- Effects on the Properties of Diketopyrrolopyrrole-based Polymer via Inserting Oxadiazoles on Their Main Chains
Rajalingam Agneeswari, Danbi Kim*, Vellaiappillai Tamilavan*, Chnan-gi Shin, Sung Heum Park*, and Youngeup Jin†

Department of Industrial Chemistry, Pukyong National University, Busan 48513, Korea
*Department of Physics, Pukyong National University, Busan 48513, Korea- Diketopyrrolopyrrole계 고분자 주사슬에 Oxadiazoles을 삽입함에 따른 고분자 특성 변화에 관한 연구
Rajalingam Agneeswari · 김단비* · Vellaiappillai Tamilavan* · 신찬기 · 박성흠* · 진영읍†

부경대학교 공업화학과, *부경대학교 물리학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
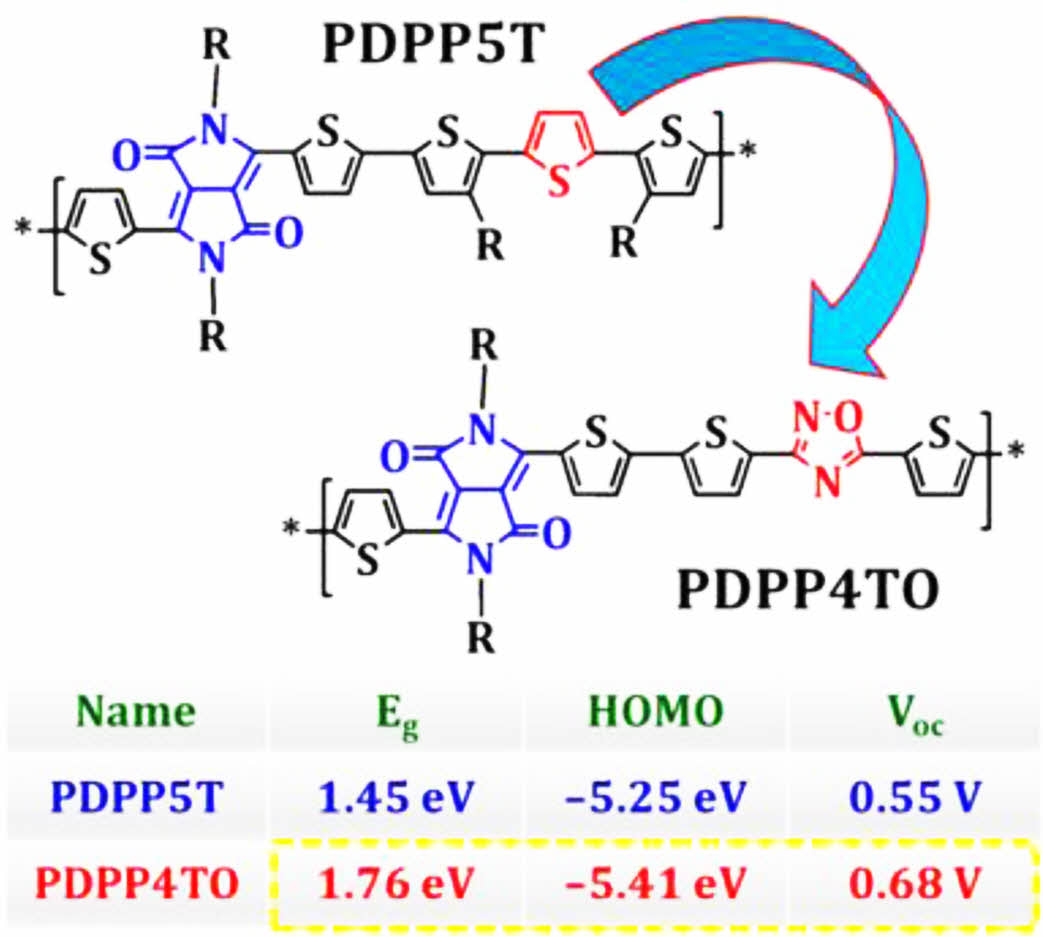
A new alternating copolymer, poly(3,6-bis(thiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-3,5-di(thiophen-2-yl)-1,2,4-oxadiazole (PDPP4TO), was prepared and their properties were compared with the reported polymer, poly(2,5-bis(2-hexyldecyl)-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-3-hexyl-2-(5-(3-hexylthiophen-2-yl)thiophen-2-yl)thiophene (PDPP5T), with the aim understanding the effects of inserting oxadiazole units on diketopyrrolopyrrole (DPP)-based polymer backbone. Surprisingly, the properties of DPP-based polymers were found to drastically change after inserting oxadiazoles on their backbone. Noticeably, PDPP4TO exhibited higher bandgap (⁓0.3 eV), and consequently showed good complementary absorption with non-fullerene acceptors, and deeper highest occupied molecular orbital (HOMO, ⁓0.16 eV) compared to those of PDPP5T. However, the photovoltaic devices made using PDPP4TO offered a lower power conversion efficiency (PCE, ⁓0.94%) compared to that of PDPP5T (⁓4.6%). Whereas, the PDPP4TO-based photovoltaic devices provided a significantly higher open-circuit voltage (Voc) than that of the PDPP5T-based devices. These results indicate that the insertion of oxadiazoles on DPP-based polymer backbones results in an enormous difference in their properties.
본 연구는 diketopyrrolopyrrole(DPP) 기반 고분자 골격에 옥사디아졸 단위를 삽입함으로써 고분자 특성에 미치는 효과를 알아보기 위해 신규 고분자 poly(3,6-bis(thiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-3,5-di(thiophen-2-yl)-1,2,4-oxadiazole(PDPP4TO)을 개발하고 이전에 보고한 고분자 poly(2,5-bis(2-hexyldecyl)-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-3-hexyl-2-(5-(3-hexylthiophen-2-yl)thiophen-2-yl)thiophene(PDPP5T)와 비교 분석하였다. DPP계 고분자는 백본에 oxadiazoles을 삽입한 후 특성이 급격히 변화됨을 확인하였다. 고분자 PDPP4TO는 PDPP5T에 비해 더 높은 밴드갭(~0.3 eV)을 가졌으며, 결과적으로 넌플러렌 수용체와 상보적으로 좋은 흡수대와 깊은 highest occupied molecular orbital(~0.16 eV)을 보였다. 그러나 PDPP4TO를 전자주개로 제작한 태양전지는 PDPP5T로 제작한 태양전지(PCE~4.6%)에 비해 상대적으로 낮은 전력 변환 효율(PCE~0.94%)을 보였다. 그러나 PDPP4TO로 제작된 태양전지는 PDPP5T 기반 태양전지보다 훨씬 높은 개방 회로 전압(Voc)을 제공했다. 이러한 결과는 DPP 기반 고분자 백본에 oxadiazoles을 삽입하면 그 성질에 큰 차이가 발생한다는 것을 나타낸다.
This study confirmed that the insertion of oxadiazole units on diketopyrrolopyrrole (DPP)-based polymer backbone results in an enormous difference in their opto-electrical and photovoltaic properties. Especially, the bandgap was increased from 1.45 to 1.76 eV and the highest occupied molecular orbital was lowered from –5.25 to –5.41 eV. As a result, the open circuit-voltage of the photovoltaic devices improved from 0.55 to 0.68 V for an oxadiazole-incorporated DPP-based polymer.
Keywords: organic solar cells, diketopyrrolopyrrole-based polymers, oxadiazole-based polymers.
This work was supported by the BB21+ Project in 2022, and this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2020R1F1A1071449 and No 2022R1F1A1075060).
The authors declare that there is no conflict of interest.
The versatile application of π-conjugated polymers on opto-electronics, such as organic solar cells (OSCs),1-4 perovskite solar cells (Per-SCs),5-7 organic thin-film transistors (OTFTs),8,9 organic light-emitting diodes (OLEDs),10,11 encourage the chemists to develop structurally new semiconducting polymers for those applications. Past few decades, we have been focused in the preparation of polymeric donors for OSCs. It is widely known that the PCE of OSCs is dependent on the properties of the materials used on their photoactive layer, which is generally made by using a blend solution containing electron-donor and electron-acceptor materials.1-4 On conventional OSCs, π-conjugated polymers are used as an electron-donor and fullerene derivatives (PC60BM or PC70BM) are utilized as an electron-acceptor for efficient light absorption and charge separation.4 The respective OSC devices gave a maximum PCE of up to 12%.12-16 However, fullerene derivatives were replaced by using electron-accepting organic small molecules, and the resulting non-fullerene acceptor-based organic solar cells (NFA OSCs) provided greatly improved PCEs in the range of 12-20%.17-22 The combined efforts such as appropriate modification on the properties of photoactive materials, ternary, and tandem device approaches improved the PCE shortly up to 20%.17-22 Noticeably, the fullerene derivative absorbs light from 300 to 700 nm, but most of the successful NFAs show their absorption band between 600 to 1100 nm.4,23 Consequently, polymers that display low bandgap are good for conventional OSCs, but polymers that exhibit large or medium bandgap are suitable for NFA OSCs. The high PCE achieved for NFA OSCs inspired the researchers to find structurally new wide or medium bandgap polymers which show good complementary absorption and suitable energy levels with NFAs.
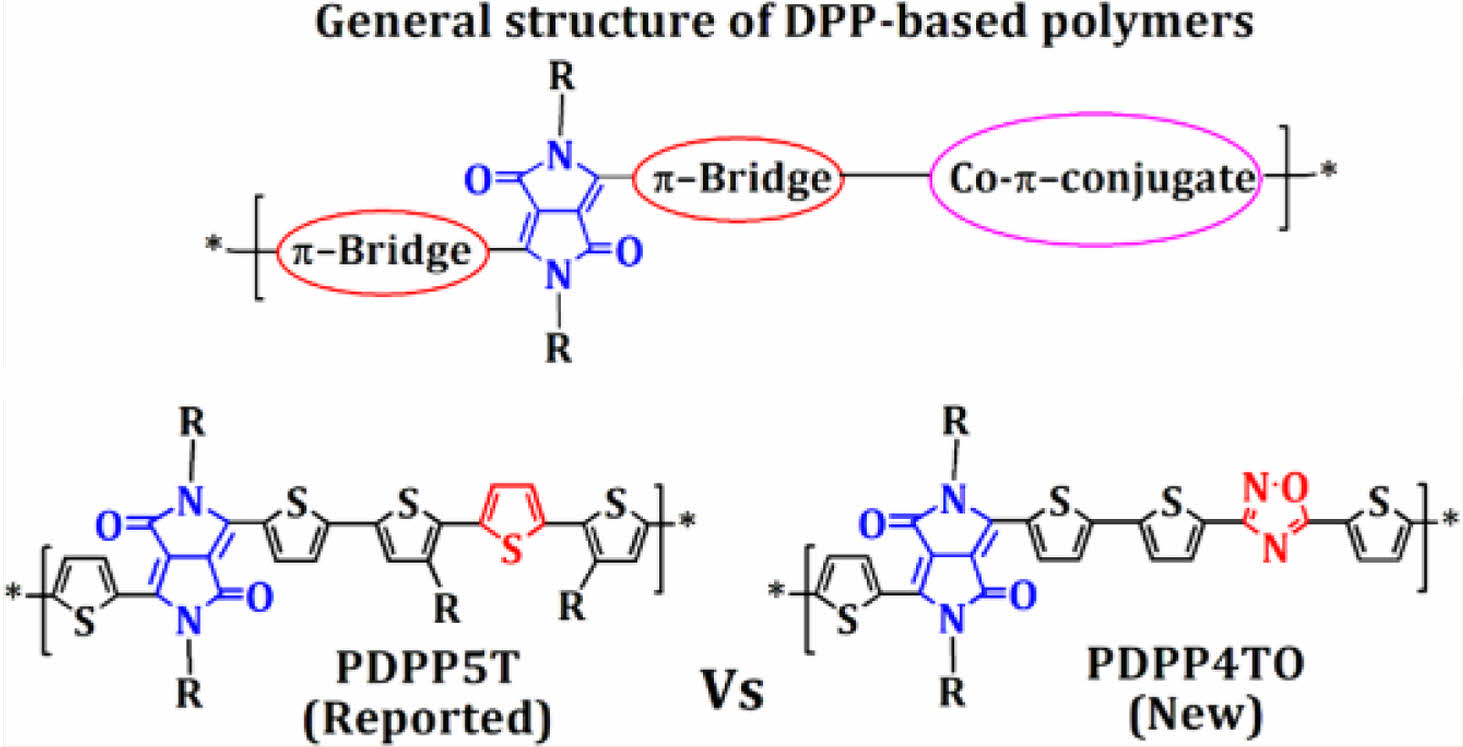
Literature suggests that the π-conjugate, namely diketopyrrolopyrrole or pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (DPP), is one of the efficient building blocks for making high energy converting low bandgap polymers for conventional fullerene-based OSCs.24-30 The advantages of DPP unit, such as strong electron-withdrawing aptitude, high charge carrier mobility due to the good crystallinity, and excellent solubility via the facile introduction of solubilizing substituents on the lactam nitrogen, insists the researchers to develop those polymers for OSCs27-29 and OTFTs.9,27-29 The common structure used for making DPP-based polymers is outlined in Figure 1, and the electron-deficient DPP unit was generally attached with π-bridges and followed by co-π-conjugates. Overall, the structural engineering of DPP-based polymers via the incorporation of different π-bridges, alkyl chains and co-π-conjugates gave a high PCE of up to 10% when it blended with PC70BM.24-30 Noticeably, most of the DPP-based polymers exhibit intense absorption between 600 and 1000 nm with an optical bandgap in the range of 1.0-1.3 eV, and thereby showed good complementary absorption from 300 to 950 nm when it blends with PC70BM.24-30 However, the utilization of DPP-based polymers as electron-donors on NFA-OSCs was mostly failed due to their mismatched absorption with NFAs and high lying-highest occupied molecular orbital (HOMO) levels. The maximum PCEs reported for the NFA-OSCs made by using DPP-based polymeric donors and NFAs were in the range of 2-5%.31-33 The polymer PDPP5T shown in Figure 1 showed a maximum PCE of 5% when it was used as an electron-donor along with narrow bandgap NFA, namely 2,2'-[[4,4,9,9-tetrakis(4-hexylphenyl)-4,9-dihydro-s-indaceno[1,2-b:5,6-b']di- thiophene-2,7-diyl]bis[[4-[(2-ethylhexyl)oxy]-5,2-thiophene- diyl]methylidyne(5,6-difluoro-3-oxo-1H-indene-2,1(3H)-diyl- idene)]]bis[propanedinitrile] (IEICO-4F).31-33
We think that the NFA-OSCs made from DPP-based polymers might give higher PCE by increasing their absorption between 300 and 700 nm along with lowering their HOMO levels below -5.40 eV. It is well known that the oxadiazoles are electron-accepting heteroaromatics and show a tendency to increase the electron mobility,34-39 and the insertion of oxadiazoles on the polymer main or side chains increasing their bandgap and notably lowing the HOMO levels.34-39 Therefore, we decided to incorporate oxadiazoles on the backbone of PDPP5T. We expect that the replacement of one thiophene using oxadiazole on the repeating unit of PDPP5T persuades blue shift absorption and lowers the HOMO level for the resulting polymer. Here, we report the synthesis of a new polymer, namely PDPP4TO, and compare their properties with those of PDPP5T to inspect the effects of inserting oxadiazole on DPP-based polymer main chain.
|
Figure 1 General structure of DPP-based polymers and the chemical structure of PDPP5T and PDPP4TO. |
Materials and Characterization. The dibromo-monomer, namely 3,5-bis(5-bromothiophen-2-yl)-1,2,4-oxadiazole(Bis- (bromo)-TOT), was synthesized according to the reports.38 On the other hand, diborane-monomer, namely 2,5-bis(2-octyldodecyl)-3,6-bis(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione(Bis- (borane)-DPP), was purchased from Sunatech, China. Whereas, IEICO-4F was purchased from One Materials, Canada. The nuclear magnetic resonance (NMR) spectra were recorded using Varian Mercury Plus spectrometer (300 MHz) in Chloroform-d (CDCl3). Gel permeation chromatography (GPC) analyses were conducted on an Agilent 1200 Infinity Series separation module using polystyrene as a standard and chloroform as an eluent to determine the molecular weight and polydispersity (PDI) of the polymers. The photophysical and electrochemical properties of PDPP4TO were studied on a JASCO V-570 spectrophotometer and a CH Instruments electrochemical analyzer, respectively.
Fabrication and Characterization of NFA-OSCs. The similar procedures40 were used for the device fabrication and characterization of NFA-OSCs. A PEDOT:PSS (Baytron PH) was spin-casted onto a pre-cleaned glass-ITO substrate and dried in air at 140 oC for 15 min. The glass-ITO/PEDOT:PSS substrate was then transferred to a glove box. The active layer was then spin cast on glass-ITO/PEDOT:PSS substrate. A blended solution of 1:1.5 (w/w) PDPP4TO:IEICO-4F in 99.5:0.5 (v/v) chlorobenzene (CB):1,8-diiodoocatne (DIO) was used for making active layer film on the glass-ITO/PEDOT:PSS layer. In the case of the Y6-based devices, a blended solution of 1:1.2 (w/w) PM6:Y6 in chloroform (CF) or 0.1:0.9:1.2 (w/w/w) PDPP4TO:PM6:Y6 in CF was used for making active layer film on the glass-ITO/PEDOT:PSS layer. The film was then dried for 30 min at RT in the glove box. A methanolic PDINO solution was then spin-cast onto the glass-ITO/PEDOT:PSS/PDPP4TO:IEICO-4F. Finally, on the top of glass-ITO/PEDOT:PSS/PDPP4TO:IEICO-4F/PDINO substrate, an aluminum (Al, 100 nm) electrode was deposited via thermal evaporation at approximately 3×10-6 Torr. The current density-voltage (J‒V) profiles of the OSC devices were acquired using a Keithley 2400 source measure unit. The solar-cell performance was determined using an Air-Mass 1.5 Global (AM 1.5 G) solar simulator with an irradiation intensity of 1000 Wm-2.
Synthesis of Poly(3,6-bis(thiophen-2-yl)-2,5-bis(2-octyl- dodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-3,5-di(thiophen-2-yl)-1,2,4-oxadiazole (PDPP4TO). The conventional Suzuki coupling reaction38 between Bis(borane)-DPP (0.33 g, 0.30 mmol) and Bis(bromo)-TOT (0.12 g, 0.30 mmol) and followed by purification by using Soxhlet apparatus afforded polymer PDPP4TO as a black solid: Yield: 0.29 g (78%). 1H NMR (300 MHz, CDCl3) d 8.92 (bs, 2 H), 8.00-7.27 (m, 6 H), 4.03 (m, 4 H), 2.01 (bs, 2 H), 1.58 (bs, 8 H), 1.21 (bs, 56 H), 0.84 (bs, 12 H). Anal. Calc. for C64H92N4O3S4: C, 70.28; H, 8.48; N, 5.12; S, 11.73. Found: C, 66.38; H, 7.43; N, 4.55; S, 11.07.
Monomer Bis(bromo)-TOT and polymer PDPP4TO were prepared as outlined in Scheme 1. Suzuki polymerization between Bis(borane)-DPP and Bis(bromo)-TOT and followed by purification using Soxhlet extraction with methanol and acetone offered a polymer PDPP4TO. The polymer PDPP4TO showed better solubility in all common chlorinated solvents at elevated temperatures. The determined 5% weight loss temperature from thermal gravimetric analysis (TGA) was 330 ⁰C for PDPP4TO. The estimated weight-average molecular weight (Mw), number-average molecular weight (Mw), and polydispersity index (PDI=Mw/Mn) were 1.06×104, 2.37×104, and 2.24 g/mol, respectively.
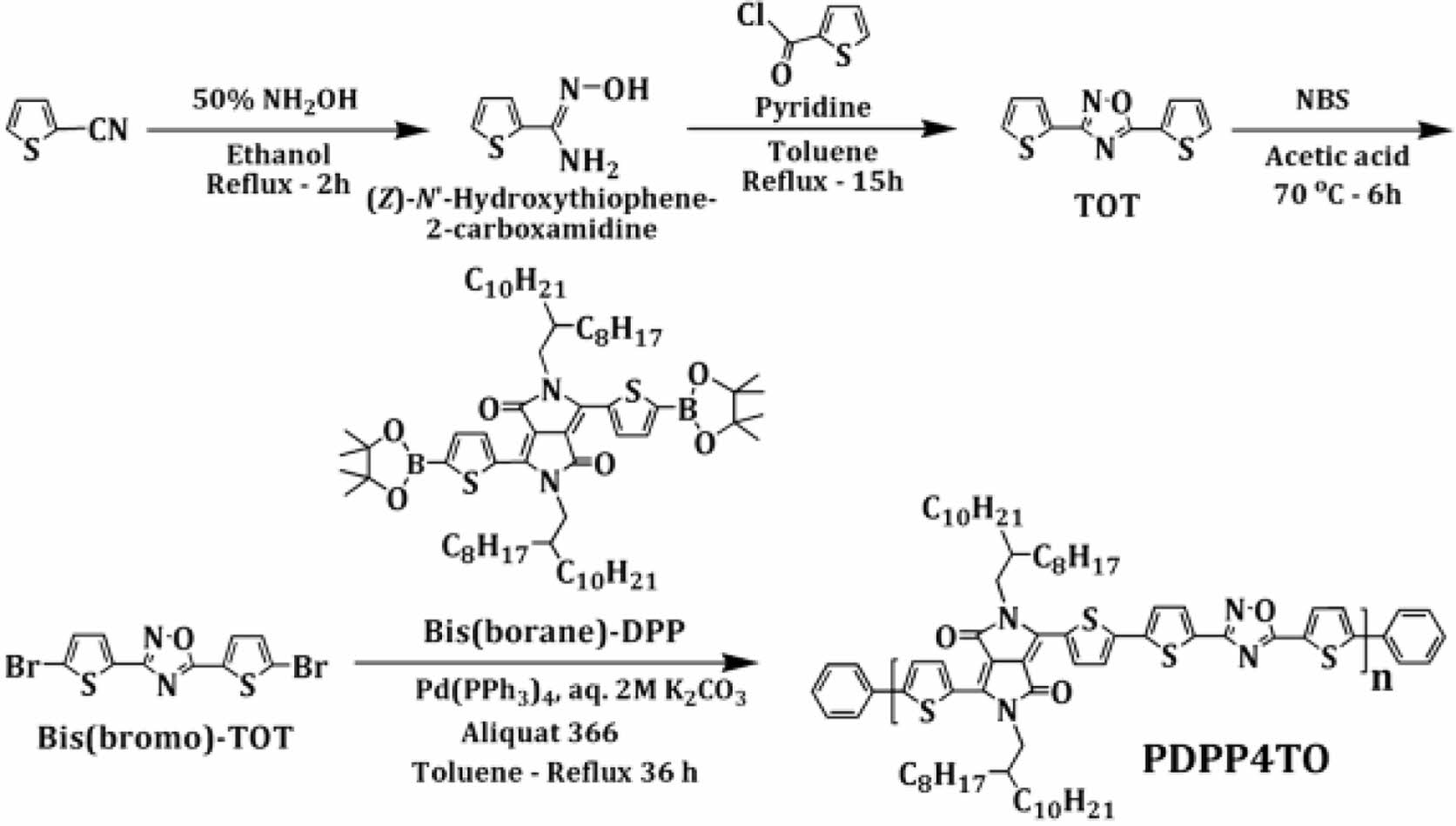
Scheme 1. Synthetic route for PDPP4TO.
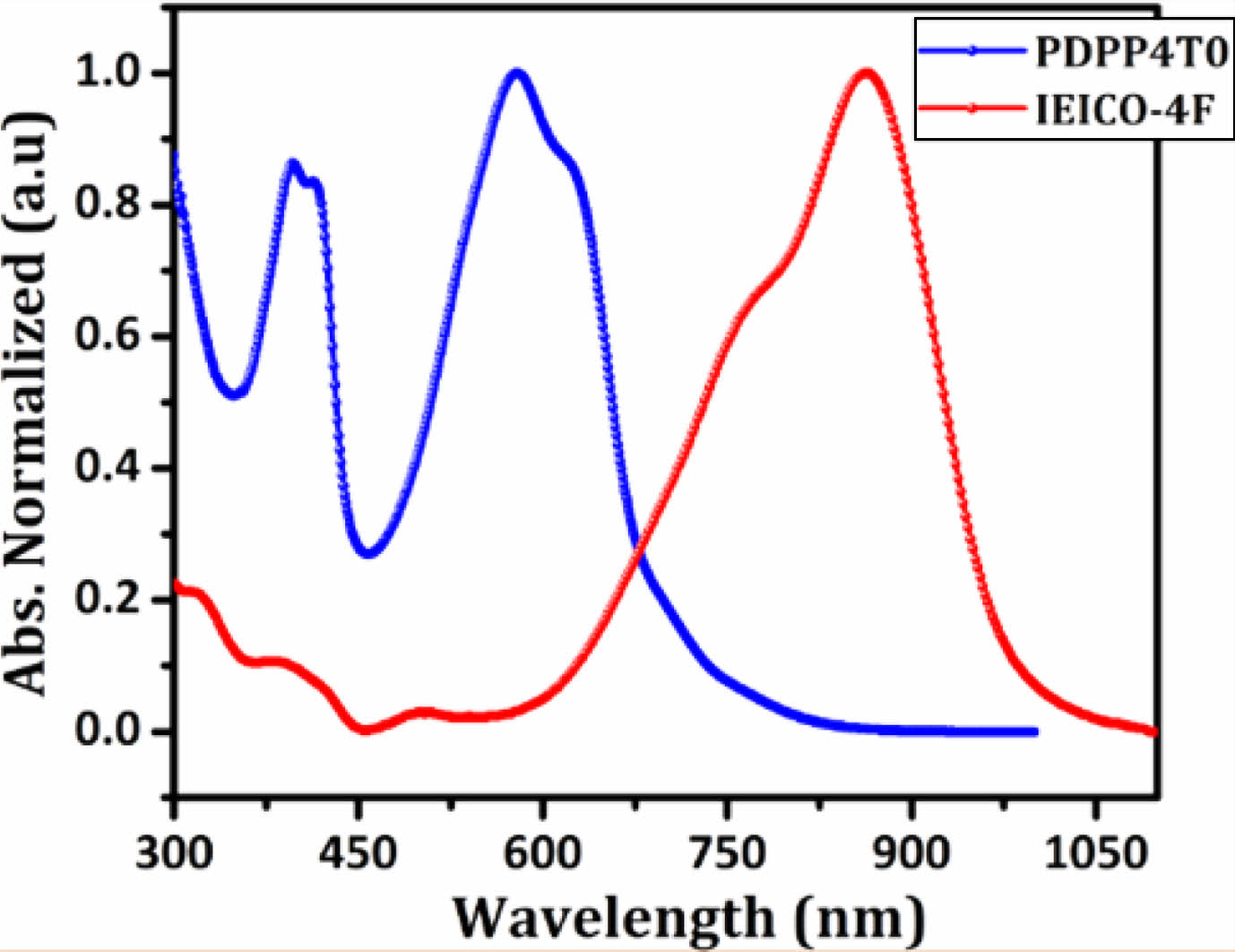
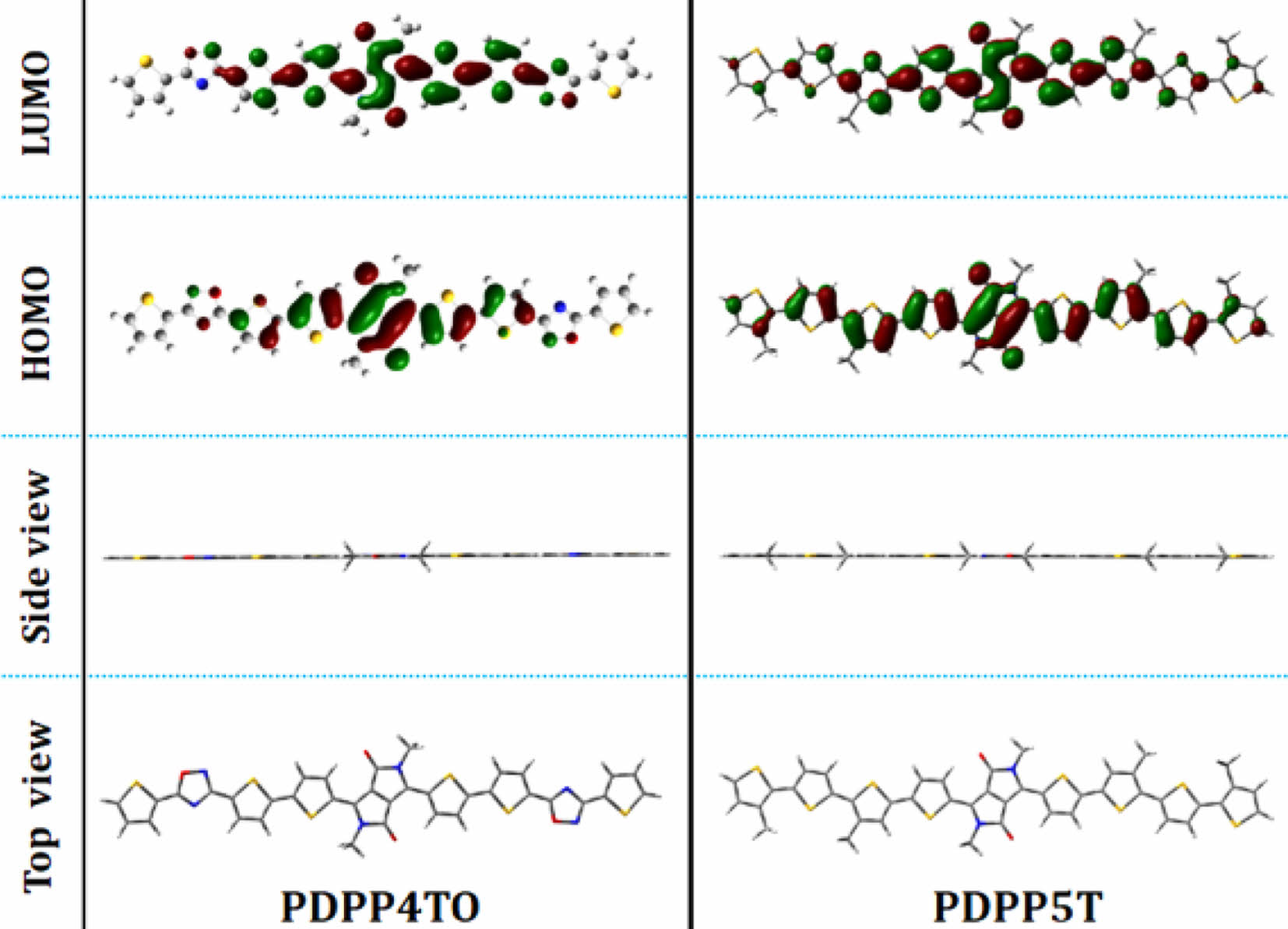
The absorption spectra for electron-donating PDPP4TO and electron-accepting IEICO-4F (film state) are shown in Figure 2, and it display strong absorption from 300 to 700 nm for PDPP4TO and between 600 and 1000 nm for IEICO-4F. These results indicate that PDPP4TO shows excellent complementary absorption from 300 and 1000 nm when it blends with IEICO-4F. Polymer PDPP4TO exhibited two distinct absorption bands with absorption maxima at 397 and 580 nm. Interestingly, each band showed additional shoulder peaks at 415 and 620 nm, respectively, which reveals that PDPP4TO chains undergo effective π-π stacking between them. The determined optical bandgap (Eg) for PDPP4TO was 1.76 eV. We think that the higher planarity of PDPP4TO could be a main reason for their strong π-π stacking ability. The optimized structure, shown in Figure 3, for the repeating units of PDPP4TO supports our arguments. The determined twist angles (q) between the aromatics of PDPP4TO were found to be incredibly low (<0.5º) which confirms that PDPP4TO exhibited a highly planar backbone.
Noticeably, the replacement of one thiophene on the PDPP5T backbone using oxadiazole was found to drastically alter their optical properties. The reports reveal that PDPP5T displayed their absorption bands from 500 to 850 nm with an Egof 1.45 eV31,32 but PDPP4TO exhibited significantly blue shifted absorption bands and higher Eg (300-700 nm, 1.76 eV) compared to those of PDPP5T. Overall, PDPP4TO showed a ⁓0.31 eV hypochromic shift in their optical band gap or onset absorption compared to PDPP5T.31,32 As seen in Figure 3, both the polymers PDPP4TO and PDPP5T displayed highly planar backbones but each polymer showed different absorption bands. The electron distributions on the HOMO and lowest unoccupied molecular orbital (LUMO) levels of PDPP4TO and PDPP5T might explain the reason for the substantial difference in their absorption. The electron density was found to be identical on the DPP units of PDPP4TO and PDPP5T, but notably varied on adjacent aromatics. It was noted that the electron density was well distributed throughout the aromatics on the PDPP5T backbone, but the same was majorly distributed only adjacent thiophenes of the DPP unit of the PDPP4TO backbone. The presence of oxadiazole on the polymer backbone somehow resists the electron distribution compared to the presence of thiophene. Overall, the better electronic distribution on PDPP5T was expected to allow efficient internal charge transfer (ICT) and thereby displayed redshift absorption band and lower band gap compared to those of PDPP4TO.
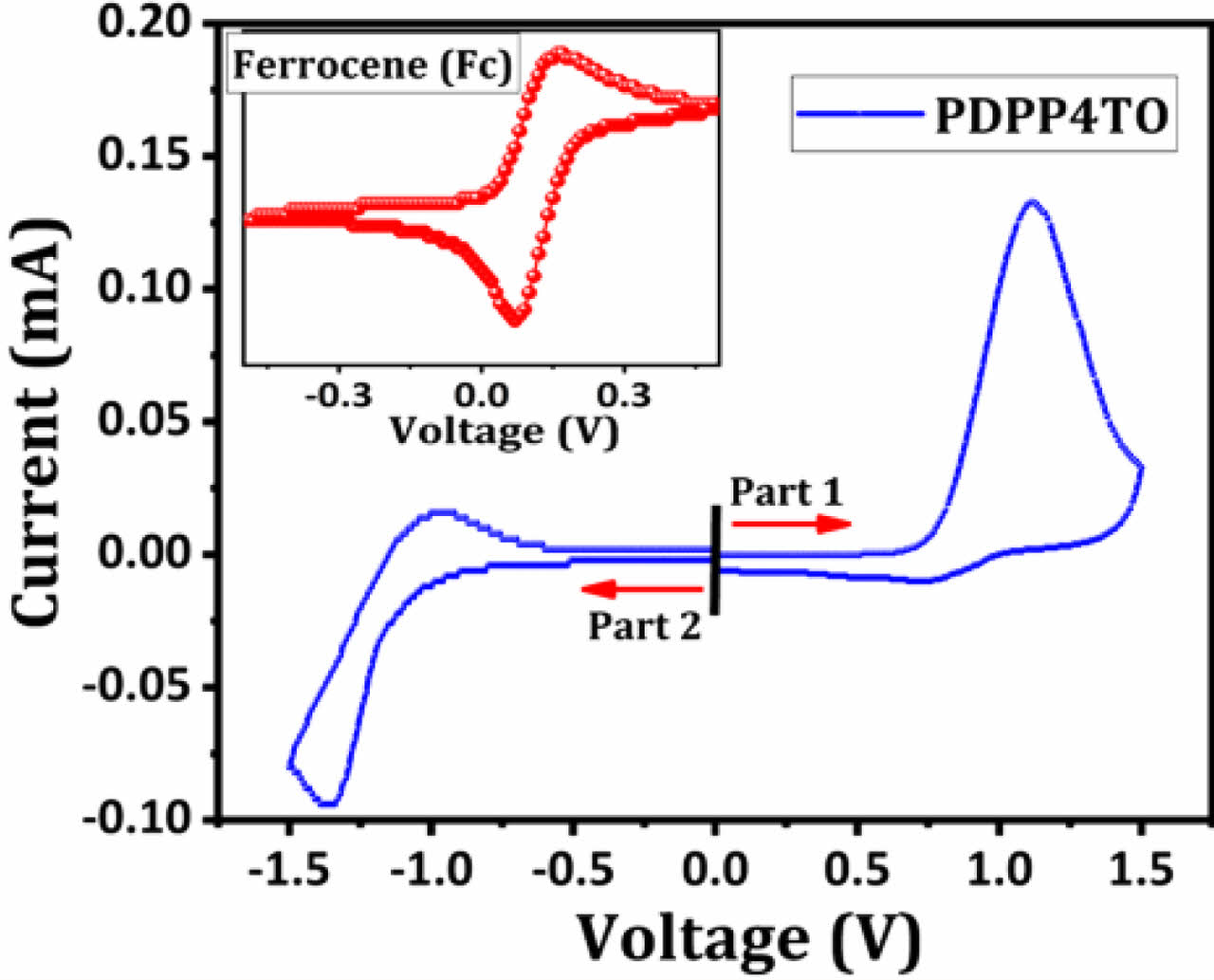
The HOMO and LUMO levels of PDPP4TO were estimated using cyclic voltammetry (CV) analysis. The CV spectrum for PDPP4TO is presented in Figure 4. The calculated onset oxidation and reduction (Eox,onset and Ered,onset) potentials for PDPP4TO were 0.73 V and -1.05 V, respectively, and the Eox,onset for ferrocene (standard) measured under identical conditions was 0.12 V. The energy levels for PDPP4TO were calculated using the standard equations41-43 and it displayed an EHOMO of -5.41 eV and an ELUMO of -3.63 eV. The electrochemical bandgap was 1.78 eV and it is well matched with their optical bandgap value. The HOMO and LUMO levels reported for PDPP5T were -5.25 eV and -3.80 eV.31,32 Noticeably, PDPP4TO exhibited lower HOMO level compared to that of PDPP5T and which helps to get higher Voc.
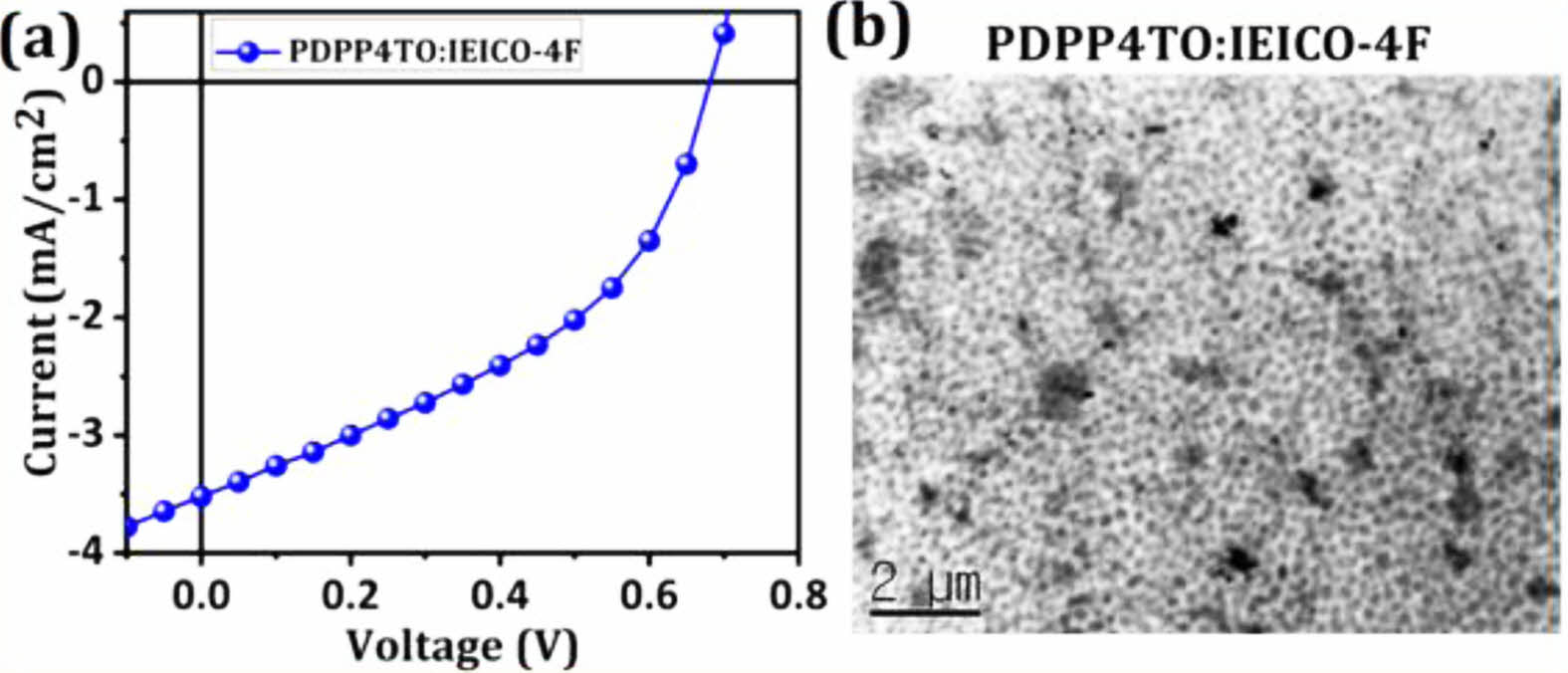
The photovoltaic properties of PDPP4TO were tested by making NFA OSCs with a device structure ITO/PEDOT:PSS/1:1.5 (w/w) PDPP4TO:IEICO-4F+0.5 vol% 1,8-diiodooctane (DIO)/PDINO/Al. The near-infrared absorbing IEICO-4F was used as an electron acceptor since PDPP4TO absorbs the light from 300 to 700 nm, and the energy levels of PDPP4TO and IEICO-4F are found to be good for charge septation. The J-V curve for PDPP4TO-based the NAF OSC device is presented in Figure 5(a), and it provided a maximum PCE of 0.94% with an open-circuit voltage (Voc) of 0.68 V, a short-circuit current (Jsc) of 3.54 mA/cm2, and an fill factor (FF) of 39%. Noticeably, the maximum PCE reported for PDPP5T: IEICO-4F blend was 4.60% (a Voc of 0.55 V, a Jsc of 15.6 mA/cm2, and an FF of 52%).32
Interestingly, the PDPP4TO-based device showed a higher Voc than that of the PDPP5T-based device and it is reliable since the PDPP4TO exhibited a deeper HOMO level than that of the PDPP5T. Whereas the PDPP4TO-based device offered much lower Jsc than that of the PDPP5T-based device, though PDPP4TO:IEICO-4F showed better complementary absorption than that of the PDPP5T:IEICO-4F blend. We think that the charge transport on the photoactive layer of PDPP4TO-based device might be significantly decreased compared to that of the PDPP5T and which limits the photocurrent generation on the photoactive layer of the PDPP4TO-based devices. The lower FF achieved for PDPP4TO-based devices supports our arguments. In addition, the optimized structures (HOMO and LUMO levels) shown in Figure 3 agree on the possibility of lower charge transfer on the PDPP4TO backbone compared to the PDPP5T backbone. It was well documented that the Jsc and FF of OSCs are strongly influenced by the charge transport that occurred on the photoactive layers of OSCs. To find out the main reason for the poor charge transport or PCE for PDPP4TO-based device, we measured the transmission electron microscope (TEM) image for PDPP4TO:IEICO-4F film. Unfortunately, the morphology of the film was found to be not good for efficient charge transport since it showed severe aggregation (see Figure 5(b)) between donor and acceptor units. As we all know, uniform blending is highly required for good charge transport and thereby high PCE. Therefore, the poor PCE of PDPP4TO is expected to originate from their poor morphology. Overall, the insertion of oxadiazoles on the DPP-based polymers notably improved the Voc but notably reduced the Jsc and FF for the resulting polymers.
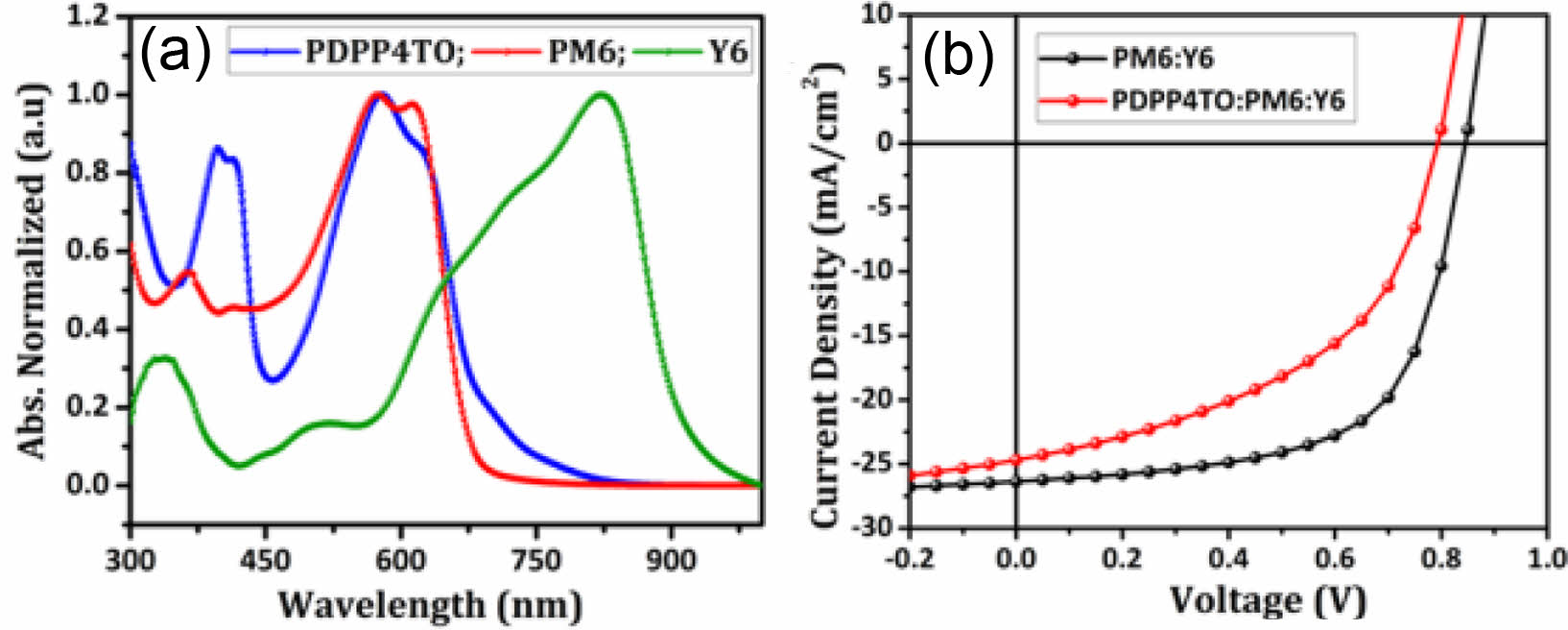
According to literature, the inclusion of a small quantity of appropriate materials to the PM6:Y6 blend further enhances the PCE of resulting ternary blend NFA-OSCs.44-48 The better complementary absorption and well-aligned energy levels of the third component with PM6:Y6 blend are the basic requirements to use any material as a third component. As seen in Figure 6(a), PDPP4TO showed good complementary absorption and well-matched energy levels with PM6:Y6. Therefore, we fabricated ternary NFA-OSCs by using 0.1:0.9:1.2 (w/w/w) PDPP4TO:PM6:Y6 blend and it displayed a PCE of 9.36% (a Voc of 0.80 V, a Jsc of 24.69 mA/cm2, and an FF of 48%). Under identical conditions, the binary device made from 1:1.2 (w/w/w) PM6:Y6 blend gave a PCE of 14.08% (a Voc of 0.85 V, a Jsc of 26.37 mA/cm2, and an FF of 63%). The respective J-V curves for binary and ternary devices are shown in Figure 6b. It is certain that the addition of PDPP4TO to PM6:Y6 blend lowers their performance, and it indicates that PDPP4TO is not working well as a third component, though it displayed good photophysical and electrochemical properties.
|
Figure 2 Absorption spectra for PDPP4TO and IEICO-4F films |
|
Figure 3 Optimized structures for the repeating unit of PDPP4TO and PDPP5T. |
|
Figure 4 CV for PDPP4TO film. |
|
Figure 5 (a) The J-V curve; (b) TEM image for the OSC device and thin film, respectively, made from 1:1.5 (w/w) PDPP4TO: IEICO-4F+0.5 vol% DIO blend. |
|
Figure 6 Absorption spectra of (a) PDPP4TO, PM6, and Y6; (b) the J-V curves for binary and ternary devices made from 1.0:1.2 (w/ w/w) PM6:Y6 and 0.1:0.9:1.2 (w/w/w) PDPP4TO:PM6:Y6 blends. |
A new polymer, namely PDPP4TO, incorporating DPP and tetrathiophene-1,2,4-oxadiazole derivatives was prepared and their properties were compared to the polymer, namely PDPP5T, containing DPP and penta-thiophene derivatives. The polymers PDPP4TO and PDPP5T showed their intense absorption bands at entirely different regions of 300-700 nm and 500-850 nm, respectively. While the HOMO level of PDPP4TO (⁓5.41 eV) was found to be deeper than that of PDPP5T (⁓5.25 eV). Finally, the NFA OSCs made from PDPP4TO:IEICO-4F blend provided a maximum PCE of 0.94% (a Voc of 0.68 V, a Jsc of 3.54 mA/cm2, and a FF of 39%) and which is lower than the value (PCE of 4.60%, a Voc of 0.55 V, a Jsc of 15.6 mA/cm2, and a FF of 52%) reported for PDPP5T:IEICO-4F blend. Interestingly, PDPP4TO gave higher Voc but lower Jsc and FF compared to those of PDPP5T. The decreased charge transporting ability of PDPP4TO is expected to be a key reason for their lower Jsc and FF. This study suggests that the insertion of oxadiazoles on DPP-based polymer backbone drastically changes the photophysical, electrochemical and photovoltaic properties for the resulting polymers. The results shown here is helpful in designing new DPP-based medium bandgap polymers for NFA OSCs.
- 1. Fu, H.; Wang, Z.; Sun, Y. Polymer Donors for High‐Performance Non‐Fullerene Organic Solar Cells. Angew. Chem. 2019, 131, 4488-4499.
-

- 2. Cho, N.; Kim, T.-D.; Jen, A. K.-Y. Reduced Recombination Losses with Enhanced Dielectric Permittivity of Donor Polymers in Polymer Solar Cells. Polym. Korea 2018, 42, 708-713.
-

- 3. Lu, L.; Zheng, T.; Wu, Q.; Schneider, A. M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 126660-12731.
-

- 4. Do, T. T.; Ha, Y. E.; Kim, J. H. Synthesis and Characterization of π-Conjugated Polymer Based on Phthalimide Derivative and its Application for Polymer Solar Cells. Polym. Korea 2013, 37, 694-701.
-

- 5. Gunasekaran, R. K.; Rana, P. J. S.; Park, S. H.; Tamilavan, V.; Karuppanan, S.; Kim, H. J.; Prabakar, K. Open Atmospheric Processed Perovskite Solar Cells Using Dopant-Free, Highly Hydrophobic Hole-Transporting Materials: Influence of Thiophene and Selenophene π-Spacers on Charge Transport and Recombi- nation Properties. Sol. Energy Mater. Sol. Cells 2019, 199, 66-74.
-

- 6. Rana, P. J. S.; Gunasekaran, R. K.; Park, S. H.; Tamilavan, V.; Karuppanan, S.; Kim, H. J.; Prabakar, K. Open Atmosphere-Processed Stable Perovskite Solar Cells Using Molecular Engineered, Dopant-Free, Highly Hydrophobic Polymeric Hole-Transporting Materials: Influence of Thiophene and Alkyl Chain on Power Conversion Efficiency. J. Phys. Chem. C 2019, 123, 8560-8568.
-

- 7. Liu, W.; Ma, Y.; Wang, Z.; Mu, Z.; Gao, W.; Fan, W.; Li, W. S.; Zhang, Q. Improving the Hole Transport Performance of Perovskite Solar Cells through Adjusting the Mobility of the As-Synthesized Conjugated Polymer. J. Mater. Chem. C 2021, 9, 3421-3428.
-

- 8. Paterson, A. F.; Singh, S.; Fallon, K. J.; Hodsden, T.; Han, Y.; Schroeder, B. C.; Bronstein, H.; Heeney, M.; McCulloch, I.; Anthopoulos, T. D. Recent Progress in High-Mobility Organic Transistors: A Reality Check. Adv. Mater. 2018, 30, 1801079.
-

- 9. Cheon, H. J.; An, T. K.; Kim, Y. H. Diketopyrrolopyrrole (DPP)-Based Polymers and Their Organic Field-Effect Transistor Applications: A Review. Macromol. Res. 2022, 30, 71-84.
-

- 10. Low, J. Y.; Aljunid Merican, Z. M.; Hamza, M. F. Polymer Light Emitting Diodes (PLEDs): An Update Review on Current Innovation and Performance of Material Properties. Mater. Today Proc. 2019, 16, 1909-1918.
-

- 11. Van der Zee, B.; Li, Y.; Wetzelaer, G. J. A. H.; Blom, P. W. M. Efficiency of Polymer Light-Emitting Diodes: A Perspective. Adv. Mater. 2022, 34, 2108887.
-

- 12. Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and Morphology Control Enables Multiple Cases of High-Efficiency Polymer Solar Cells. Nat. Commun. 2014, 5, 5293.
-

- 13. Jin, Y.; Chen, Z.; Xiao, M.; Peng, J.; Fan, B.; Ying, L.; Zhang, G.; Jiang, X. F.; Yin, Q.; Liang, Z.; Huang, F.; Cao, Y. Thick Film Polymer Solar Cells Based on Naphtho[1,2-c:5,6-c]Bis[1,2,5]- Thiadiazole Conjugated Polymers with Efficiency over 11%. Adv. Energy Mater. 2017, 7, 1700944.
-

- 14. Lee, J.; Tamilavan, V.; Rho, K. H.; Keum, S.; Park, K. H.; Han, D.; Jung, Y. K.; Yang, C.; Jin, Y.; Jang, J. W.; Jeong, J. H.; Park, S. H. Overcoming Fill Factor Reduction in Ternary Polymer Solar Cells by Matching the Highest Occupied Molecular Orbital Energy Levels of Donor Polymers. Adv. Energy Mater. 2018, 8, 1702251.
-

- 15. Tamilavan, V.; Liu, Y.; Lee, J.; Jung, Y. K.; Son, S.; Jeong, J.; Park, S. H. Highly Crystalline New Benzodithiophene-Benzothiadiazole Copolymer for Efficient Ternary Polymer Solar Cells with an Energy Conversion Efficiency of over 10%. J. Mater. Chem. C 2018, 6, 4281-4289.
-

- 16. Aqoma, H.; Park, S.; Park, H. Y.; Hadmojo, W. T.; Oh, S. H.; Nho, S.; Kim, D. H.; Seo, J.; Park, S.; Ryu, D. Y.; Cho, S.; Jang, S. Y. 11% Organic Photovoltaic Devices Based on PTB7-Th: PC71BM Photoactive Layers and Irradiation-Assisted ZnO Electron Transport Layers. Adv. Sci. 2018, 5, 1700858.
-

- 17. Hong, S.; Song, C. E.; Lim, E. Polymer Solar Cells based on a Furan-containing Asymmetric Nonfullerene Acceptor. Polym. Korea 2020, 44, 741-746.
-

- 18. Qin, J.; Zhang, L.; Zuo, C.; Xiao, Z.; Yuan, Y.; Yang, S.; Hao, F.; Cheng, M.; Sun, K.; Bao, Q.; Bin, Z.; Jin, Z.; Ding, L. A Chlorinated Copolymer Donor Demonstrates a 18.13% Power Conversion Efficiency. J. Semicond. 2021, 42, 010501.
-

- 19. Jin, K.; Xiao, Z.; Ding, L. 18.69% PCE from Organic Solar Cells. J. Semicond. 2021, 42, 060502.
-

- 20. Zhan, L.; Li, S.; Xia, X.; Li, Y.; Lu, X.; Zuo, L.; Shi, M.; Chen, H.; Liu, F.; Zhou, L.; Liu, W. W.; Zhou, Z.; Yue, Q.; Zheng, W.; Sun, R.; Liu, W. W.; Xu, S.; Fan, H.; Feng, L.; Yi, Y.; Zhang, W.; Zhu, X. Organic Solar Cells with 18% Efficiency Enabled by an Alloy Acceptor: A Two-in-One Strategy. Adv. Mater. 2021, 33, 2100830.
-

- 21. Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171-184.
-

- 22. Wang, J.; Zhang, M.; Lin, J.; Zheng, Z.; Zhu, L.; Bi, P.; Liang, H.; Guo, X.; Wu, J.; Wang, Y.; Yu, L.; Li, J.; Lv, J.; Liu, X.; Liu, F.; Hou, J.; Li, Y. An Asymmetric Wide-Bandgap Acceptor Simultaneously Enabling Highly Efficient Single-Junction and Tandem Organic Solar Cells. Energy Environ. Sci. 2022, 15, 1585-1593.
-

- 23. Wadsworth, A.; Moser, M.; Marks, A.; Little, M. S.; Gasparini, N.; Brabec, C. J.; Baran, D.; McCulloch, I. Critical Review of the Molecular Design Progress in Non-Fullerene Electron Acceptors towards Commercially Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596-1625.
-

- 24. Dou, L.; Gao, J.; Richard, E.; You, J.; Chen, C. C.; Cha, K. C.; He, Y.; Li, G.; Yang, Y. Systematic Investigation of Benzo- dithiophene- and Diketopyrrolopyrrole- Based Low-Bandgap Polymers Designed for Single Junction and Tandem Polymer Solar Cells. J. Am. Chem. Soc. 2012, 134, 10071-10079.
-

- 25. Dou, L.; You, J.; Yang, J.; Chen, C.-C.; He, Y.; Murase, S.; Moriarty, T.; Emery, K.; Li, G.; Yang, Y. Tandem Polymer Solar Cells Featuring a Spectrally Matched Low-Bandgap Polymer. Nat. Photonics 2012, 6, 180-185.
-

- 26. Dou, L.; Chang, W. H.; Gao, J.; Chen, C. C.; You, J.; Yang, Y. A Selenium-Substituted Low-Bandgap Polymer with Versatile Photovoltaic Applications. Adv. Mater. 2013, 25, 825-831.
-

- 27. Zhao, C.; Guo, Y.; Zhang, Y.; Yan, N.; You, S.; Li, W. Diketo- pyrrolopyrrole-Based Conjugated Materials for Non-Fullerene Organic Solar Cells. J. Mater. Chem. A 2019, 7, 10174-10199.
-

- 28. Bao, W. W.; Li, R.; Dai, Z. C.; Tang, J.; Shi, X.; Geng, J. T.; Deng, Z. F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 679.
-

- 29. Lim, E.; Influence of CN Substitution on DPP-furan-based Small-molecule Acceptors for Polymer Solar Cells. Polym. Korea 2020, 44, 408-414.
-

- 30. Rasool, S.; Hoang, Q. V.; Vu, D. Van; Song, C. E.; Lee, H. K.; Lee, S. K.; Lee, J. C.; Moon, S. J.; Shin, W. S. High-Efficiency Single and Tandem Fullerene Solar Cells with Asymmetric Monofluorinated Diketopyrrolopyrrole-Based Polymer. J. Energy Chem. 2021, 64, 236-245.
-

- 31. Jiang, X.; Xu, Y.; Wang, X.; Wu, Y.; Feng, G.; Li, C.; Ma, W.; Li, W. Non-Fullerene Organic Solar Cells Based on Diketopyrrolo- pyrrole Polymers as Electron Donors and ITIC as an Electron Acceptor. Phys. Chem. Chem. Phys. 2017, 19, 8069-8075.
-

- 32. van der Pol, T. P. A.; Li, J.; van Gorkom, B. T.; Colberts, F. J. M.; Wienk, M. M.; Janssen, R. A. J. Analysis of the Performance of Narrow-Bandgap Organic Solar Cells Based on a Diketopyrro- lopyrrole Polymer and a Nonfullerene Acceptor. J. Phys. Chem. C 2021, 125, 5505-5517.
-

- 33. Kranthiraja, K.; Murotani, K.; Hamada, F.; Saeki, A. Diketo- pyrrolopyrrole-Based Chlorinated Bithiophene Polymers for Organic Solar Cells: Effect of Thiophene or Pyridine Flank. ACS Appl. Electron. Mater. 2022, 4, 2086-2094.
-

- 34. Hamciuc, E.; Hamciuc, C.; Cazacu, M. Poly(1,3,4-Oxadiazole-Ether-Imide)s and Their Polydimethylsiloxane-Containing Copolymers. Eur. Polym. J. 2007, 43, 4739-4749.
-

- 35. Vellis, P. D.; Mikroyannidis, J. A.; Cho, M. J.; Choi, D. H. Carbazolevinylene-Based Polymers and Model Compounds with Oxadiazole and Triphenylamine Segments: Synthesis, Photo- physics, and Electroluminescence. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5592-5603.
-

- 36. Nie, Y.; Zhao, B.; Tang, P.; Jiang, P.; Tian, Z.; Shen, P.; Tan, S. Synthesis and Photovoltaic Properties of Copolymers Based on Benzo[1,2-b:4,5-B′]Dithiophene and Thiophene with Electron-Withdrawing Side Chains. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3604-3614.
-

- 37. Agneeswari, R.; Tamilavan, V.; Song, M.; Kang, J. W.; Jin, S. H.; Hyun, M. H. Synthesis of Polymers Containing 1,2,4-Oxadiazole as an Electron-Acceptor Moiety in Their Main Chain and Their Solar Cell Applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2131-2141.
-

- 38. Agneeswari, R.; Tamilavan, V.; Hyun, M. H. Synthesis and Characterization of 1,2,4-Oxadiazole-Based Deep-Blue and Blue Color Emitting Polymers. Bull. Korean Chem. Soc. 2014, 35, 513-517.
-

- 39. Agneeswari, R.; Tamilavan, V.; Song, M.; Hyun, M. H. Property Modulation of Benzodithiophene-Based Polymers via the Incorporation of a Covalently Bonded Novel 2,1,3-Benzo- thiadiazole-1,2,4-Oxadiazole Derivative in Their Main Chain for Polymer Solar Cells. J. Mater. Chem. C 2014, 2, 8515-8524.
-

- 40. Tamilavan, V.; Kim, D.; Yang, H.-S.; Shin, I.; Kim, J.; Lee, B. R.; Park, S. H. Enhanced Photovoltaic Performance of Benzothiadi- azole-Based Polymers by Controlling their Backbone Planarity for Organic Solar Cells. Macromol. Chem. Phys. 2022, 223, 2200222.
-

- 41. Tamilavan, V.; Song, M.; Jin, S.-H.; Hyun, M. H. Synthesis of Conjugated Polymers with Broad Absorption Bands and Photo- voltaic Properties as Bulk Heterojuction Solar Cells. Polymer 2011, 52, 2384-2390.
-

- 42. Tamilavan, V.; Song, M.; Ban, T.-W.; Jin, S.-H.; Hyun, M. H. Synthesis and Photovoltaic Properties of 1-(2,6-Diisopropyl- phenyl)-2,5-di(2-thienyl)pyrrole-based Low-bandgap Polymers. Polym. Bull. 2012, 69, 439-454.
-

- 43. Tamilavan, V.; Song, M.; Kim, S.; Agneeswari, R.; Kang, J.-W.; Hyun, M. H. Synthesis of N-[4-Octylphenyl]dithieno[3,2-b:2′,3′-d]pyrrole-based Broad Absorbing Polymers and Their Photovoltaic Applications. Polymer 2013, 54, 3198-3205.
-

- 44. Yan, T.; Ge, J.; Lei, T.; Zhang, W.; Song, W.; Fanady, B.; Zhang, D.; Chen, S.; Peng, R.; Ge, Z. 16.55% Efficiency Ternary Organic Solar Cells Enabled by Incorporating a Small Molecular Donor. J. Mater. Chem. A 2019, 7, 25894-25899.
-

- 45. Du, X.; Yuan, Y.; Zhou, L.; Lin, H.; Zheng, C.; Luo, J.; Chen, Z.; Tao, S.; Liao, L. S. Delayed Fluorescence Emitter Enables Near 17% Efficiency Ternary Organic Solar Cells with Enhanced Storage Stability and Reduced Recombination Energy Loss. Adv. Funct. Mater. 2020, 30, 1909837.
-

- 46. Li, D.; Zhu, L.; Liu, X.; Xiao, W.; Yang, J.; Ma, R.; Ding, L.; Liu, F.; Duan, C.; Fahlman, M.; Bao, Q. Enhanced and Balanced Charge Transport Boosting Ternary Solar Cells Over 17% Efficiency. Adv. Mater. 2020, 32, 2002344.
-

- 47. Wang, D.; Qin, R.; Zhou, G.; Li, X.; Xia, R.; Li, Y.; Zhan, L.; Zhu, H.; Lu, X.; Yip, H. L.; Chen, H.; Li, C. Z. High-Performance Semitransparent Organic Solar Cells with Excellent Infrared Reflection and See-Through Functions. Adv. Mater. 2020, 32, 2001621.
-

- 48. Chang, L.; Sheng, M.; Duan, L.; Uddin, A. Ternary Organic Solar Cells Based on Non-Fullerene Acceptors: A Review. Org. Electron. 2021, 90, 106063..
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(1): 79-86
Published online Jan 25, 2023
- 10.7317/pk.2023.47.1.79
- Received on Oct 20, 2022
- Revised on Nov 23, 2022
- Accepted on Nov 28, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Youngeup Jin
-
Department of Industrial Chemistry, Pukyong National University, Busan 48513, Korea
- E-mail: yjin@pknu.ac.kr
- ORCID:
0000-0001-9878-0882








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.