- Catechol/aldehyde Moiety-based Bioadhesive Thermosensitive Poly(organophosphazenes) Hydrogel System
Sung Hoon Kim*, **,#, Gu Won Jeong*, **,#, and Young-Min Kim*, **,†

*Center for Biomaterials, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea
**Division of Biomedical Science and Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Korea- 카테콜/알데히드기 기반 생체접착용 온도감응성 폴리포스파젠 하이드로젤 시스템
*한국과학기술연구원 생체재료연구센터, **과학기술연합대학원 대학교 의공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Injectable tissue adhesives using catechol have been raised as problems such as reduced effectiveness, dispersion into surrounding tissues and toxicity due to catechol groups. To overcome these problems, we designed that a thermo-sensitive and adhesive hydrogel with special characteristics, 1) rapid gelation to reduce the dispersion of the adhesive within body, 2) temperature-dependent hydrogel network formation to improve the adhesive effect by increased the number of cohesive networks and tissue-adhesive catechol moieties due to replacement of cohesive networks between catechol moieties, and 3) reduction the toxicity by catechol group. Here, the adhesive hydrogel system was developed by mixing the synthesized polymers. The structure of the polymer complex was analyzed using 1H nuclear magnetic resonance spectroscopy and Fourier transform infrared spectroscopy. In addition, the sol-gel transition and biocompatibility of the polymer were confirmed. Finally, it was confirmed that the potential of tissue adhesive through the adhesion test on various materials.
카테콜을 이용한 주입형 조직접착제는 주변조직으로의 분산으로 인한 접착효과 저하와 카테콜기로 인한 독성으로 문제가 제기되어 왔다. 이러한 문제점을 극복하기 위해 온도 감응성 네트워크 형성으로 인한 접착 하이드로젤의 빠른 젤화로 1) 온몸에 분산되는 것을 줄이고, 2) 카테콜 간 코헤시브 결합으로 인해 생기는 네트워크를 대체함으로 코헤시브 네트워크와 조직 접착 카테콜 형태의 수를 증가시켜 접착능력을 향상시키고, 3) 접착을 위해 상대적으로 필요한 카테콜 기의 수를 줄이며 생체 적합한 고분자를 이용함으로 생체 적합하면서도 접착능력이 있는 온도 감응성 접착 하이드로젤 시스템을 개발하고자 하였다. 합성된 고분자는 1H nuclear magnetic resonance spectroscopy와 Fourier transform infrared spectroscopy를 통해 구조를 분석하였다. 또한, 특정 온도에서 온도 감응성 하이드로젤의 솔-젤 전이 현상과 폴리머의 독성 및 생체 적합성을 확인하였다. 최종적으로 여러 재질에 접착테스트를 통해 접착 가능성 및 접착 강도의 확인을 통해 조직 접착제에 대한 가능성을 확인하였다.
Adhesive hydrogels were developed by simple mixing with thermo-sensitive poly(organophosphazene) (PPZ) and catechol-induced oxidized alginate. This biocompatible and adhesive hydrogel exhibits the thermal sol-gel transition and exhibits adhesive effect by the increased cohesive and adhesive ability of catechol.
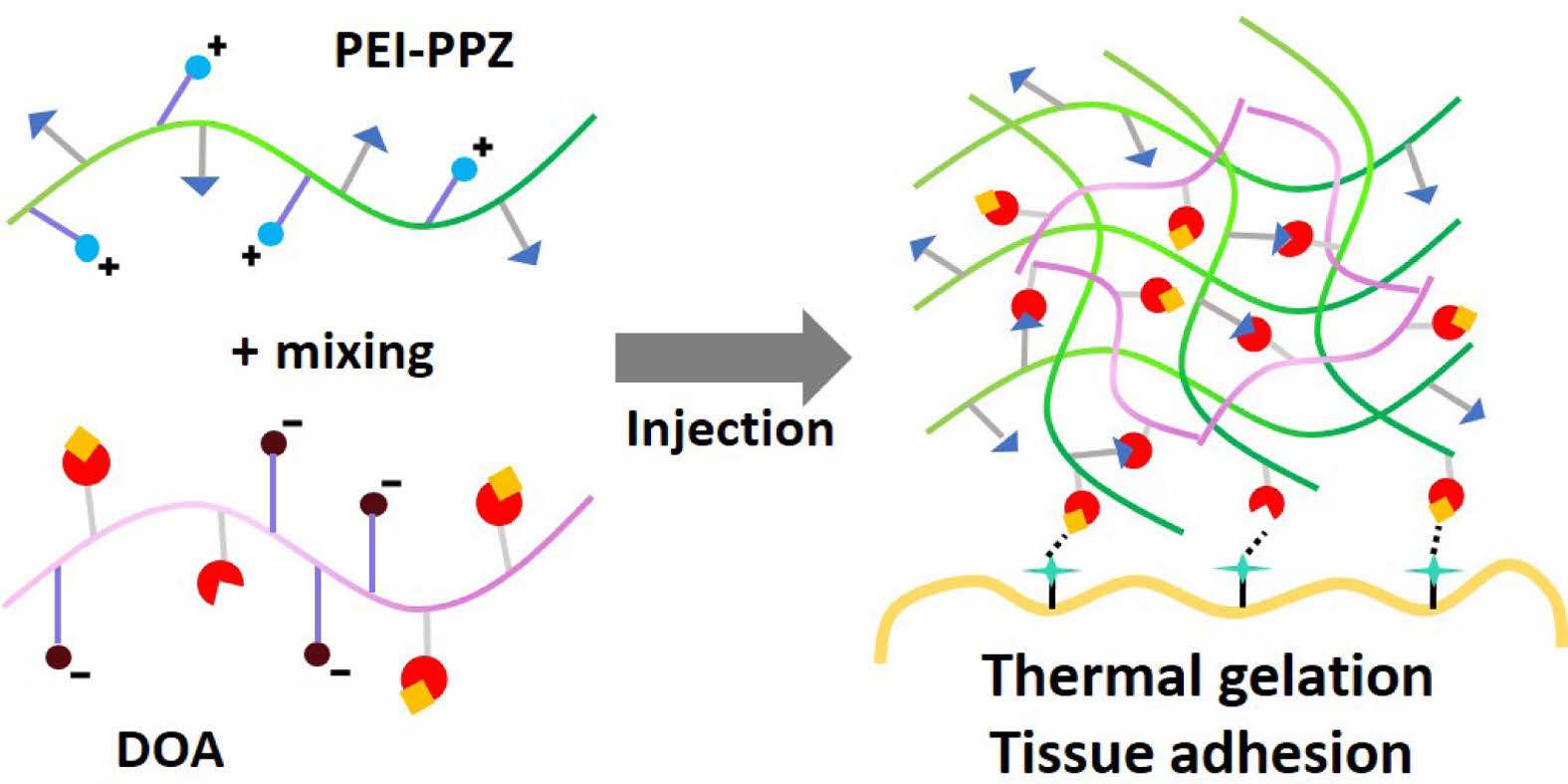
Keywords: poly(organophosphazene), thermosensitive hydrogels, tissue adhesive, catechol.
This research was supported by the Korea Institute of Science and Technology (2E31603) and the National Research Foundation of Korea (2021R1C1C1009620).
The authors declare that there is no conflict of interest.
Excessive bleeding after tissue damage causes mortality and bacterial invasion.1 Suture surgery has been widely used as a traditional method to stop bleeding by closing the wound site. However, it has some limitations such as 1) post-traumatic stress disorder after suture surgery, 2) bacterial infection, and 3) increased pain due to additional tissue damage by the needle.2,3
Hemostatic sealants have been developed to treat tissue damage with less blood loss and safer application. Cyanoacrylate glue and fibrin, the representative glues have been used as sealants, but they caused high toxicity and immune response.4,5 Besides, the adhesive effect of the sealants was significantly decreased in wet conditions.6 On the other hand, catechol, a major component for mussel adhesive proteins (MAPs) in various marine organisms, can adhere to diverse substrates in a wet environment.7,8 Oxidized catechol groups can form ortho-quinone groups and induce adhesive ability via interaction with -NH2, -COOH, -OH groups of tissues in alkaline conditions.9,10 Catechol based-natural adhesive polymers have been studied because of adhesive ability in wet conditions and less immune responses.11
However, the substitution of high concentrations of catechol groups to increase adhesive strength has been limited because the substituted ratio of catechol and toxicity are proportional.12 Besides, the polymer solution was spread out through the body because the time to form a three-dimensional structure via cohesive interaction cost a few minutes and resulted in less adhesive ability and higher side effects.6 It was assumed that a three-dimensional network formation via hydrophobic interaction for catechol-based adhesives would be advantageous for good adhesive ability with fewer catechol groups by replacing catechol-based cohesive ability. In addition, it can reduce immune response due to fewer numbers of catechol groups.13,14
Herein, we constructed adhesive hydrogel with biocompatible and thermo-sensitive properties using thermo-sensitive poly(organophosphazene) (PPZ) and catechol-induced oxidized alginate. The PPZ with catechol moiety exhibits the sol-gel transition which occurs rapidly as the temperature increases, forming a three-dimensional network structure. Thus, it can selectively interact with target tissue without dispersion of catechol groups and induce increased cohesive and adhesive ability (Scheme 1). In brief, we synthesized a biocompatible PPZ showing sol-gel transition at a certain temperature via hydrophobic interaction. The low molecular weight of polyethyleneimine (PEI) was conjugated with PPZ via an amide coupling reaction to induce catioinc propery for ionic interaction (PEI-PPZ). The catechol group was applied to sodium alginate by oxidation via a Schiff base reaction. As the PEI-PPZ and dopamine-conjugated oxidized-alginate (DOA) showed attractive ionic interaction, complexation would be assessed easily via ionic interaction by simple mixing (PPDOA = PEI-PPZ + DOA). Therefore, these systems can generate adhesive ability and biocompatible properties for biomedical applications including adhesive tissue regenerative systems.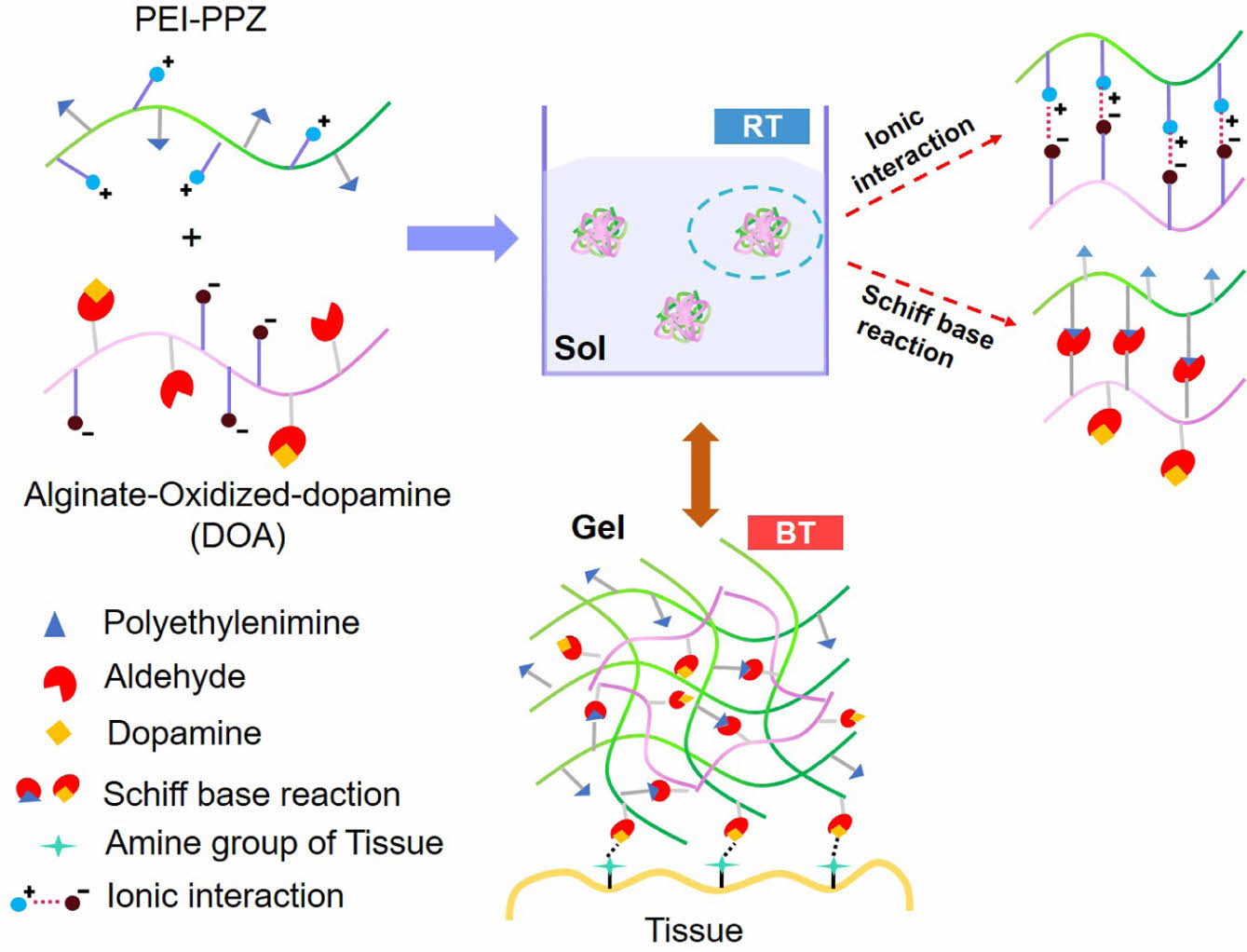
Scheme 1. Schematic representation of a biocompatible, thermosensitive adhesive hydrogel using the complexation with PEI-PPZ
and DOA via ionic interaction and Schiff base reaction (PPDOA).
Materials. Hexachlorocyclotriphosphazene (Sigma Aldrich, Saint Louis, MO, USA) was purified by sublimation at 55 ℃ under vacuum (about 0.1 mmHg). Poly(dichlorophosphazene) was prepared as described previously.15,16 It was prepared from hexachlorocyclotriphosphazene using aluminum chloride (AlCl3) as a catalyst at 250 ℃ for 5 h. L-Isoleucine ethyl ester hydrochloride (IleOEt·HCl) was purchased from A&Z food additives (Hangzhou, China). α-Amino-ω-methoxy-poly(ethylene glycol) with a molecular weight of 750 Da (AMPEG750) was synthesized according to previously reported.17 Polyethyleneimine with molecular weights of 423, 2-aminoethanol (AEtOH), sodium alginate, sodium periodate (NaIO4), dopamine hydrochloride, isobutyl chloroformate, ethylene glycol, hydroxylamine hydrochloride, sodium hydroxide and glutaric anhydride were purchased from Sigma-Aldrich. MES buffer (pH: 5.5) was obtained from Biosesang. Tetrahydrofuran (THF) and triethylamine (TEA) were distilled by reflux under dry nitrogen over sodium metal/benzophenone and barium oxide, respectively. All other reagents were obtained from commercial suppliers and used as received.
Synthesis of PEI-poly(organophosphazene) Conjugates.
OH-PPZ: [NP(IleOEt)1.47 (AEtOH)0.25 (AMPEG)0.28]n: Distilled THF and TEA were added to the dried IleOEt·HCl (23.47 g, 119.9 mmol) flask. Poly(dichlorophosphazene) (10 g, 86.2 mmol) dissolved in distilled THF was added to the flask. The reaction mixture was stirred at -70 ℃ for 24 h and then reacted at room temperature for 24 h. AEtOH (1.29 g, 21.5 mmol) was added dropwise to mixture solutions and then AMPEG750 (23.29 g, 31.06 mmol) dissolved in dry THFs was added through stirring at 45 ℃ for 24 h. Thereafter, AMPEG750 (23.29 g, 31.06 mmol) dissolved in distilled THF was added to the solutions and mixed at 45 ℃ for 24 h. Then, concentrated reaction mixture was poured into n-hexane to obtain a precipitate, which was re-precipitated twice in the same solvent system. The polymer product was further purified by dialysis with a dialysis membrane (Spectra/Por, MWCO: 10-12 kDa) against methanol for 4 days at room temperature and against distilled water for 4 days at 4 ℃. The dialyzed polymer solution was lyophilized (Scheme 2). Yield: 80%. 1H NMR (CDCl3), δ (ppm): 0.8-1.0 (s, 6H), 1.1-1.3 (b, 3H), 1.3-1.6 (b, 2H), 1.6-1.9 (b, 1H), 2.8-3.3 (b, 2H), 3.4-3.8 (b, 73H), 3.9 (s, 1H), 4.0-4.3 (b, 3H).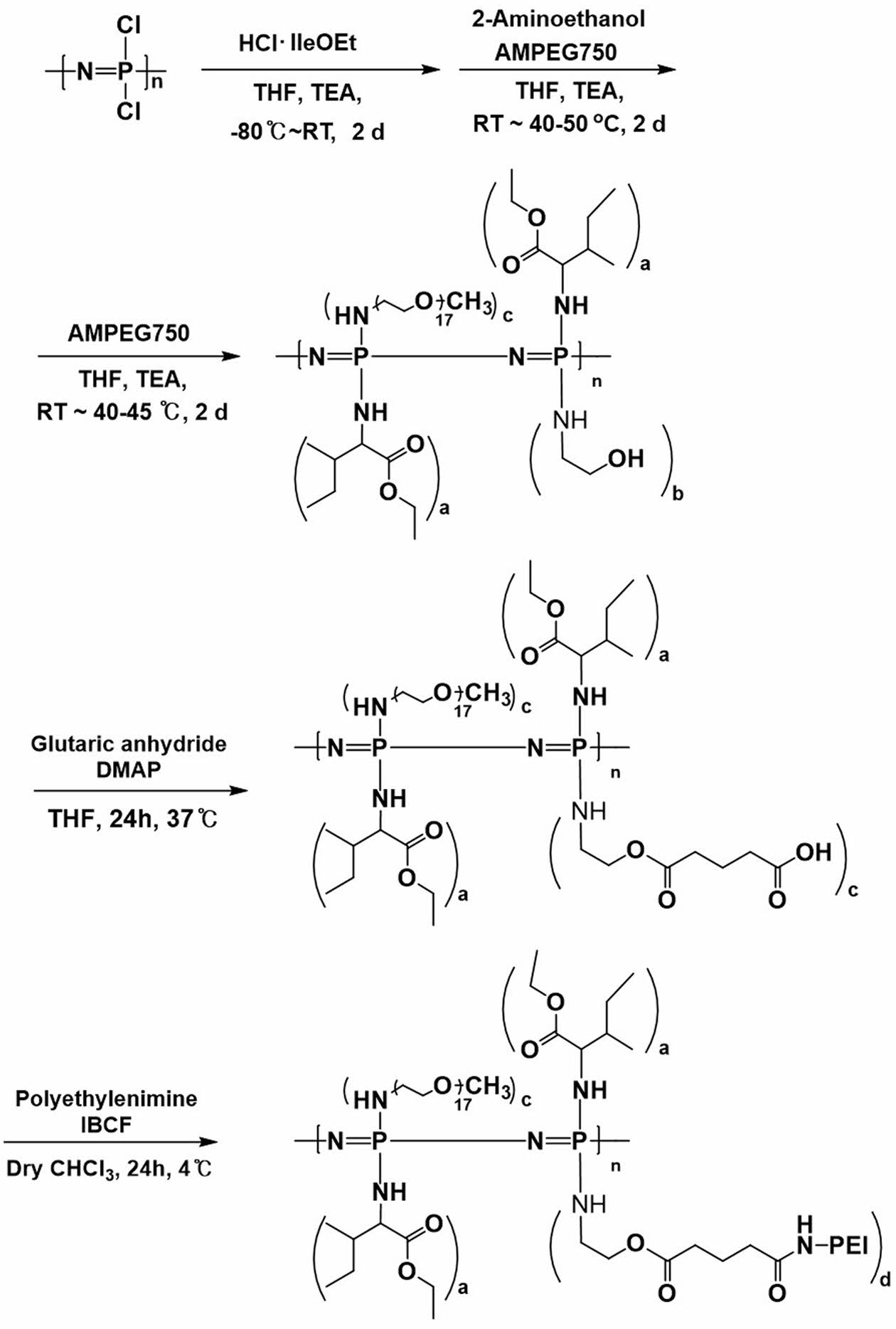
Scheme 2. Synthesis scheme of the PEI-PPZ.
GA-PPZ: [NP(IleOEt)1.47 (Glutaric acid)0.1 (AEtOH)0.25 (AMPEG)0.28]n: OH-PPZ (10.05 g, 6.68 mmol) dissolved in distilled THF. Glutaric anhydride (3.04 g, 26.7 mmol) and 4-(dimethylamino)pyridine (DMAP) (3.26 g, 26.7 mmol) dissolved in THF separately and then were added individually into reaction mixture solution through stirring at 45 ℃ for 72 h. The products were purified by using dialysis membrane (Spectra/Por, MWCO: 10-12 kDa) with methanol for 3 days and deionized water for 3 days. After dialysis, the products were lyophilized. Yield: 90%. 1H NMR (CDCl3), δ (ppm): 0.8-1.0 (s, 6H), 1.1-1.3 (b, 3H), 1.3-1.6 (b, 2H), 1.6-1.9 (b, 1H), 2.5-2.8 (b, 5H), 2.8-3.3 (b, 2H), 3.4-3.8 (b, 73H), 3.9 (s, 1H), 4.0-4.3 (b, 3H).
PEI-PPZ: [NP(IleOEt)1.47 (Glutaric acid)0.07 (PEI423)0.03 (AEtOH)0.25 (AMPEG)0.28]n: The carboxylic acid-ended polymer (GA-PPZ) (5 g, 2.43 mmol) was dissolved in chloroform. Thereafter, in order to activate carboxyl groups of polymer, isobutyl chloroformate (0.332 g, 2.43 mmol) and TEA (0.67 mL, 4.87 mmol) were added separately at 0 ℃ through stirring for 30 min. PEI423 (7.21 g, 17.0 mmol) dissolved in chloroform was added into the reaction mixture through stirring at 0 ℃ for 24 h and then incubated at 45 ℃ for 48 h. After filtration and evaporation, the products were precipitated by using a centrifuge with 1 M KF solution. Thereafter, the products were dialyzed with a dialysis membrane (Spectra/Por, MWCO: 10-12 kDa) with deionized water at 4 ℃ for 3 days and freeze-dried. Yield: 80%. 1H NMR (CDCl3), δ (ppm): 0.8-1.0 (s, 6H), 1.1-1.3 (b, 3H), 1.3-1.6 (b, 2H), 1.6-1.9 (b, 1H), 2.5-2.8 (b, 5H), 2.6-2.9 (b, 49H), 2.8-3.3 (b, 2H), 3.4-3.8 (b, 73H), 3.9 (s, 1H), 4.0-4.3 (b, 3H).
Synthesis of Dopamine Conjugated Oxidized-alginate (DOA). Alginate (10 g, 46.25 mmol) was dissolved in MES buffer (pH: 5.5) through vigorous stirring for 1 h in the dark flask. Thereafter, NaIO4 (11.13 g, 52.8 mmol) was added into alginate solutions and then stirred for 24 h. After the finished reaction, in order to remove unreacted NaIO4, ethylene glycol (3.28 g, 52.8 mmol) was added into mixture solutions for 1 h. The products were dialyzed with dialysis membrane (Spectra/Por, MWCO: 10-12 kDa) with deionized water for 4 days at room temperature and lyophilized. Yield: 30%.
Oxidized-alginate (1 g, 4.62 mmol) was dissolved in deionized water (pH: 4.4), and dopamine hydrochloride (0.87 g, 4.62 mmol) was added via stirring at 45 ℃ for 48 h. The polymers were dialyzed against deionized water at pH 4.4 with dialysis membrane (Spectra/Por, MWCO: 10-12 kDa) at 4 ℃ for 3 days and freeze-dried. Yield: 90%.
Preparation of the PPDOA Hydrogel Solution. 10% (by weight) of the PEI-PPZ polymer in DW solution and 1.25% (by weight) of the DOA in DW solution were used for experiments after filtration using 0.2 µm cellulose acetate syringe filter, respectively. A series of experiments were performed with the PPDOA solution by mixing the PEI-PPZ and DOA solutions to obtain final concentrations of 10% and 1.25%, respectively.
Characterizations of the PEI-PPZ and DOA. The structures of PEI-PPZ and DOA were estimated by measuring 1H nuclear magnetic resonance spectroscopy (1H NMR, Bruker advance 400 MHz Fourier transform mode with CDCl3 and D2O, Germany) and Fourier transform infrared spectroscopy (FTIR, Spectrum GX FTIR, Perkin-Elmer, Atlanta, GA, USA). The substituted amount of amine groups in PEI423 was checked by ninhydrin assay (Pierce, Rockford, IL, USA).
The rheology test was performed by using a rheometer (MSC 102, Anton paar) with PEI-PPZ, PEI-PPZ with NaIO4, PEI-PPZ and DOA with NaIO4. All of the measurements were performed with an oscillating frequency of 1Hz, 10% of the oscillating strain and temperature in 4 and 60 ℃. The storage modulus (G') and loss modulus (G'') were measured by the instrument's software.
The size and zeta potentials of the PEI-PPZ, DOA, PPDOA were measured by using Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) at room temperature. The final concentration of polymer was 10 µg/mL. They were measured in triplicate.
The mechanical property of the polymer (PEI-PPZ, DOA, and PPDOA) were measured by using a rheometer (MSC 102, Anton Paar) in the frequency sweep mode of 0.1-100Hz with 5.0% strain at 45 ℃. The final concentration of polymer was 10% respectively.
In Vitro Adhesion Test. NIH3T3 cell lines (mouse fibroblasts) were prepared from Korean Cell Line Bank (KCLB, Seoul, Korea). The cells (1×104 cells/well) were seeded in 96-well plates (SPL, Pocheon, Korea) and incubated overnight in complete RPMI1640 culture media supplemented with 10% FBS and 1% penicillin-streptomycin. Then the culture media were replaced with serum-free media containing different concentration of conjugates and PEI. After 24 hours, the media were changed to serum free media containing thiazolyl blue tetrazolium bromide (MTT, 98%, Alfa Aesar, Ward Hill, MA, USA; 100 μg/well) and incubated for 3 hrs in order to form formazan crystals. After the removal of supernatants, the formazan crystals were dissolved in 100 µl of dimethylsulfoxide (DMSO) and the absorbance of the solution was observed at 570 nm by Spectramax 340 Microplate Reader (Molecular Devices, USA).
Synthesis and Characterization of PEI-PPZ Conjugate. Poly(organophosphazene) bearing carboxyl group at the terminal end (PPZ-COOH), GA-PPZ was prepared as shown in the previous literature.18 In brief, as shown in the Scheme 2, we synthesized GA-PPZ by substitution with hydrophobic IleOEt and hydrophilic AMPEG, to enable thermosensitive gelation by hydrophobic interaction19 and substituted with AEtOH to provide terminal carboxyl group for conjugation with PEI for functionalization.20
Sol-gel transition was observed by increased hydrophobic interaction due to showing lower critical solution temperature (LCST) of the polymer. At low temperatures, the hydrogen bonding of the polymer was caused by water molecules, while as temperature increases, the hydrogen bonding becomes broken and water molecules are released from the polymer by the increased hydrophobic interaction between molecules. We esterified the hydroxyl group of OH-PPZ to provide a hydrolyzable ester linkage and a terminal carboxylic acid group to conjugate with PEI. Finally, PEI was conjugated with the carboxylic acid group of poly(organophosphazene) (GA-PPZ) via amide coupling reaction. The structure of PEI-PPZ was estimated by using 1H NMR and ninhydrin assay.
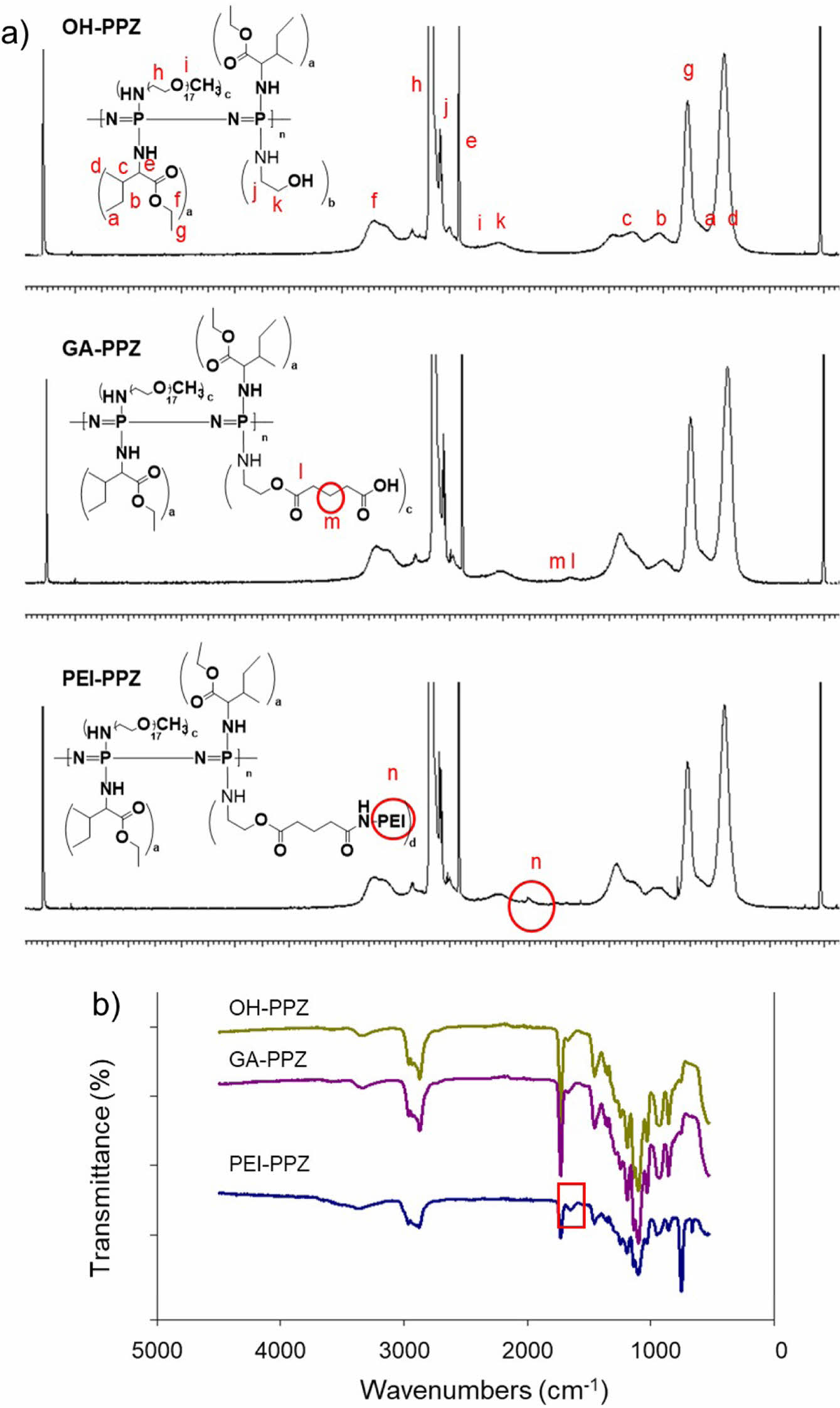
The structures of the OH-PPZ, GA-PPZ, and PEI-PPZ were determined by 1H NMR and FTIR. We confirmed the inherence peaks of PEI at 2.7 ppm (Figure 1(a)). As shown in Figure 1(b), we also confirmed C=O stretching of the amide bond in PEI-PPZ at the 1640 cm-1. These results indicated that PEI-PPZ was successfully conjugated via amide coupling reaction to the polymer at the carboxylic acid terminal.
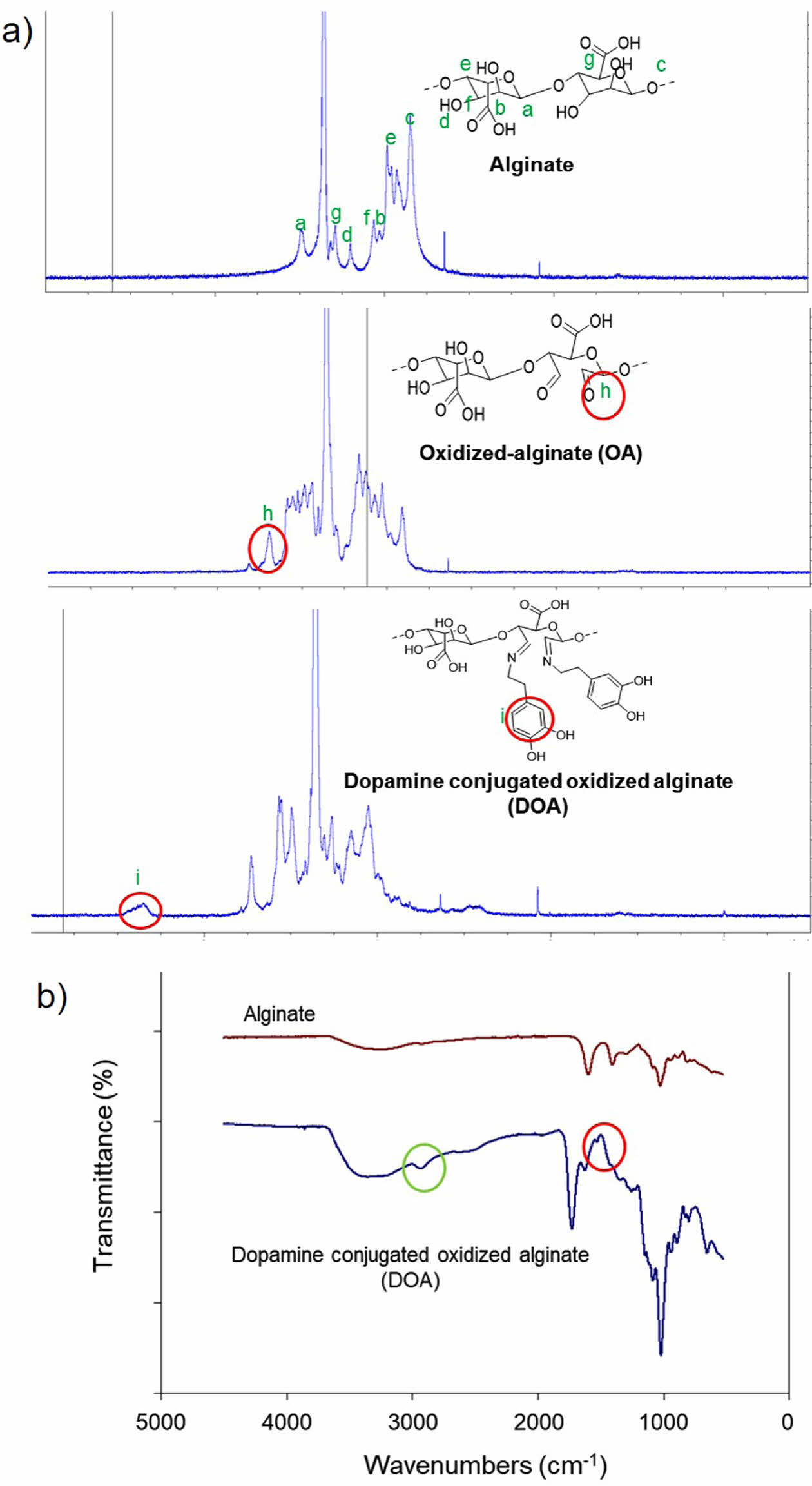
Synthesis and Characterization of the DOA. Alginate was oxidized by using NaIO4 (Scheme 3). In brief, NaIO4 converts the cis-diol group of saccharide to the di-aldehyde group via oxidation reaction and make aldehyde-bearing saccharide. Aldehyde group contents of the DOA was determined by using the hydroxylamine hydrochloride titration method and the aldehyde group content was about 80% of the alginate backbone.21 Thereafter, dopamine hydrochloride as a catechol moiety was conjugated with oxidized-alginate (OA) by using a Schiff base reaction. The reaction was conducted under nitrogen-protected atmosphere and pH was adjusted between 4.4 and 5 to prevent the oxidation and self-polymerization of the catechol groups. The structures of the OA and dopamine conjugated with oxidized-alginate (DOA) were checked after synthesis (Figure 2). The conjugated ratios of dopamine to OA were about 50% after calculation of integration of the specific of the catechol group at 6.8 ppm. The existence of the aromatic ring’s C-H bond of dopamine and C=N imine bond at 2950 cm-1 and 1534 cm-1 in FTIR data were also confirmed, respectively.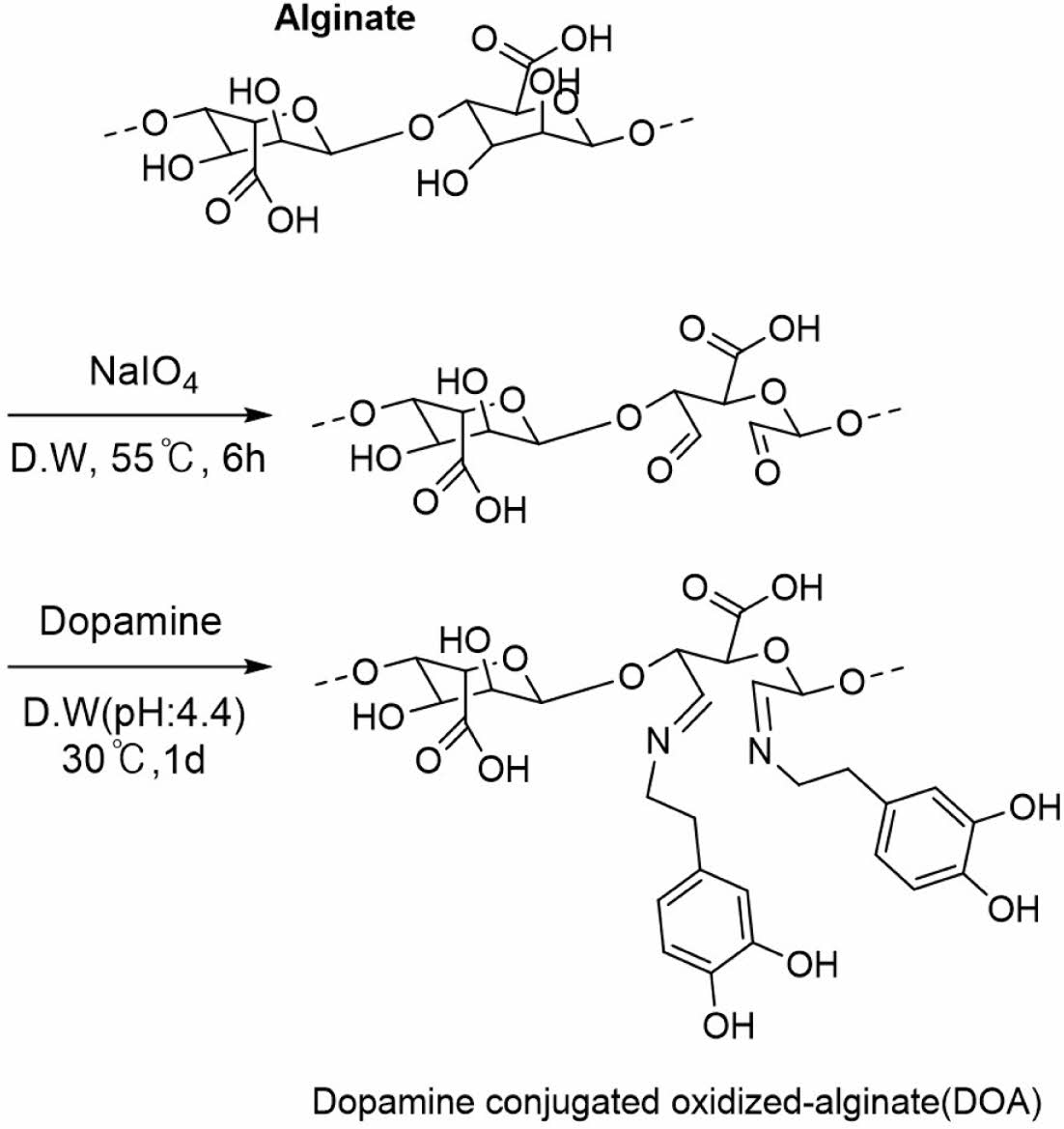
Scheme 3. Synthesis scheme of DOA.
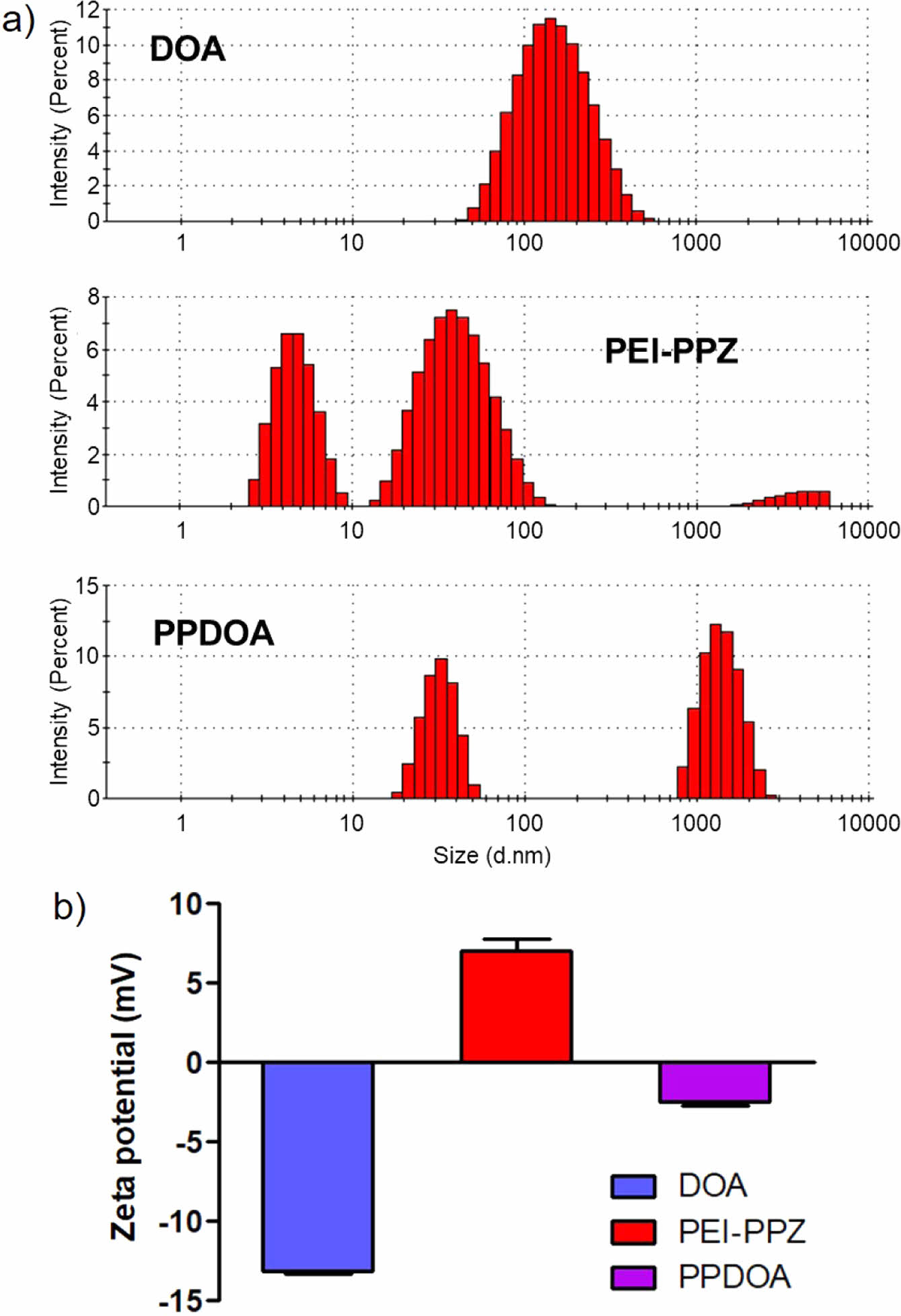
Preparation of the Complex Between PEI-PPZ and DOA. The complexation of the PEI-PPZ and DOA was confirmed by size differences and surface charge changes. In contrast to the DOA group showing homogeneous one size, the PEI-PPZ groups showed three different size distributions due to partial crosslinking of the PEI-PPZ and aggregation of the polymer (Figure 3(a)). After mixing with the DOA, only two peaks with different sizes were observed. It was assumed that the cationic PEI-PPZ interacted with anionic DOA via ionic interaction and resulted in increased size of the PEI-PPZ/DOA complexes (PPDOA) than the PEI-PPZ only group because positively charged surface of the PEI-PPZ micelles could be coated with negatively charged DOA (Figure 3(a)). As shown in Figure 3(d), the DOA showed a negative charge of about -15 mV and PEI-PPZ indicated a positive charge of about +7 mV. In the PPDOA group, the surface charge was -2 mV, increased than in the DOA group (Figure 3(b)). Therefore, the two polymers derived complexation is well-formed via ionic interaction between the PEI-PPZ and DOA.
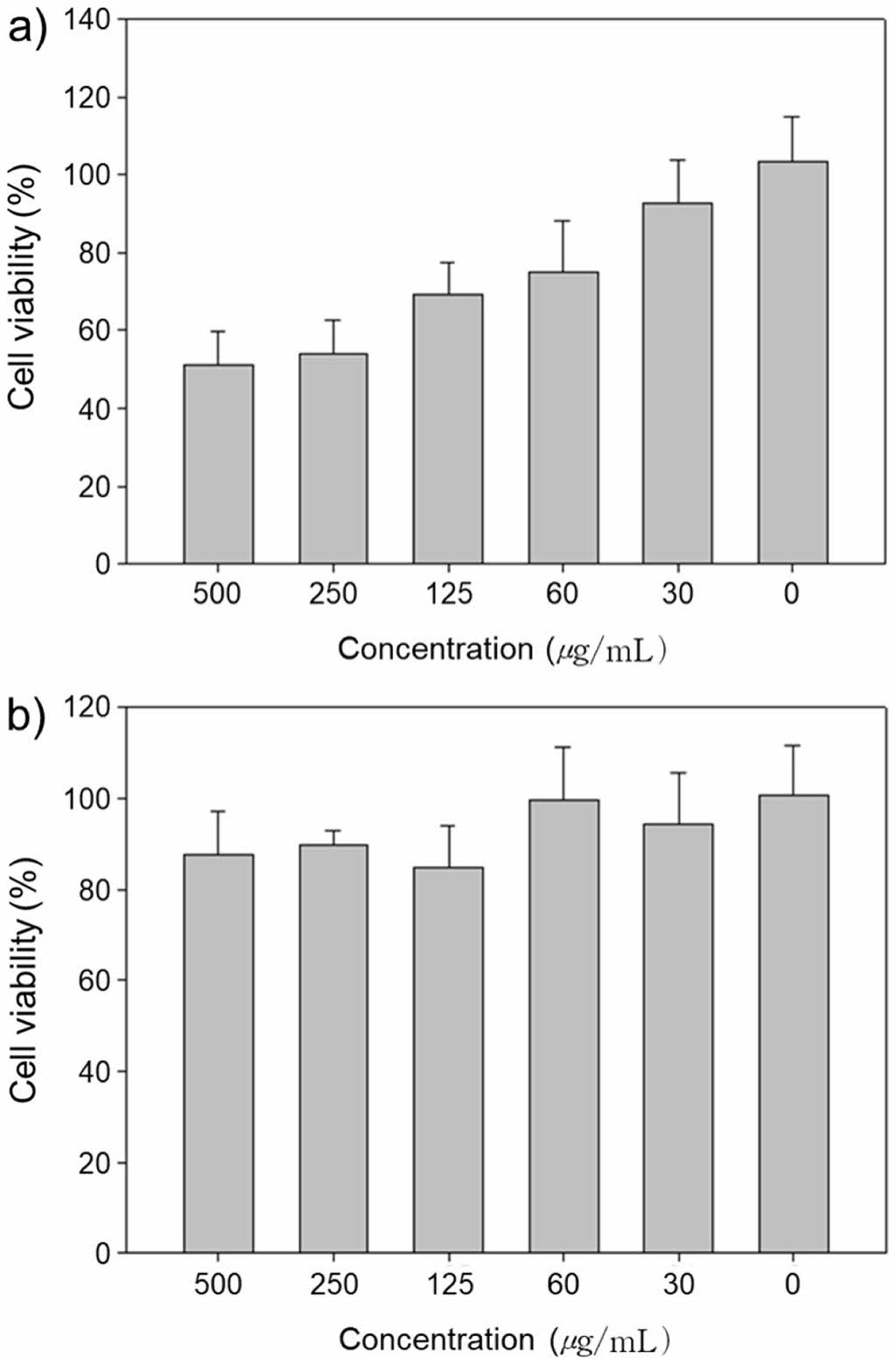
Evaluation of Biocompatibility. The thermo-sensitive adhesive hydrogel (PPDOA) was assumed as a biocompatible material because polyphosphazene and alginate were known as biocompatible materials.22,23 In order to check the biocompatibility of thermo-sensitive adhesive polymers, biocompatibility test was conducted by MTT assay in vitro. In the case of the DOA group, the cell viability was about 50% with 500 μg/mL due to a lot of catechol and aldehyde groups, however, the PEI-PPZ /DOA were mixed group, showed increased cell viability, about 80% with same concentration with DOA group (Figure 4). It explained that it can alleviate the toxicity of catechol due to shielding effect of surrounded the PEI-PPZ, and disappear remained aldehyde groups of the DOA due to interaction with amino group of the PEI-PPZ via Schiff base reaction.24
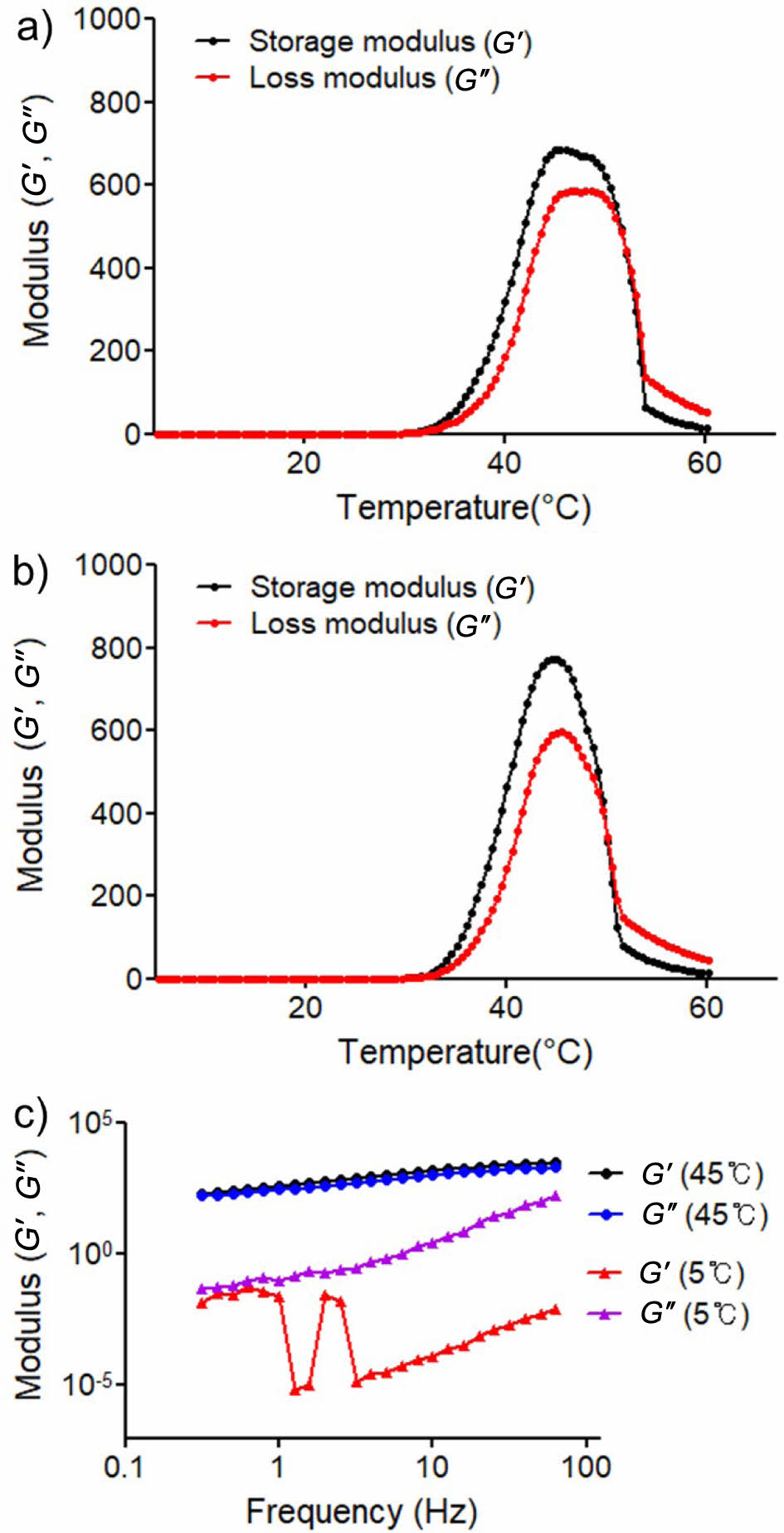
Mechanical Properties of the PEI-PPZ and PPDOA. The PPDOA has adhesive and thermo-sensitive ability showing the three-dimensional structure of sol-gel transition dependent on temperature. In order to check if it can be applied to the body, mechanical properties of adhesive hydrogel were measured by the rheometer. The temperature-dependent sol-gel transition behaviors of the PEI-PPZ were measured by monitoring storage modulus (G') and loss modulus (G''). In the PEI-PPZ group, sol-gel transition occurred at 34 ℃ and the G' value indicated a 600 Pa at 45 ℃ (Figure 5(a)). similarly, when NaIO4 was mixed with the PEI-PPZ (10% w/w), G' was increased than the PEI-PPZ only group at the 45 ℃ (Figure 5(b)). It was correlated with the previous research that NaIO4 might induce entanglement of the networks.25
As shown in Figure 5(c), it was assumed that relative increased hydrophilic portion of the polymer by addition of hydrophilic alginate polymers affected the temperature for sol-gel transition. We measured the mechanical property according to the frequency at constant temperature. In the PPDOA group, the loss modulus (G″) was higher than the storage modulus (G′) at a cool tempera- ture (5 ℃) whereas G′ value was greater than G″ at 45 ℃. Because amino groups of the PEI-PPZ interacted with remained aldehyde group of the DOA via Schiff base reaction, it can enhance crosslinking of the network. Also, the cohesive and adhesive ability of catechol moieties could be was increased by forming a three-dimensional network of the hydrogel.
Therefore, it demonstrated that mechanical property is increased because the three-dimensional network was formed at a certain temperature by PEI-PPZ instead of catechol. Also, the cohesive and adhesive ability of the catechol group is enhanced due to replacement of the cohesive ability of catechol.
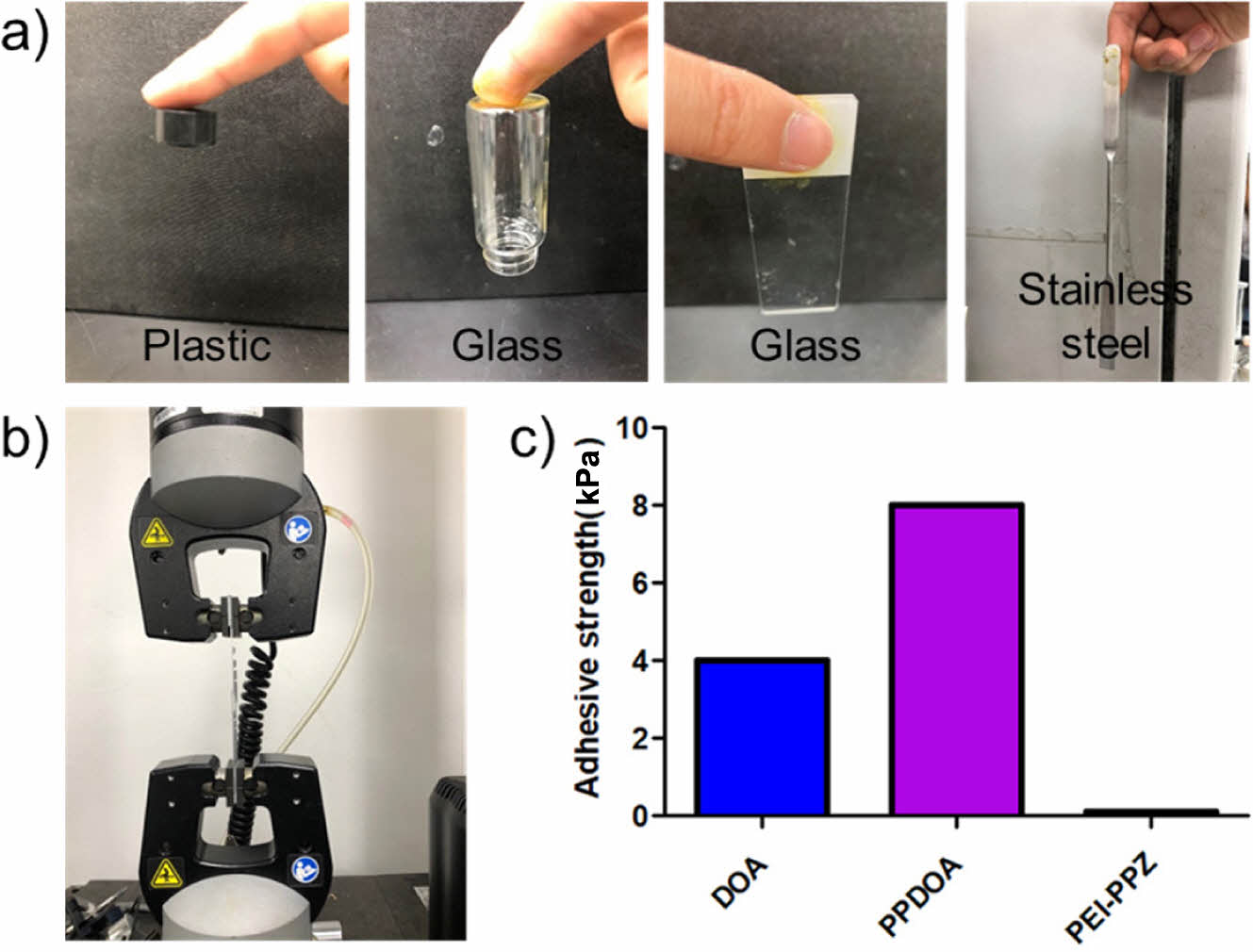
In Vitro Adhesion Test of the PPDOA. Adhesion test of the PPDOA was conducted dependent on the diverse substrates at 45 ℃ such as plastic, glass, and stainless steel. The PPDOA was perfectly adhered to diverse substrates respectively (Figure 6(a)). To compare exact adhesive parameters, the PPDOA, PEI-PPZ, and DOA groups were assessed through the tensile-strength method by using an Instron machine respectively (Figure 6(b)). After mixing between the PEI-PPZ and DOA, adhesive strength was 8 kPa, which was increased 2-fold higher than the DOA group (Figure 6(c)). While the adhesive strength was not observed in the PEI-PPZ group because of no existence of catechol groups.
It demonstrated that adhesive strength was increased due to increasing cohesive and adhesive ability by using thermo-sensitive hydrogel and enhancing the covalent bond between the polymers and substrates.
|
Figure 1 Characterizations of structure of the polymers by (a) 1 H NMR; (b) FTIR. |
|
Figure 2 Characterizations of DOA: (a) 1 H NMR spectra of the alginate, OA, and DOA; (b) FTIR spectra of the alginate and DOA. |
|
Figure 3 (a) Size distributions of DOA, PEI-PPZ and PPDOA respectively; (b) Zeta-potentials of the DOA, PEI-PPZ, and PPDOA. |
|
Figure 4 In vitro biocompatibility test of (a) DOA; (b) PPDOA. |
|
Figure 5 (a) Thermo-sensitive gelation properties of PEI-PPZ; (b) PEI-PPZ with sodium periodate (PPS); (c) The storage modulus (G′) and loss modulus (G″) of the PPDOA. |
|
Figure 6 (a) Adhesive ability tests in diverse substrates such as plastic, glass, and stainless steel; (b) Process of the tensile-strength test; (c) Tensile-strength tests of the DOA, PPDOA, and PEI-PPZ. |
In summary, we prepared a thermo-sensitive, and adhesive hydrogel for a novel adhesive system showing injectability, adhesive ability, and biocompatibility. This system has several advantages; 1) fast formation of a three-dimensional network of the hydrogel-based, minimized the dispersion of solution by sol-gel property of hydrogel, 2) tissue adhesive effect by the increased cohesive and adhesive ability of catechol and, 3) superior biocompatible property by using the safe materials and fewer contents of catechol groups. It was expected that this system can be a promising material for adhesive tissue regeneration.
- 1. Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; Zou, Y.; Yu, D.; Arnot, D. E.; Zou, X.; Zhu, L.; Zhang, S.; Ouyang, H. A Strongly Adhesive Hemostatic Hydrogel for the Repair of Arterial and Heart Bleeds. Nat. Commun. 2019, 10, 2060.
-

- 2. Blunt, J. W. Sutures for Trauma. Am. J. Surg. 1958, 95, 512-517.
- 3. Mahesh, L.; Kumar, V. R.; Jain, A.; Shukla, S.; Aragoneses, J. M.; González, J. M. M.; Fernández-Domínguez, M.; Calvo-Guirado, J. L. Bacterial Adherence around Sutures of Different Material at Grafted Site: A Microbiological Analysis. Materials (Basel). 2019, 12, 2848.
-

- 4. Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; Román, J. S.; Bellón, J. M. Cytotoxicity of Cyanoacrylate-Based Tissue Adhesives and Short-Term Preclinical In Vivo Biocompatibility in Abdominal Hernia Repair. PLoS One 2016, 11, e0157920.
-

- 5. Yan, S.; Wang, W.; Li, X.; Ren, J.; Yun, W.; Zhang, K.; Li, G.; Yin, J. Preparation of Mussel-Inspired Injectable Hydrogels Based on Dual-Functionalized Alginate with Improved Adhesive, Self-Healing, and Mechanical Properties. J. Mater. Chem. B 2018, 6, 6377-6390.
-

- 6. Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; Luo, Y.; Yu, P.; Ning, C.; Tan, G. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457.
-

- 7. Priemel, T.; Palia, R.; Babych, M.; Thibodeaux, C. J.; Bourgault, S.; Harrington, M. J. Compartmentalized Processing of Catechols during Mussel Byssus Fabrication Determines the Destiny of DOPA. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 7613-7621.
-

- 8. Quan, W. Y.; Hu, Z.; Liu, H. Z.; Ouyang, Q. Q.; Zhang, D. Y.; Li, S. D.; Li, P. W.; Yang, Z. M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586.
-

- 9. Kim, Y. M.; Kim, C. H.; Park, M. R.; Song, S. C. Development of an Injectable Dopamine-Conjugated Poly(Organophophazene) Hydrogel for Hemostasis. Bull. Korean Chem. Soc. 2016, 37, 372-377.
-

- 10. Lee, D.; Bae, H.; Ahn, J.; Kang, T.; Seo, D. G.; Hwang, D. S. Catechol-Thiol-Based Dental Adhesive Inspired by Underwater Mussel Adhesion. Acta Biomater. 2020, 103, 92-101.
-

- 11. Ryu, J. H.; Hong, S.; Lee, H. Bio-Inspired Adhesive Catechol-Conjugated Chitosan for Biomedical Applications: A Mini Review. Acta Biomater. 2015, 27, 101-115.
-

- 12. De Oliveira, D. M.; Pitanga, B. P. S.; Grangeiro, M. S.; Lima, R. M. F.; Costa, M. F. D.; Costa, S. L.; Clarêncio, J.; El-Bacha, R. S. Catechol Cytotoxicity In Vitro: Induction of Glioblastoma Cell Death by Apoptosis. Hum. Exp. Toxicol. 2010, 29, 199-212.
-

- 13. Van Der Heijden, C. D. C. C.; Groh, L.; Keating, S. T.; Kaffa, C.; Noz, M. P.; Kersten, S.; Van Herwaarden, A. E.; Hoischen, A.; Joosten, L. A. B.; Timmers, H. J. L. M.; Netea, M. G.; Riksen, N. P. Catecholamines Induce Trained Immunity in Monocytes In Vitro and In Vivo. Circ. Res. 2020, 127, 269-283.
-

- 14. Flierl, M. A.; Rittirsch, D.; Huber-Lang, M.; Vidya Sarma, J.; Award, P. Catecholamines - Crafty Weapons in the Inflammatory Arsenal of Immune/Inflammatory Cells or Opening Pandora’s Box? Mol. Med. 2008, 14, 195-204.
-

- 15. Hong, K. H.; Kim, Y. M.; Song, S. C. Fine-Tunable and Injectable 3D Hydrogel for On-Demand Stem Cell Niche. Adv. Sci. 2019, 6, 1900597.
-

- 16. Sohn, Y. S.; Cho, Y. H.; Baek, H.; Jung, O.-S. Synthesis and Properties of Low Molecular Weight Polyphosphazenes. Macromolecules 1995, 28, 7566-7568.
-

- 17. Bromberg, L.; Magner, E. Release of Hydrophobic Compounds from Micellar Solutions of Hydrophobically Modified Polyelectrolytes. Langmuir 1999, 15, 6792-6798.
-

- 18. Hong, K. H.; Song, S. C. 3D Hydrogel Stem Cell Niche Controlled by Host-Guest Interaction Affects Stem Cell Fate and Survival Rate. Biomaterials 2019, 218, 119338.
-

- 19. Lee, B. H.; Song, S. C. Synthesis and Characterization of Biodegradable Thermosensitive Poly(Organophosphazene) Gels. Macromolecules 2004, 37, 4533-4537.
-

- 20. Kim, Y. M.; Park, M. R.; Song, S. C. Injectable Polyplex Hydrogel for Localized and Long-Term Delivery of siRNA. ACS Nano 2012, 6, 5757-5766.
-

- 21. Wang, L.; Hou, Y.; Zhong, X.; Hu, J.; Shi, F.; Mi, H. Preparation and Catalytic Performance of Alginate-Based Schiff Base. Carbohydr. Polym. 2019, 208, 42-49.
-

- 22. Lee, B. H.; Lee, Y. M.; Sohn, Y. S.; Song, S. A Thermosensitive Poly(Organophosphazene) Gel. Macromol. 2002, 35, 3876-3879.
-

- 23. Sahoo, D. R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30.
- 24. Mo, C.; Xiang, L.; & Chen, Y. Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun., 2021, 42, 2100025.
-

- 25. Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225-18234.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(2): 241-248
Published online Mar 25, 2023
- 10.7317/pk.2023.47.2.241
- Received on Dec 27, 2022
- Revised on Jan 12, 2023
- Accepted on Jan 19, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Young-Min Kim
-
*Center for Biomaterials, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea
**Division of Biomedical Science and Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Korea - E-mail: davidkim@kist.re.kr
- ORCID:
0000-0002-0912-3010









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.