Res. 2011, 331, 133-138.
-

- 40. Tateishi, Y.; Kai, N.; Noguchi, H.; Uosaki, K.; Nagamura, T.; Tanaka, K. Local Conformation of Poly(methyl methacrylate) at Nitrogen and Water Interfaces. Polym. Chem. 2010, 1, 303-311.
-

- 41. Lin, Y.; Bilotti, E.; Bastiaansen, C. W. M.; Peijs, T. Transparent Semi‐crystalline Polymeric Materials and Their Nanocomposites: A Review. Polym. Eng. Sci. 2020, 60, 2351-2376.
-

- 42. Saad-Gouider, N.; Estevez, R.; Agnon, C.; Seguela, R. Calibration of a Viscoplastic Cohesive Zone for Crazing in PMMA. Eng. Fract. Mech. 2006, 73, 2503-2522.
-

- 43. Xu, S.; Wang, Z.; Cao, M.; Dai, X.; Zhang, S.; Liu, L. Preparation and Properties of Anionic Nylon-6-b-polyether Amine Copolymers with Varying Soft Chain Length. Polym. Plast. Technol. Eng. 2018, 57, 1430-1439.
-

- 44. Xu, S.; Wang, Z.; Liu, L.; Dai, X.; Zhang, S.; Cao, M. Respective Contribution Research of Soft Component and Macroinitiator on Synthesis and Performance of MCPA-PEA Materials. Polym. Eng. Sci. 2018, 58, 1353-1361.
-

- 45. Lu, Z.; Guan, W.; Tang, L. High Performances Polyurethane-urea Polyacrylate Hybrid Emulsion Coatings with Multiple Crosslinking Structures. Prog. Org. Coat. 2019, 132, 328-335.
-

- 46. Ren, G.; Miao, Y.; Qiao, L.; Qin, Y.; Wang, X.; Wang, F. Toughening of Amorphous Poly(propylene carbonate) by Rubbery CO2-based Polyurethane: Transition from Brittle to Ductile. RSC Adv. 2015, 5, 49979-49986.
-

- 47. Zhu, Y. P.; Wang, L. S.; Zhang, A. Q.; Wang, X.; Cai, T.; Zeng, X. Toughening Effect of Poly(ethene-co-1-butene)-graft-methyl Methacrylate and Acrylonitrile on Styrene-acrylonitrile Copolymer (SAN). J. Macromol. Sci., Part B: Phys. 2010, 49, 757-769.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2022; 46(5): 566-576
Published online Sep 25, 2022
- 10.7317/pk.2022.46.5.566
- Received on Apr 11, 2022
- Revised on Jul 1, 2022
- Accepted on Jul 7, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Bin Yang
-
*College of Chemistry & Chemical Engineering, Key Laboratory of Environment-Friendly Polymeric Materials of Anhui Province, Anhui University, Hefei 230601, Anhui, P.R. China
**Anhui Zhongding Sealing Parts Co., Ltd., Ningguo 242399, Anhui, P.R. China - E-mail: yangbin@ahu.edu.cn
- ORCID:
0000-0003-4972-9196










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.