- Designing Rating Scale to Measure Daily Stain Prevention and Removal Capability and Its Application in New Polymeric Phosphate-Containing Whitening Mouthwashes
Ji Young Kim, Seongwoo Bak, Soonran Song, Brian Chung, Kyoung Hee Oh, Shun Hyu Kim*, Yongju Jung*,†
 , and Wonho Ha†
, and Wonho Ha† 
LG Science Park, LG Household & Health Care Ltd., Seoul 07795, Korea
*School of Energy Materials Chemical Engineering, KOREATECH, Cheonan 31253, Korea- 생활 착색 예방 및 제거 능력의 신속한 측정을 위한 평가 척도 개발 및새로운 고분자 인산염을 포함한 미백 양치액의 성능 평가
LG 생활건강 기술연구원, *한국기술교육대학교 에너지신소재화학공학부
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Facile and quantitative methods for evaluating daily stain prevention and removal efficacy were designed for developing daily whitening mouthwashes. A new daily staining index was defined as a measurement of stains. Hydroxyapatite powder was used as a tooth substitute and the degree of staining was quantitatively evaluated. Mouthwashes containing polymeric phosphates, sodium hexametaphosphate (SHMP), showed significantly higher stain prevention efficacy with just one treatment than those containing common pyrophosphate chelating agents. The newly developed daily whitening mouthwash containing 1% SHMP without hydrogen peroxide showed excellent daily stain prevention and removal efficacy compared with a typical commercial mouthwash. We expect that consumers with teeth sensitive to hydrogen peroxide can effectively use this SHMP-containing mouthwash as a teeth-whitening mouthwash. In addition, we believe that the new approaches introduced in this study can contribute to opening up new avenues in the field of teeth-whitening oral care products.
치아 미백 양치액 개발을 위해 착색 방지 및 예방 효과를 정량적으로 평가할 수 있는 방법이 고안되었다. 착색을 유발하는 물질은 대표적인 치아 착색 음식 중에서 선정되었고, 새로운 착색 지수가 착색의 척도로서 새롭게 정의되었다. 하이드록시아파타이트 분말이 착색 방지 및 착색 제거 평가에 치아 대체물로 사용되었다. 착색의 정도는 크로마 미터를 이용하여 정량적으로 평가하였다. 파이로포스페이트계 킬레이팅 물질보다는 고분자 인산염(SHMP)을 포함하는 양치액이 매우 뛰어난 착색 예방 효능을 보였다. 본 연구에서 개발된 1% SHMP를 포함한 비과수 양치액이 시판되는 양치액 대비 매우 뛰어난 착색 예방 및 제거 효능을 보였다. 이 결과로부터 SHMP를 사용하는 치아 미백 양치액이 과수에 민감한 소비자들에게 효과적으로 사용될 수 있으리라 기대한다.
Mouthwashes containing polymeric phosphates, sodium hexametaphosphate, showed significantly higher stain prevention efficacy with just one treatment than those containing common pyrophosphate chelating agents.
Keywords: stain prevention, stain removal, teeth whitening mouthwashes, polymeric phosphate, sodium hexametaphosphate.
The authors declare that there is no conflict of interest.
Information is available regarding the digital images of the HAP powders before and after stain removal tests, and the schematic illustration of stain removal of SHMP. The materials are available via the Internet at http://journal.polymer-korea.or.kr.
PK_2023_047_03_303_Supporting_Information_template.pdf (772 kb)
Supplementary Information
Maintaining white teeth and a bright smile is an important beauty factor because it has been a representative symbol of youth and beauty. The scale and trend of the teeth whitening market as well as the government regulations on whitening vary. Moreover, the preference for whitening products strongly depends on the customers. For example, Americans favor professional-level whitening within a short time by actively using high-concentration peroxide products. In the US, teeth-whitening products are considered cosmetics with no limits on the peroxide concentration.1-5 Conversely, the Korean Food and Drug Association regulates the peroxide concentration in oral hygiene products as <0.75% by quasi-drug safety and thus, customers have less preference for extra teeth whitening products.6 However, according to the customer survey in Korea, the customers wish to whiten their teeth using daily oral hygiene products.
Depending on their deposited locations, dental discoloration can be classified into extrinsic and intrinsic strains.7 The extrinsic stain can be formed via the adhesion of chromogens when ingesting foods and beverages such as coffee, tea, and wine. The chromogens of coffee, tea, and wine are tannins containing polyphenols such as catechins and leucoanthocyanidin. These chromogens have a conjugated double bond, and it has been widely reported that they bind to the tooth surface via an ion exchange mechanism.
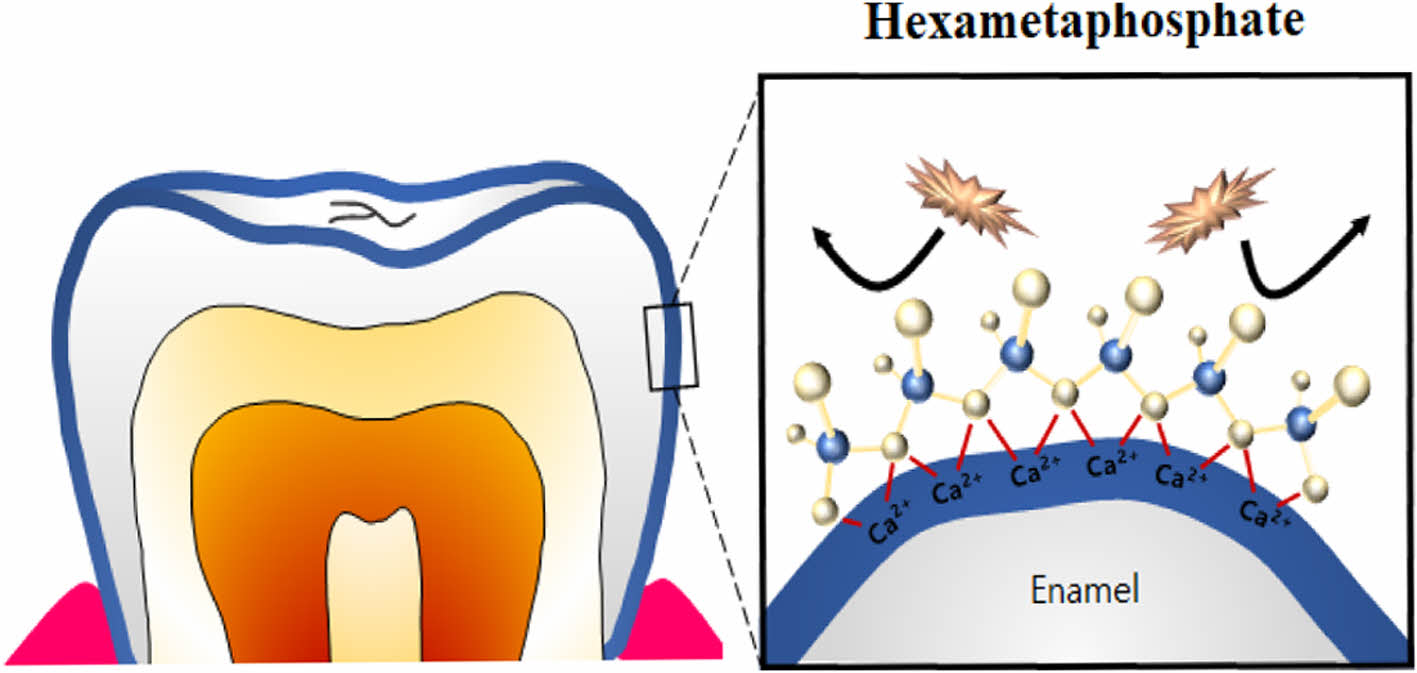
In addition, the foods and beverages, such as coffee, tea, and curry, may generate stains by physically depositing their chromogens on the tooth surface. The attractive forces include electrostatic, van der Waals forces, hydration forces, hydrophobic interactions, dipole-dipole forces, and hydrogen bonds. Electrostatic forces can be dominant in the oral cavity, because the enamel is negatively charged, consequently resulting in the selective adhesion of positive chromogens.7 Moreover, the chromogens can be attached to the enamel surface via a calcium bridge of hydroxyapatite (96% components of dentinal enamel),8 as illustrated in Figure 1.7 Such pigmentation tends to darken over time and is more challenging to remove. The darkening of the color is associated with the subsequent formation of a metal bridge between the hydroxyl group of polyphenol and the metal ion in the oral cavity. In other words, the polyphenolic components and metal ions in the saliva or foods stack to form a layer-by-layer structure on teeth surfaces. Thus, it is considerably challenging to remove the stain.7
Few whitening efficacy evaluation methods are used in the teeth-whitening field. The pellicle cleaning ratio (PCR) procedure has been widely used in the field to assess the stain removal performance of whitening toothpastes.9,10 The PCR procedure can be performed by obtaining the enamel samples from bovine permanent incisors or human enamel and embedding them in a rectangular acrylic resin with the enamel surface exposed. The staining solution can be prepared using coffee, tea, ferric chloride, and gastric mucin with oral bacteria, and the samples should be stained for at least 10 days for mimicking the aged teeth stains using Stookey’s method.10 To initiate the PCR method, a brushing machine is required for evaluation. The difference between the pre-brushing and post-brushing tests for each color factor (L, a, b) represents the ability of the test toothpastes to remove the stain and whiten the teeth. The overall color change in a stain can be calculated using the following equation:

This PCR evaluation requires time-consuming, cumbersome, and complex process, because it is designed to mimic aged stains. In addition, the PCR method is not appropriate for daily staining removal or prevention. Particularly, this method does not apply to the assessment of mouthwashes that do not use an abrasive.
Stookey’s staining method can lead to different results owing to the deviation between the bovine and human enamel specimens. To overcome this limitation, the hydroxyapatite (HAP) disc specimens as artificial teeth have been used instead of the bovine or human enamel specimens. Ahn et al. investigated HAP discs to develop the color index of teeth for the most popular Korean foods and the stained samples for 7 days for quantitative tests.11 These models can be adopted to simulate the aged stains, but this approach is inadequate for a daily stain assessment.
Baig et al. evaluated the stain removal efficacy of whitening toothpastes using the HAP powder.12 However, a large content of abrasives (10-65 wt%) that are water-insoluble particles, such as silica, calcium carbonate, sodium bicarbonate, and dicalcium phosphate dehydrate, are included in toothpastes. Moreover, when the color of the HAP powder specimens is directly measured for analyzing whitening efficacy, the white-colored abrasives cause bias in measuring the true color of the samples. Thus, they introduced an indirect method to obtain more accurate and quantitative values as a measurement of stain removal efficacy.12 Baig et al. dispersed stained HAP powders in a concentrated ethylenediamine tetraacetic acid (EDTA) solution, centrifuged, and evaluated the removal efficacy of the toothpastes using a colorimeter to measure the solution color of the supernatant. However, this method requires a time-consuming step, which significantly limits its widespread application to evaluate the whitening efficacy of oral care products.
In this study, we designed new testing methods for evaluating the stain prevention and removal efficacy of the mouthwashes based on direct color measurements of the HAP powders. Considering consumer requirements, we developed a new method for quantitatively measuring teeth stains, and assessed the whitening efficacy of two types of mouthwashes (with and without hydrogen peroxide), which have been used as teeth whiteners in dentistry for over 100 years.13 In this work, for the first time, a new composition of mouthwash containing polymeric phosphate (SHMP) was developed for customers who do not use peroxide-containing products owing to the peculiar irritation and distinctive tastes of peroxide.

|
Figure 1 Schematic of mechanism of dental stain formation associated with caffeic acid. |
Materials and Preparation. Hydroxyapatite (HAP) powder with an average particle size of 3 um was purchased from OCI (Korea). black coffee (Folgers Classic Roast Coffee, J. M. Smucker Co., USA), black tea (Lipton Pyramid English Breakfast Tea, Unilever, Poland), chocolate (Nestle Hot Choco Original, Nestle, Korea), curry (Ottogi Curry Powder Mild, Ottogi, Korea), kimchi stew (Bibigo Pork Kimchi Stew, CJ Cheiljedang, Korea), and instant noodles (Shin Ramen, Nongshim, Korea) were used as teeth-staining foods. Chelating agents tested in this work, tetrasodium pyrophosphate (TSPP, molar mass = 266 g/mol), sodium acid pyrophosphate (SAPP, molar mass = 222 g/mol), and sodium hexametaphosphate (SHMP, molar mass = 611 g/mol), were purchased from Seodo BNI (Korea). All other chemicals used were of analytical or reagent grade. For the preparation of a peroxide mouthwash, a flavor and a solubilizer were mixed by a disperser. Then, 0.02% sodium fluoride, 1% sodium hexametaphosphate (SHMP), a sweetener, a pH adjuster, and purified water are added and mixed with 0.55% hydrogen peroxide until all ingredients were completely dissolved. A non-peroxide mouthwash was prepared using the same procedure as the peroxide mouthwash, except for adding 0.5% tetrasodium pyrophosphate (TSPP) instead of 0.55% hydrogen peroxide. As a control, a commercial mouthwash (Garglin Zero), which contains 0.02% NaF without TSPP, SHMP, and peroxide, was examined.
Preparation of Staining Solutions. Instant noodle soups, curry, and kimchi stew were prepared following the recipes provided on the packaging, and their solid ingredients were removed via centrifugation before adding them to the HAP powder. Commercial Lipton tea was brewed. Typically, 2 bags were immersed in 200 g of boiled water for 3 min. Coffee powder (14 g) was dissolved in hot water (196 g). Hot chocolate powder (48 g) was dissolved in 120 g of hot water for 5 min and then the remaining insoluble substances were removed via centrifugation.
Evaluation Method of Coloring. The color of samples was quantified using the CIELab color space. Each color was represented by the color coordinates (L*, a*, b*) of the color point. A chroma meter (CR-321, Minolta Co., Japan) was used to identify the L, a, and b values, which are defined as:

For quantitative analysis, approximately 0.2 g of the powder was transferred onto a slide glass and covered by a cover glass. The color change was directly measured using a chroma meter.
Measurements of Staining Index. In particular, the staining degree of teeth is highly dependent on the experimental conditions (e.g., temperature and time), so they were adjusted to mimic typical eating and drinking habits. In this regard, the optimal contact time for the staining of HAP powders was determined to be 1 min using beverages such as coffee, tea, and hot chocolate. The adequate contact time was set to be 10 min for foods, such as instant noodles, curry, and kimchi stew. After contacting the HAP powders with the staining food samples for a fixed time, they were washed with distilled water and completely dried. To measure the staining index using a chroma meter, the stained HAP powders were sandwiched between a slide glass and cover glass. The staining index (ΔE), defined as {(ΔL)2 + (Δa)2 + (Δb)2}1/2) in this work, was calculated from ΔL (Lstained − Linitial), Δa (astained – ainitial), and Δb (bstained – binitial).
Stain Prevention Efficacy Tests. To measure stain prevention efficacy, the HAP powder was pre-treated with solutions of different chelating agents. First, 2 g of the HAP powder was transferred to conical tubes. Then, 40 mL of mouthwashes were added and vortexed for 1 min. Subsequently, the mixture was then centrifuged at 4000 rpm for 15 min, and then, the supernatant was discarded. Further, 40 mL of water was added to the wet powder to remove the residual solutions. The washing process was repeated twice. Finally, the powders were vortexed and then centrifuged. The resulting powders were stained by a coffee solution with the highest staining index among the representative teeth-staining foods. A coffee solution was prepared by dissolving 14 g of coffee powder in 196 g of hot water. After 40 mL of hot coffee solution was added into a conical tube with 2 g of pre-treated HAP powders, and the tube was vortexed for 1 min to homogeneously disperse the powders in the coffee solution. The coffee solution was then centrifuged for 15 min at 4000 rpm and washed twice with 40 mL of water. After centrifugation, the supernatant was removed, and then, the powders in the conical tube were dried at 70 ℃ overnight. After drying, the powders were transferred to a plastic dish for complete drying. For quantitative tests, L, a, and b values were measured using a chroma meter using approximately 0.2 g of sample powders. The efficacy of stain prevention was calculated as reduction percentage versus water using the following equation:
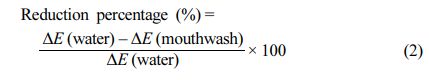
Stain Removal Efficacy Tests. To monitor stain removal efficacy, the pre-stained HAP powders were used after complete drying. The samples were exposed to 40 mL of mouthwashes for 1 min, and the mixture was centrifuged. The resulting powders were washed with water at least three times. Finally, the resulting color changes in the HAP powders were intensively examined using a chroma meter.
Method of Indexing Stain using CIELab Chroma Meter. The staining indexes of the representative food samples were calculated through a direct color measurement of HAP powder using a chroma meter. To obtain the daily staining index, the reasonable contact times for beverages and foods were determined as 1 and 10 min, respectively. The highest-staining food was observed to be coffee, as shown in Figure 2. The staining indexes of tea, kimchi stew, curry, and instant noodle soup were similar to each other (Figure 2). In particular, chocolate exhibited the lowest staining index, indicating that it causes relatively less teeth-staining.
The teeth-staining is attributable to calcium ions (Figure 1), whichcombined with the polyphenol species (staining ingredients of coffee or tea), strongly adsorb onto the surfaces with just one treatment on the tooth surface. It is considered that the higher staining index of coffee, compared with that of tea, is due to the oily components in coffee.14 Ahn et al. reported the staining effect of the oily component on the HAP disc by comparing kimchi and kimchi stew.11 Presumably, the hydrophobic interaction between HAP powders and oily molecules in coffee may form strong bonding, resulting in a higher staining index.To examine the efficiency of chelating agents as a teeth whitener, SHMP, SAPP, and TSPP with phosphate functional groups were tested (Figure 3). Among them, pyrophosphates have been widely used in oral hygiene products. Recently, SHMP has been embedded in whitening toothpastes as a substitute for hydrogen peroxide, which has been used as a popular teeth whitener for a long time.12,15
To evaluate stain prevention efficacy, coffee with the highest staining index was selected, and the quantitative results of stain prevention against coffee are listed in Table 1. The stain prevention efficacies of TSPP and SAPP were largely similar. Notably, SHMP showed excellent prevention efficacy against coffee. This can be explained by the wide and strong coverage capability of SHMP over the teeth surface (Figure 4). The enamel, the thin outer layer of the tooth, is composed mainly of HAP, constituting 96 wt% of enamel. HAP, Ca10(PO4)6(OH)2, has abundant calcium ions with six repeating phosphate groups. For TSPP and SAPP with a bidentate structure, they can complex with one calcium ion, so they have a more limited coverage, compared with SHMP. However, SHMP, a polymeric phosphate with a multi-dentate structure, can complex with multiple calcium ions, leading to a highly strong affinity over wide teeth surfaces. Based on these results, we selected SHMP as a key component of daily teeth-whitening mouthwashes.
Daily Stain Prevention Efficacy of Mouthwashes. Typical teeth-whitening agents in oral care products, such as toothpaste and mouthwash, used in Korea are hydrogen peroxide and TSPP. Hydrogen peroxide and TSPP can brighten teeth via stain removal when used for a long time via bleaching and chelating mechanisms, respectively. However, a long time is required for customers to notice their whitening effect. The most effective daily stain prevention agent, SHMP, was selected as the primary active ingredient of our experimental mouthwashes. Despite the strong bleaching properties of hydrogen peroxide, numerous customers do not prefer hydrogen peroxide-containing mouthwashes because of their drawbacks of distinctive taste, allergic reaction, and hypersensitivity.16 Hence, we developed both a hydrogen peroxide-containing mouthwash and a non-peroxide mouthwash, as shown in Figure 5. It was found that the efficacy of mouthwashes was very similar in the range of 1.0 to 2.5%. As the concentration of phosphates increased, however, the possibility that phosphates stimulate teeth greatly increased, causing a teeth sensitivity issue. In this work, considering consumers with sensitive teeth to phosphates, 1.0% SHMP was included in the mouthwashes. Typical mouthwashes in Korea contain 0.02% NaF as a cavity protection agent. To evaluate the daily stain prevention efficacy of the three types of mouthwash, the HAP powder-based staining index method developed in this study was used. As shown in Figure 6, both the peroxide mouthwash and non-peroxide mouthwash with SHMP exhibited considerably higher whitening efficacy than the control. The stain prevention percentage by non-peroxide mouthwash was observed to be 62.1, 62.5, and 14.7% for curry, kimchi stew, and instant noodles, respectively, and 69.5, 79, and 41.6% for coffee, tea, and chocolate, respectively.
Daily Stain Removal Efficacy of Mouthwashes. The digital images of the HAP powders before and after stain removal tests are shown in Figure S1. Two SHMP-containing mouthwashes exhibit brighter images compared with the control mouthwash, indicating that the stain is effectively removed. The two mouthwashes containing SHMP showed higher stain removal efficacy for the representative staining foods compared with the commercial mouthwash (Figure 7). The high efficacy may be arising from the considerably stronger bonding of the phosphate groups with calcium ions than that of tannic acid (stain). It can most reasonably be inferred from the difference in the bond strength that the stains on the outer surfaces of teeth are replaced by SHMP, as illustrated in Figure S2.
Notably, the phosphate mouthwash without peroxide showed excellent stain removal efficacy comparable with peroxide mouthwash.

|
Figure 2 Staining index of the representative food samples selected among highly teeth-staining Korean foods. |

|
Figure 3 Structures of TSPP, SAPP and SHMP. |

|
Figure 4 Comparison of stain prevention efficacy of pyrophosphate (left) and hexametaphosphate (right) |

|
Figure 5 The active ingredients of two mouthwashes and commercial mouthwash (Garglin). |

|
Figure 6 Stain prevention efficacy of three kinds of mouthwashes against the teeth-staining foods. |

|
Figure 7 Stain removal efficacy of three types of mouthwashes against the representative teeth-staining foods. |
|
Table 1 Comparison of Daily Stain Prevention Efficacy of TSPP, SAPP, and SHMP Versus Water |

The colors of the stained HAP powders selected as a teeth substitute were rapidly and precisely measured using a CIELab chroma meter. The daily staining index as a measurement of teeth discoloration was newly designed using the color coordinates (L*, a*, b*) of the color point. A new method for evaluating the daily stain prevention capability was established. SHMP exhibited the highest prevention ability among the chelating agents tested. A newly developed daily whitening mouthwash containing 1% SHMP without hydrogen peroxide showed excellent daily stain prevention and removal efficacy compared with a typical commercial mouthwash. Notably, the phosphate mouthwash without peroxide showed excellent stain removal efficacy compared with that of peroxide mouthwash. We expect that the phosphate-based teeth whitening mouthwash can help consumers with teeth sensitive to hydrogen peroxide. In addition, we believe that the new approach proposed in this study can contribute to facilitating the optimal design and adequate evaluation of teeth-whitening mouthwash products.
- 1. Koradas, M.; Hatipoglu, O. Efficacy of Mouthwashes Containing Hydrogen Peroxide on Tooth Whitening. Sci. World J. 2015, 961403.
-

- 2. Shahidi, H.; Barker, M. L.; Sagel, P. A.; Robert, R. W. Randomized Controlled Trial of 10% Hydrogen Peroxide Whitening Strips. J. Clin. Dent. 2005, 16, 91-95.
- 3. Gerlach, R. W.; Zhou, X. Vital Bleaching with Whitening Strips: Summary of Clinical Research on Effectiveness and Tolerability. J. Compend Dent. Pract. 2001, 2, 1-15.
-

- 4. Sharma, N.; Galustians, J.; Qaquish, J.; Rustoqi, K. N.; Petrone, M. E.; Chaknis, P.; DeVizio, W.; Volpe, A. R.; Proskin, H. M. A Six-Week Clinical Efficacy Study of Three Commercially Available Dentifrices for the Removal of Extrinsic Tooth Stain. J. Clin. Dent. 1999, 111, 111-114.
- 5. Swift, E. J.; Miguez, P.; Barker, M. L. Three-week Clinical Trial of a 14% Hydrogen-Peroxide, Strip-based Bleaching System. Compend Contin. Educ. Dent. 2004, 25, S27-S42.
- 6. Woo, G. J.; Kim, E. K.; Jeong, S. H.; Song, K. B.; Goo, H. J.; Jeon, E. S.; Choi, Y. H. Comparison of the Whitening Effect of Toothpastes Containing 0.25% Hydroxyapatite and 0.75% Hydrogen Peroxide. J. Korean Acad. Oral Health 2014, 38, 3-9.
-

- 7. Nathoo, S. A. The Chemistry and Mechanisms of Extrinsic and Intrinsic Discoloration. J. Am. Dent. Assoc. 1997, 128, 6S-10S.
-

- 8. Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhano, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; Liu, Z.; Tang, R. Repair of Tooth Enamel by a Biomimetic Mineralization Frontier Ensuring Epitaxial Growth. Sci. Adv. 2019, 5, eaaw9569.
-

- 9. Wang, C.; Lucas, R.; Smith, A. J.; Cooper, P. R. An In Vitro Screening Assay for Dental Stain Cleaning. BMC Oral Health 2017, 17, 37.
-

- 10. Stookey, G. K.; Burkhard, T. A.; Schemehorn, B. R. In Vitro Removal of Stain with Dentifrices. J. Dent. Res. Dent. 1982, 61, 1236-1239.
-

- 11. Ahn, H. K.; Park, J. H.; Kim, J. E.; Kwon, H. J.; Lee, B. R.; Kim, C. H.; Kim, D. K.; Kim, W. G.; Park, Y. D.; Hwang. K. S. Development of Colored Index of Teeth Due to the Korean Favorite Food Intake. Int. J. Clin. Prev. Dent. 2014, 10, 219-225.
-

- 12. Baig, A. A.; Kozak, K. M.; Cox, E. R.; Zoladz, J. R.; Mahony, L.; White, D. J. Laboratory Studies on the Chemical Whitening Effects of a Sodium Hexametaphosphate Dentifrice. J. Clin. Dent. 2002, 13, 19-24.
- 13. Féliz-Matos, L.; Hernández, L. M.; Abreu, N. Dental Bleaching Techniques; Hydrogen-carbamide Peroxides and Light Sources for Activation, an Update. Mini Review Article Open Dent. J. 2014, 8, 264-268.
-

- 14. Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y. F., Eds.; John Wiley & Sons, Blackwell Publishing Ltd.: London, 2012, pp 21-58.
- 15. Cho, M. J. The Tooth Whitening Effect of Toothpaste Containing High Cleaning Silica and Sodium Hexametaphosphate and the Preventive Effect of Staining by Coffee, Tea and Wine. Int. J. Clin. Prev. Dent. 2020, 16, 192-199.
-

- 16. Watt, B. E.; Proudfoot, A. T.; Vale, J. A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 53-57.
- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2023; 47(3): 303-308
Published online May 25, 2023
- 10.7317/pk.2023.47.3.303
- Received on Dec 1, 2022
- Revised on Feb 2, 2023
- Accepted on Feb 7, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Yongju Jung* , and Wonho Ha
-
LG Science Park, LG Household & Health Care Ltd., Seoul 07795, Korea
*School of Energy Materials Chemical Engineering, KOREATECH, Cheonan 31253, Korea - E-mail: yjung@koreatech.ac.kr, hha@lghnh.com
- ORCID:
0000-0001-5611-6166, 0000-0002-3222-1452








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.