- Drug-Releasing Type Teeth Anti-Aging Coating Agent Using Shellac and Polyvinyl Acetate Composites
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- 셀락과 폴리비닐아세테이트를 이용한 약물 방출형 치아 안티-에이징 코팅제
단국대학교 화학공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
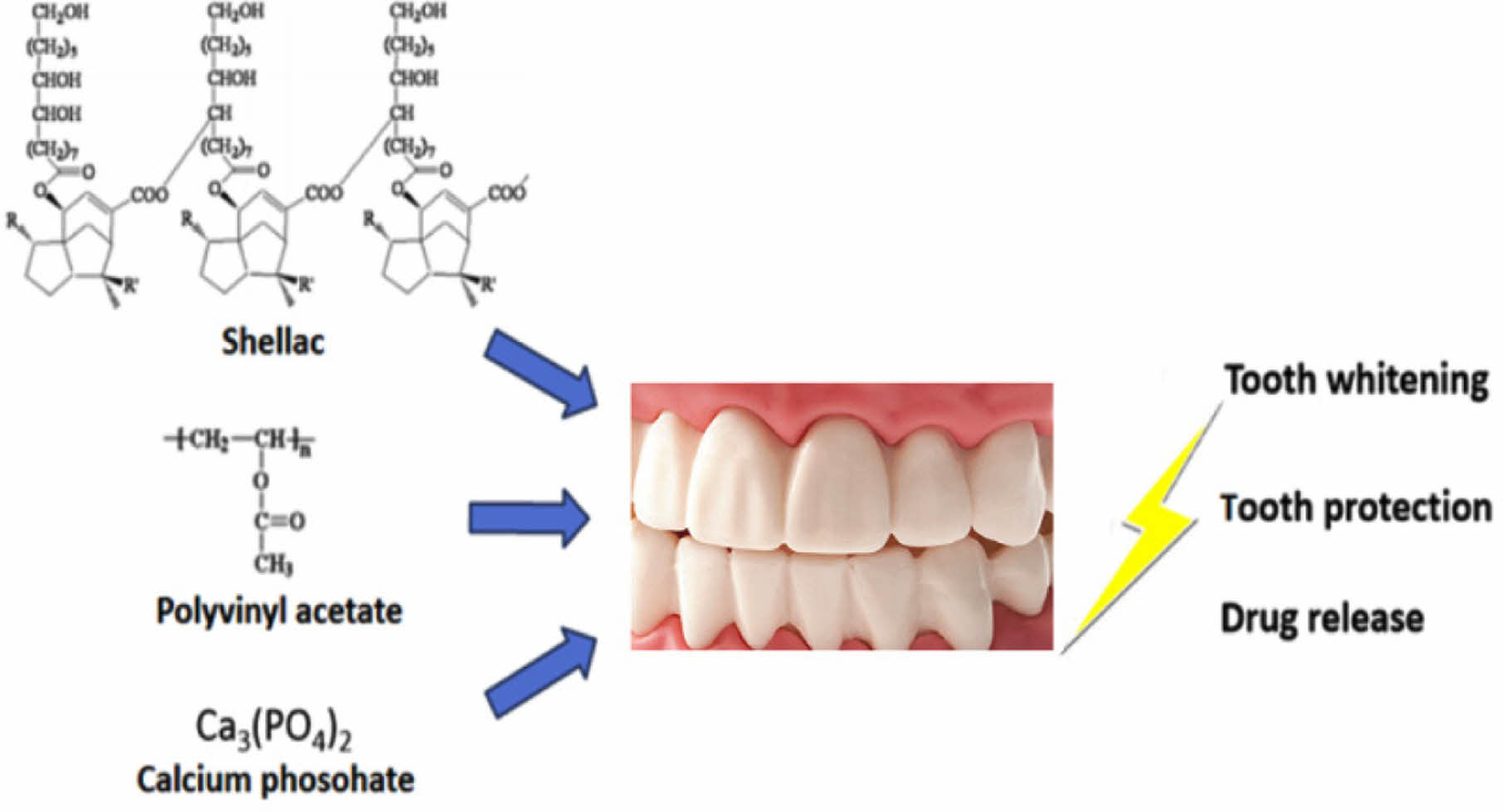
Shellac and polyvinyl acetate (PVAc) composites were prepared to protect teeth and obtain a drug release effect. When shellac is used alone, low friction resistance and a easily broken film are formed, but by added polyvinyl acetate and calcium phosphate, the friction resistance is greatly improved, and a stable film state is obtained. The optimal PVAc and calcium phosphate concentration could be obtained through tests using various concentrations. Drug release behavior was tested at neutral and acidic pH through a dye release test, and the death of U-87MG cells was observed in a release test using temozolomide. The composite prepared in this study can be used as a multi-purpose tooth coating agent that can achieve the effect of protecting teeth, coloring specific colors, and releasing drugs.
치아를 보호하고 약물방출 효과을 얻기 위해 셀락과 폴리비닐아세테이트 복합체를 제조하였다. 셀락을 단독으로 사용시 마찰에 대한 낮은 저항성과 잘 부서지는 피막이 형성되었으나 폴리비닐아세테이트와 칼슘포스페이트를 혼합해준 후 마찰 저항성이 크게 향상되었으며, 안정적인 피막 상태를 얻을 수 있었다. 다양한 농도를 이용한 시험을 통하여 최적의 폴리비닐아세테이트와 칼슘포스페이트 농도를 구할 수 있었다. 염료 방출 시험을 통하여 중성 pH에서 약물방출 거동을 시험하였고, 테모졸로마이드를 활용한 방출 시험에서는 U-87MG 세포의 사멸을 관찰 할 수 있었다. 본 연구에서 제조된 복합물은 치아 보호 효과와 특정 색상의 착색 효과, 그리고 약물 방출효과, 이렇게 1석 3조의 효과를 거둘 수 있는 다목적 치아 코팅제로 적용이 가능할 것이다.
Shellac and polyvinyl acetate composites were prepared to protect teeth and obtain a drug release effect. When shellac is used alone, low friction resistance and a easily broken film are formed, but by added polyvinyl acetate and calcium phosphate, the friction resistance is greatly improved, and a stable film state is obtained.
Keywords: coating agent, anti-aging, teeth, shellac, polyvinyl acetate.
The present research was conducted by the research fund of Dankook University in 2022
The authors declare that there is no conflict of interest.
Dental sealants are commonly used in clinical practice in hospitals. As a passive and defensive concept, dental sealant is a thin, plastic coating painted on the chewing surfaces of teeth to prevent tooth decay. The sealant quickly bonds into the depressions and grooves of the teeth, forming a protective layer over the enamel of each tooth. Also, sealants protect these vulnerable areas from tooth decay by “sealing out” plaque and food.1-3 Different types of coatings are used in dentistry to prevent the development of carious lesions, and tooth surfaces are coated with their ions varnishes4 and/or resins.5 In the case of fluorides, fluoride ions may diffuse from the coating into the surface of the tooth, thereby improving the acid resistance of the tooth surface.6 By placing a coating on some surfaces one may also be able to modify a color and make a surface less receptive to staining.7
On the other hand, an active and aggressive concept of tooth coating is based on coating whole tooth. The whole tooth coating is a preemptive dental treatment applied before serious erosion, wear or caries occur on the teeth.
One of the most important advantages of tooth coating is that it extends the life of the tooth significantly. Of course, having aesthetically positive results is another advantage of dental coating treatments.8,9 Despite various advantages, there is a clear limit to applying the existing materials for tooth coating to the whole tooth coating. For example, glass ionomers can bind to the local Umbrella region of the tooth, but the setting time is too short to apply on the whole tooth, and the impact strength is low, which can cause coating membrane to break during conversation or by food.
In the case of photopolymerized acryl resins, the release of unreacted monomer is burdensome to proceed with photopolymerization on the whole tooth due to the large amount of unhealthy resin monomers.10,11 Also, all of these methods are very foreign when applied to the teeth, making it inconvenient for users to use. Therefore, studies on polymer materials for coating the entire teeth have been actively conducted. Nevertheless, when a polyvinyl alcohol, polyvinyl pyrrolidone, or cellulose-based water-soluble polymer is used, a problem that a coated film melts occurs, and it is impossible to make a crosslinking reaction on the tooth surface. In addition, most of the polymers dissolved in ethanol have weak physical properties, and there are not many types.12,13 Therefore, due to the type of solvent that can be used and the limitations of the physical properties of the polymer, the development of materials that can be expected to be commercialized is not well developed.14-18
Shellac is a natural, nontoxic, biocompatible, and biodegradable polymer that is generally recognized as a safe substance by the FDA. Figure 1 shows the molecular structure of the shellac. As shown in the figure, shellac is known as a natural polymer with acidity. The polymer is a resinous exudate from the Laccifer lacca insect inhabiting tropical forests. Commercially, shellac is available as an alkaline aqueous solution, which is applied as an enteric or sustained release coating polymer. Formerly, shellac was used mostly as an alcoholic solution. In recent years, it has gained considerable attention as a biomaterial with diverse applications in tissue engineering owing to its low cost, large-scale availability and biocompatibility.19-21
In this study, a composite polymer comprising shellac, polyvinyl acetate as an strength enhancer, and calcium phosphate was selected because of its stability, film-forming properties, and enhanced strength.
Thus, the objectives of this study were i) to design novel dental coating composite based on shellac and polyvinyl acetate ii) to evaluate the mechanical properties of membrane and drug release potential, and iii) to evaluate the GBM (U-87MG) cell cytotoxicity by drug release effect.

|
Figure 1 Chemical structure of shellac. |
Materials. In this study, 31.4% (w/v) shellac solution (ES Food Co, Gyeong-gi-do, Korea) in ethanol was used as the base for the dental coating base resin. Calcium phosphate [Ca3(PO4)2], ethanol, polyvinyl acetate, and temozolomide were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade. Ultrapure water from a Milli-Q water system was used to prepare the aqueous solutions.
Fabrication of Shellac Composites and Measurement of Mechanical Properties. Shellac, calcium phosphate, and polyvinyl acetate were prepared in the same ratio as shown in Table 1. The solvent was ethanol, and the final volume of each sample was 20 mL. 2 mL of the resulting solutions were injected into a Teflon plates (30 × 30 × 5 mm) with an overhead projector (OHP) film at the bottom, dried at room temperature for 1 week, and then vacuum dried for 24 hours. The obtained film was washed with pure water for 2 hours and then vacuum-dried.
For the crack occurrence test at various bending angles, shellac composite films coated on the overhead projector (OHP) films were prepared using calcium phosphate and polyvinyl acetate of the composition shown in Table 1. A 5 N load cell with a crosshead speed of 1 mm/min (bending angle = 180°) was used for this purpose. While bending the film for each angle, the film was visually checked for cracks, and the bending angle was measured when cracks occurred.
The surface hardness of the shellac composite films were determined by performing a hardness test using an Imoto IMC-1552 pencil scratch hardness tester according to the JIS K5600-5-4 standard (equivalent to ISO/DIS 15184). In this study, the pencil scratch hardness test was conducted based on 5B, which allows stable formation of membrane when applied to teeth, but can be cleaned when brushing teeth.
Surface Morphology, and Dispersion. Surface morphology, and dispersion state of calcium phosphate were observed using a digital microscope (digital microscope: A-2111; Dino-Lite, Taiwan).
Measurement of Shear Bond Strength. The shear bond strength to dentin was measured as a function of teeth protection coating agent. To prepare a dentin surface with embedded acryl resin, freshly extracted human molar teeth were polished with 600 grit sand paper using a polishing machine (RotoPol-25; Struers, Ballerup, Denmark). Then, each composites solution was placed in a Teflon mold (diameter = 4 mm, height = 7 mm) laid on a polished dentin surface. After 2 weeks at 37 ℃, the mold was removed from the dried composite sample. The specimens were then immersed in distilled water at 37 ℃ for 1 h. The shear bond strength was measured using a universal testing machine. The crosshead speed was set to 1 mm/min, and the load required to debone the specimen from the dentin was determined.
Measurement of Cell Cytotoxicity. Cytotoxicity tests of the shellac composite were performed using a previously described method.22 For this, the shellac composite films were pre-wetted with a medium [Dulbecco’s modified Eagle’s medium supplemented with 2 mM L-glutamine and 10% fetal bovine serum] and incubated at 37 ℃ in a 5% CO2 environment. After 12 hours, the medium was aspirated, and NIH3T3 cells (ATCC-L929, Manassas, VA, USA) were plated directly on each film in a 200 µL media suspension. The cell density was set at 3×104 cells per well. After another 1 hour, 1800 µL medium was added to each well, and the initial adhesion and proliferation were quantified using a WST-8 assay
Dye Release Test from Shellac Composite Film. Drug release from the shellac composite film was simulated using the dye acid blue-25. In Table 1, acid blue-25 (0.5 mg/mL) was mixed with the composition of Sample No. 4 to prepare a film. Without going through a separate washing step, the film was incubated and recovered at 37 ℃ at 50 mL of pH 7.0 PBS and pH 4.5 sodium acetate buffer for 0, 1, 2, 3, 5, 7, and 9 hours, concentrated, and put into a 96-well plate. The amount of the released dye was determined by measuring the absorbance at 600 nm using a microplate reader.
Temozolomide (TMZ) Release Test and Cytotoxicity. Drug release from the shellac composite was also simulated using TMZ. In Table 1, TMZ (1 mg/mL) was mixed with the composition of Sample 4 to prepare a film. Without going through a separate washing step, the film was incubated in the cell culture plate. The U-87GM cells were added directly to each plate in 200 µL of media (Dulbecco’s modified Eagle’s medium with 2 mM l-glutamine and 10% fetal bovine serum) suspension. The cell density was set at 2 × 104 cells per well. After 1 h, 1800 µL of the medium was added to each well and incubated at 37 ℃ and 5% CO2. The proliferation was quantified after 4, 24, 48, 72, and 96 hours using a WST-8 assay.
|
Table 1 Mechanical properties according to polyvinyl acetate (PVAc), shellac, and calcium phosphate (CP) contents |

×: No scratche, ○: Scratch occurred. |
Mechanical Properties According to the Composition of the Composite. The surface hardness and bending angle of a film manufactured with various compositions with different amounts of shellac, polyvinyl acetate, and calcium phosphate. As shown in Table 1, when calcium phosphate and polyvinyl acetate were generally contained, surface hardness and bending angle tended to increase. First of all, for polyvinyl acetate, an improvement in physical properties was observed in about 9% or more, and the properties were improved in accordance with the content of calcium phosphate. On the other hand, in case of 0.5 mg/mL of calcium phosphate, it is expected that the pencil hardness test result or bending angle is generally low due to an increase in surface roughness caused by a decrease in particle dispersion. Therefore, it is judged that more than 9% of polyvinyl acetate and 0.1 mg/mL of calcium phosphate are appropriate through this test. We suggest that polyvinyl acetate and shellac are partially anionic polymers, and calcium phosphate forms ionic bonds between these polymer chains, which are considered to have increased strength due to the crosslinking effect.
Measurement of Dispersion State of Calcium Phosphate Particles. The dispersion state of calcium phosphate particles and the surface morphology of the films were studied using a digital microscope. The test was conducted with different amounts of calcium phosphate according to the composition of Sample No. 4 in Table 1. As shown in Figure 2, the dispersion states of calcium phosphate particles on the shellac composites at 0, 0.1, and 0.2 mg/mL of calcium phosphate were similar in a homogeneous state. Meanwhile, composites with more than 0.5 mg/mL concentration showed an increase in clumps and surface roughness because of the lower dispersion efficiency of the white calcium phosphate particles. We suggest that the increased clumps and surface roughness are due to the increased amount of calcium phosphate particles and the cohesion phenomenon between each other. Therefore, in this dispersion test, if the concentration of calcium phosphate is less than 0.5 mg/mL, the dispersion state is generally good.
Result of Measurement of Shear Bond Strength. Shear bond strength was measured in the same composition as the dispersion test. The composite by calcium phosphate 0.1 mg/mL showed more than 1.8-fold increase in the bond strength compared with the composite by calcium phosphate 0.5 mg/mL, as shownin Figure 3. The high bond strength of the specimens of the composite by calcium phosphate 0.1 mg/mL was because of the enhanced strength by ionic crosslinking effect by calcium phosphate and ionic bond between shellac as an anionic polymer and calcium on dentin. Meanwhile, lower bond strength was observed in the composites of 0.2 and 0.5 mg/mL. This lower strength might have been because of the decreased bond strength with shellac by competitive reaction between calcium on dentin and calcium phosphate. In addition, in the case of 0.5 mg/mL, the lowest binding strength was indicated. It is believed to be the result of the effect of particles clumping together with the competitive reaction between ions. Therefore, as a result of this shear bond strength test with dentin, 0.1 mg/mL composition is considered to be a suitable composition for improving adhesion to the tooth surface.
Cytotoxicity of Shellac Composites. For cell toxicity evaluation, NIH3T3 cells were cultured on composite films with different amounts of calcium phosphate according to the composition of Sample No. 4 in Table 1. The initial cell adhesion and proliferation were affected by the calcium phosphate concentration, except in case of the shellac/PVAc film prepared without calcium phosphate. However, low cell adhesion and proliferation were observed in the shellac/PVAc composite prepared without calcium phosphate (Figure 4). The low cell adhesion and proliferation efficiency is expected to be attributed to the shellac's slightly swollen properties and smooth surfaces in neutral aqueous solutions. On the other hand, it was found that as the amount of calcium phosphate increased, the initial adhesion efficiency and proliferation of cells improved. It is determined that this is a result of increasing the roughness of the film surface as the amount of calcium phosphate increases. In addition, cytotoxicity was not observed in the overall composition.
Dye Release Behavior from Shellac Composite. The emission behavior of the dye was investigated under pH 4.5 and 7.
At pH 4.5, dye release from composite film was not observed, whereas drug release began to increase gradually by increasing the pH of the solution (pH 7) (Figure 5). According to a previous study,23 the pKa of shellac is approximately 6, and the pH of the pure shellac solution is 4. We hypothesized that dye release from shellac was due to ionized shellac at pH values greater than 7. Also, it was confirmed that the drug release behavior can be adjusted according to pH, and in this study, a study was conducted based on pH 7.
Therefore, a detailed cell investigation was conducted to confirm the effectiveness of the drug.
Cytotoxicity by Emission of Temozolomide (TMZ) from Shellac Composite Film. TMZ is a glioblastoma cytotoxic drug licensed by the U.S. FDA and has been used in clinical practice since early 2000. While culturing U-87MG cells using a human glioblastoma cells (GBM), experiments were conducted on the release of drugs and cytotoxicity of the shellac composite film containing TMZ. As shown in Figure 6, unlike in the absence of drugs, cytotoxic effects were confirmed on drugs released in a neutral pH environment, and differences in cell survival rate were clearly observed after 24 hours. Unlike the dye release test, we suggest that the reason for the clear difference after 24 hours is that the released drug flows into the cell and it takes time to react with DNA.

|
Figure 2 Surface morphological study and dispersion state of shellac composite according to calcium phosphate using a digital microscope calcium phosphate: (a) 0; (b) 0.1; (c) 0.2; (d) 0.5 mg/mL, ×250, scale bar: 400 µm. |

|
Figure 3 Shear bond strength of shellac composites according to calcium phosphate (n=5, * P < 0.05). |

|
Figure 4 Cell viability of shellac composites according to calcium phosphate (TCP: tissue culture plate, n=5, * P < 0.05). |

|
Figure 5 Differences in the behavior of Acid blue-25 release from shellac/PVAc/CP composite film at 37 ℃ (n=5). |

|
Figure 6 Differences in the cytotoxicity by temozolomide release from shellac/PVAc/CP composite film. Picture in the diagram shows a photograph of a cell after 96 hours: (a) without a drug; (b) with a drug, ×40, n=5, * P < 0.05, **P < 0.05. |
Dental anti-aging coating agent was prepared by a simple method using a shellac, polyvinyl acetate, and calcium phosphate in ethanol solution. The shear bond strength, surface hardness, crack angle, drug release, and cytotoxicity of the composites were studied to determine the optimal composition for tooth anti-aging coating agent applications. It was found from the mechanical property tests that 9% PVAc, 91% shellac, and 0.1 mg/mL calcium phosphate was the most effective composition to increase the strength and adhesive properties of the tooth coating. In future research, it is required that more sophisticated drug release tests, application of biological evaluation. Through this study, the shellac composite shows promise for use for tooth whitening, and as protecting agent in the field of dentistry.
- 1. Kloukos, D.; Pandis, N.; Eliades, T. In Vivobisphenol-A Release from Dental Pit and Fissure Sealants: A Systematic Review. J. Dent. 2013, 41, 659-667.
-

- 2. Oong, E. M.; Griffin, S. O.; Kohn, W. G.; Gooch, B. F.; Caufield, P. W. The Effect of Dental Sealants on Bacteria Levels in Caries Lesions: A Review of the Evidence. J. Am. Dent. Assoc. 2008, 139, 271-278.
-

- 3. Zhao, X.; Pan, J.; Malmstrom, H. S.; Ren, Y. F. Protective Effects of Resin Sealant and Flowable Composite Coatings Against Erosive and Abrasive Wear of Dental Hard Tissues. J. Dent. 2016, 49, 68-74.
-

- 4. Daneshkazemi, P.; Sadeghian, S.; Khodaei, M. Shear Bond Strength of Orthodontic Brackets on Intact and Demineralized Enamel After Application of Resin Infiltrant, Fluoride Varnish and Casein Phosphopeptide-amorphous Calcium Phosphate Remineralizing Agents: In Vitro Study. Int. Orthod. 2021, 19, 259-268.
-

- 5. Söderholm, K. J. M. Coatings in Dentistry-A Review of Some Basic Principles. Coatings 2012, 2, 138-159.
-

- 6. Cochrane, N. J.; Cai, F.; Huq, N. L.; Burrow, M. F.; Reynolds, E. C. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187-1197.
-

- 7. Lung, C. Y. K.; Matinlinna, J. P. Aspects of Silane Coupling Agents and Surface Conditioning in Dentistry: An Overview. Dent. Mater. 2012, 28, 467-477.
-

- 8. Praveen, A. S.; Arjunan, A.; Baroutaji, A. Coatings for Dental Applications. Encyclopedia of Smart Materials 2022, 1, 426-435.
-

- 9. Lim, J. I. Tooth Whitening and Protecting Effect Using TiO2/PLCL Biodegradable Polymer Composites. Polym. Korea 2021, 45, 421-427.
-

- 10. Pérez-Mondragón, A. A.; Cuevas-Suárez, C. E.; González-López, J. A.; Trejo-Carbajal, N.; Meléndez-Rodríguez, M.; Herrera-González, A. M. Preparation and Evaluation of a BisGMA-free Dental Composite Resin Based on a Novel Trimethacrylate Monomer. Dent. Mater. 2020, 36, 542-550.
-

- 11. He, J.; Kopperud, H. M. Preparation and Characterization of Bis-GMA-free Dental Composites with Dimethacrylate Monomer Derived from 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene. Dent. Mater. 2018, 34, 1003-1013.
-

- 12. Wang, Y.; Zhu, M.; Zhu, X. X. Functional Fillers for Dental Resin Composites. Acta Biomater. 2021, 122, 50-65.
-

- 13. Alavi, S. E.; Panah, N.; Page, F.; Gholami, M.; Dastfal, A.; Sharma, L. A.; Shahmabadi, H. E. Hydrogel-based Therapeutic Coatings for Dental Implants. Eur. Polym. J. 2022, 181, 111652-111657.
-

- 14. Cebe, M. A.; Cebe, F.; Cengiz, M. F.; Cetin, A. R.; Arpag, O. F.; Ozturk, B. Elution of Monomer from Different Bulk Fill Dental Composite Resins. Dent. Mater. 2015, 31, e141-e149.
-

- 15. Amirouche-Korichia, A.; Mouzalib, M.; Watts, D. C. Shrinkage Strain – Rates Study of Dental Composites Based on (BisGMA/TEGDMA) Monomers. Arab. J. Chem. 2017, 10, S190-S195.
-

- 16. Habib, E.; Wang, R.; Zhu, X. X. Correlation of Resin Viscosity and Monomer Conversion to Filler Particle Size in Dental Composites. Dent. Mater. 2018, 34, 1501-1508.
-

- 17. Srivastava, R.; Liu, J.; He, C.; Sun, Y. BisGMA Analogues as Monomers and Diluents for Dental Restorative Composite Materials. Mater. Sci. Eng. C 2018, 88, 25-31.
-

- 18. Wu, X.; Dai, S.; Chen, Y.; He, F.; Xie, H.; Chen, C. Reinforcement of Dental Resin Composite via Zirconium Hydroxide Coating and Phosphate Ester Monomer Conditioning of Nano-zirconia Fillers. J. Mech. Behav. Biomed. Mater. 2019, 94, 32-41.
-

- 19. Kumpugdee-Vollrath, M.; Tabatabaeifar, M.; Helmis, M. New Coating Materials Based Onmixtures of Shellac and Pectin for Pharmaceutical Products. Int. Scholar Sci. Res. Innov. 2014, 8, 21-29.
- 20. Chauhan, O. P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P. S. Shellac and Aloe Vera Gel Based Surface Coating for Shelf Life Extension of Tomatoes. J. Food Sci. Technol. 2015, 52, 1200-1205.
-

- 21. Phaechamud, T.; Setthajindalert, O. Antimicrobial In Situ Forming Gels Based on Bleachedshellac and Different Solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285-293.
-

- 22. Landegren, T.; Risling, M.; Persson, J. K. E.; Sondén, A. Cyanoacrylate in Nerve Repair: Transient Cytotoxic Effect. Int. J. Oral Maxillofac. 2010, 39, 705-712.
-

- 23. Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M. H.; Zhang, X.; Li, B.; Li, L. Shellac: A Promising Natural Polymer in the Food Industry. Trends Food Sci. Technol. 2021, 109, 139-153.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2023; 47(3): 326-331
Published online May 25, 2023
- 10.7317/pk.2023.47.3.326
- Received on Jan 6, 2023
- Revised on Mar 8, 2023
- Accepted on Mar 9, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jin Ik Lim
-
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- E-mail: limjinik@dankook.ac.kr
- ORCID:
0000-0003-4803-0455









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.