- Influence of Polycaprolactone/Collagen Ratio and Microstructure of Fibrous Membranes on Mechanical Properties and Anti-Platelet Properties
Jia-Shuai Zhang*,#, Hui-Lin Mo*,#, Shun-Yang Shen*,#, Du-Han Zhang*, Wei Zhang*, **,†
 , Yu Ren*, **,†
, Yu Ren*, **,†  , and Mei-Xian Li*, **,†
, and Mei-Xian Li*, **,† 
*School of Textile and Clothing, Nantong University, Nantong 226019, China
**National and Local Joint Engineering Research Center of Technical Fiber Composites for Safety and Protection, Nantong University, Nantong 226019, China- Polycaprolactone/Collagen 비율과 섬유 막의 미세 구조가 기계적 특성 및 항혈소판 특성에 미치는 영향 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this paper, polycaprolactone (PCL)/collagen (COL) nanofibrous membranes were prepared by electrospinning to study their mechanical properties as well as biocompatibility. The surface morphology was characterized by scanning electron microscopy (SEM) to access the effect of microstructure on the mechanical properties, cytocompatibility, and enzymatic degradation behaviors. In addition, the effect of hydrophilicity of membranes on the antithrombotic properties was also investigated. The results show that tensile strength of PCL/COL10 is higher than that of other samples due to smaller fiber diameters and tight spatial interlacing of PCL/COL10 membranes. In addition, the hydrophobic surface of PCL becomes hydrophilic after the addition of collagen, which affect platelet adhesion and cytocompatibility of nanofibrous membrane samples. The addition of collagen could promote the cell proliferation as well as the enzymatic degradation rate. Overall, PCL/COL10 has good morphology, the best mechanical properties, appropriate degradation rate, and the best antithrombotic effect as well as biocompatibility.
Polycaprolactone (PCL)/collagen (COL) nanofibrous membranes with different COL concentrations were prepared via electrospinning. In the presence of an appropriate concentration of collagen, the mechanical properties, cytocompatibility, and the enzymatic degradation rate increases. PCL/COL with 10 wt% of COL has good morphology, the best mechanical properties, appropriate degradation rate, and the best antithrombotic effect as well as biocompatibility.
Keywords: polycaprolactone, collagen, electrospinning, nanofibrous membrane, biocompatibility, antithrombosis.
This work was supported by Natural Science Foundation of Jiangsu Province (BK20220613), the Key R&D project of Jiangsu Province (BE2023740), the Nantong Science and Technology Project (JC12022080), Provincial College Students' Innovation and Entrepreneurship Training Program (202210304117Y), and Large Instruments Open Foundation of Nantong University.
The authors declare that there is no conflict of interest.
Natural and synthetic polymers have been widely used as implants or substitutes in clinical fields, including vascular grafts, dressing, cartilage, and so on.1,2 These polymers used as biomedical materials should meet appropriate mechanical properties, biocompatibility, and a low immune response.3,4 Furthermore, some blood-contacting biomedical materials require anti-protein adsorption and antithrombotic properties. Meanwhile, tissue regeneration requires the implants or substitutes made from natural and synthetic polymers to provide a suitable microenvironment for cell adhesion, migration, and proliferation.5
Polycaprolactone (PCL), as a synthetic polyester-based biopolymer material, has good biocompatibility and mechanical properties.6 Meanwhile, PCL scaffold with a certain porosity does not have an antigen reaction to the human body, and could sustain cell growth and promote cell adhesion as well as proliferation.7,8 Yin et al.9 prepared PCL/bioactive glass composite porous scaffolds, exhibiting high expression of osteogenic differentiation and accelerating cranial bone regeneration. Shi et al.10 constructed heparin loaded PCL/gelatin hybrid vascular grafts via electrospinning, promoting endothelialization and regulating smooth muscle regeneration. Although PCL has many advantages, there are also its own drawbacks. The inherent defects of poor hydrophilicity, low bioactivity, and slow degradation of PCL limit its wider application.11 One of the effective ways to solve these problems is to modify PCL by blending natural polymer such as collagen, gelatin, silk fibroin for its wider application.12-14
Collagen, a protein with a unique structure, widely exists in mammals accounting for more than 25% of proteins in the whole body,15 especially in bone, tendon sheath, muscle, ligament, muscle membrane, cartilage and skin part, supporting the organs and protecting the muscle. Collagen has widely used as a promising tissue engineering substitute due to its non-toxicity, weak antigenicity, superior biocompatibility, and relatively low price.16. Besides that, collagen is the most important functional protein in the intercellular substance, therefore, it is used widely in the biomedical fields.17 However, it is usually used in the combination with other synthetic polymers owing to its fast degradation rate and low mechanical properties.18 Electrospinning technology is a method of preparing ultra-fine fiber by using electrostatic force as a traction. Nanofibrous membrane prepared via electrospinning show large surface areas and porosity to facilitate to mimic the extracellular matrix for cell attachment and proliferation, exhibiting excellent cytocompatibility.19,20 Therefore, it is widely used in tissue engineering applications.21,22 Several studies on the biocompatibility of PCL/collagen membranes with bioactive materials have been undertaken, but the data for mechanical properties and anti-platelet properties of PCL/collagen mixtures with different ratios without bioactive materials remain insufficient.
The aim of this study was to investigate the effect of collagen contents and the microstructures of PCL and PCL/COL nanofibrous membranes on the mechanical properties and cytocompatibility. In addition, mechanical performance, hydrophilicity, platelet adhesion, cell viability, degradation behavior was investigated, respectively.
Materials. Polycaprolactone (PCL, Mw 45000) was purchased from Macklin (China), and collagen type I (COL) and cell counting kit-8 (CCK-8) were purchased from FeiyuBio Co., Ltd. (China). Lipase was purchased from Bidepharm Co., Ltd., (China), and hexafluoroisopropanol (HFIP, 99.5%) was provided from Aladdin (China) and employed without further purification.
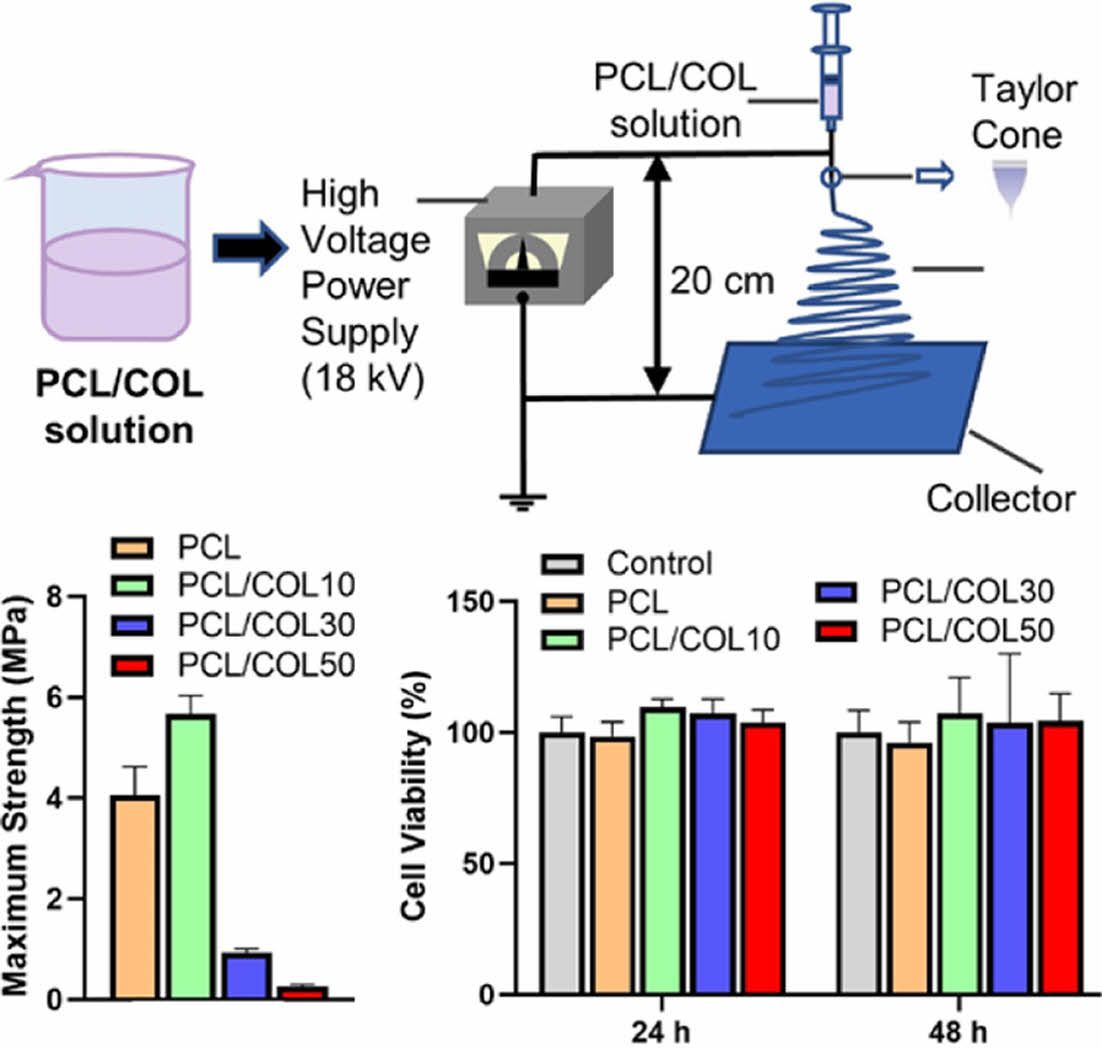
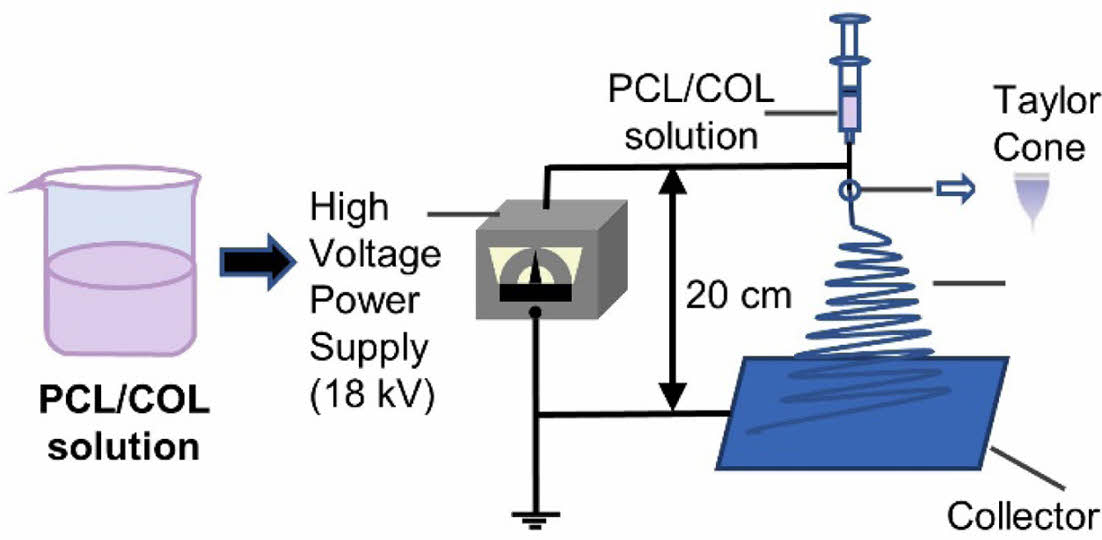
Electrospinning. PCL/COL solution (15 w/v%) was prepared using HFIP as a solvent with 0, 10, 30, and 50 wt% of collagens, and named PCL, PCL/COL10, PCL/COL30, and PCL/COL50, respectively. The electrospinning solution was stirring at room temperature for 4 h, followed by placed in a 10 mL syringe without voids. PCL/COL nanofibrous membranes were collected with a grounded collector with the voltage power of 18 kV and the distance of 20 cm as well as at a flow rate of 1 mL/h (Figure 1). Finally, samples were dried in a vacuum oven to eliminate remnant solvents.
Characterization. The surface morphology of nanofibrous membranes was observed by field-emission scanning electron microscopy (FE-SEM, JSM-6510, JEOL Ltd., Japan) with an accelerating voltage of 5 kV. The hydrophilicity PCL/COL nanofibrous membranes was evaluated using optical contact angle measuring instrument (OCA15EC, DataPhysics Instruments GmbH, Germany). A 2-μL droplet of pure distilled water was dropped on the PCL/COL nanofibrous membranes, and the time was measured from the droplet dropped on the membrane to the contact angle became zero. The mechanical properties of the PCL/COL fibrous membranes were investigated using an Instron 5969 (USA). The specimen has a width of 10 mm, a length of 50 mm, a gauge length of 25 mm, and a load cell of 50 N was used at a crosshead speed of 50 mm/min.23 For each measurement, 3 samples were measured and both the mean value and standard deviation were recorded.
Platelet Adhesion Test. Platelet rich plasma (PRP) was diluted with fresh frozen plasma (FFP) to form a predetermined concentration (1×105 plt/μL). The PCL and PCL/COL nanofibrous membranes were separately placed in 24-well plates and sterilized with 70% ethanol and UV light for 10 min, following by hydrated with PBS solution for 1 h at 37 ℃. After removing solution, 1 ml of platelet solution was added immediately to each well and incubated at 37 ℃ for 2 h. After that, the samples were washed three times with PBS solution to remove residual platelets and then immersed in 2.5% glutaraldehyde solution for 1 h at room temperature. Finally, the samples were dehydrated in a series of ethanol/distilled water solutions (50, 70, 90, 95, and 100% ethanol for 5 min each) and dried completely in a vacuum desiccator for 24 h. Subsequently, the morphology of adherent platelets was observed under field emission scanning electron microscope (FE-SEM, JSM-6510, Japan). For the quantification of adhered platelets, the samples were treated with 2% Triton-X100 solution and incubated at 37 ℃ for 15 min, followed by carried out lactate dehydrogenase (LDH) activity assay.
Cell Viability. The mouse fibroblasts L929 cells (NCTC Clone 929, China) was used to evaluate cell viability on the base of the International Organization for Standardization (ISO 10993-5). In brief, the PCL and PCL/COL nanofibrous membranes were cut into 0.5 cm × 0.5 cm and placed in 96-well plates before sterilization under UV irradiation. After that, L929 cells were seeded on the membranes with the density of 1 × 104 cells/well and incubated at 37 ℃ for a determined time. After that, the medium with 10% CCK-8 solution was added to each well and incubated at 37 ℃ for 2 h. The supernatant optical density (OD) was measured with an Elisa Reader (Infinite 200 Pro, Austria) at 450 nm wavelength, and the relative cell viability was calculated via the eq. (1).
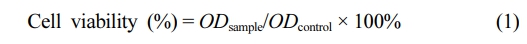
where ODsample and ODcontrol represented the measured absorbance of cells with collagen in different concentration and control group, respectively.
Enzymatic Biodegradation Test. The enzymatic degradation of the PCL and PCL/COL nanofibrous membranes were examined in the presence of lipase (200 U/mL), and weight change of the samples were monitored as a function of time. In brief, the samples were vacuum dried at room temperature for 48 h to a constant weight and weighed (W0), after which they were immersed in PBS containing 200 U/mL of lipase. At predetermined time points, the samples were rinsed three times with distilled water, and then vacuum dried for 48 h to a constant weight and weighed as W1. The weight loss was calculated according to the following formula (eq. (2)):24
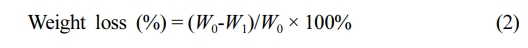
All experiments were performed in triplicate, and the data were expressed as the mean ± standard deviation
Statistical Analysis. The data were stated as mean value ± standard deviation (SD). The one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used to analyze the significance of differences between experimental groups. A value of p*<0.01, **<0.005 was considered to be statistically significant.
|
Figure 1 Schematic diagram of electrospinning apparatus. |
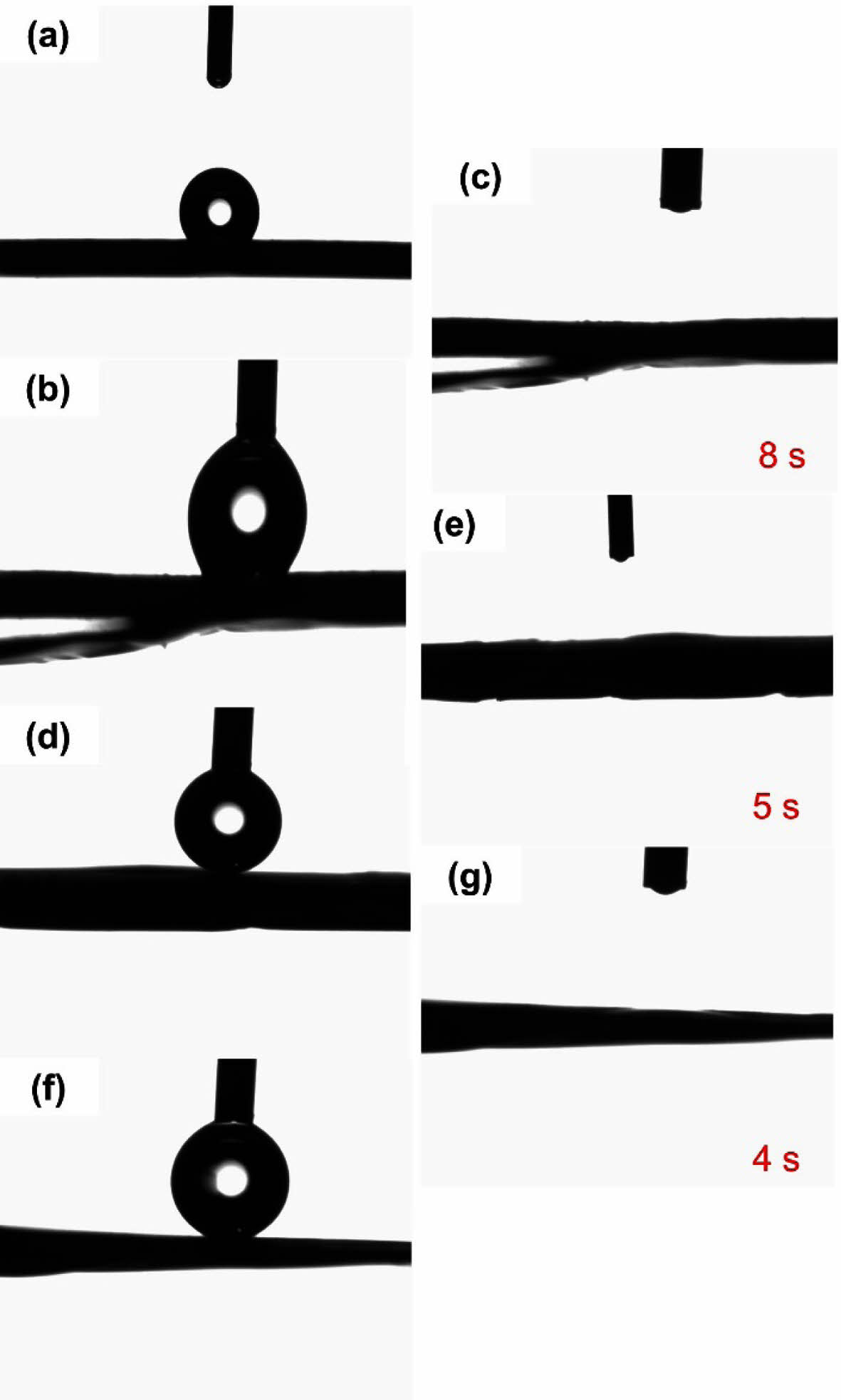
The hydrophilicity of the PCL and PCL/COL nanofibrous membranes was evaluated by determining their water contact angles. As shown in Figure 2, the water contact angle of pure PCL is about 124.9° exhibiting hydrophobicity. However, the contact angle of PCL/COL nanofibrous membranes becomes zero when the water droplet falls on the samples, and it takes 8 s, 5 s, and 4 s for PCL/COL10, PCL/COL30, PCL/COL50 samples, respectively, which indicates that the hydrophilicity is more obvious with the increase of collagen weight percent. Generally, pure PCL contains five methylene groups, causing undesired wettability and prolonged degradation rate, thus it limits its wide application in tissue engineering. In contrast, collagen contains a large number of hydrophilic groups in the chain and exhibits strong hydrophilicity. Therefore, the addition of collagen could improve the hydrophilicity of PCL, and as the weight percent of collagen increases, wetting speed is getting faster.
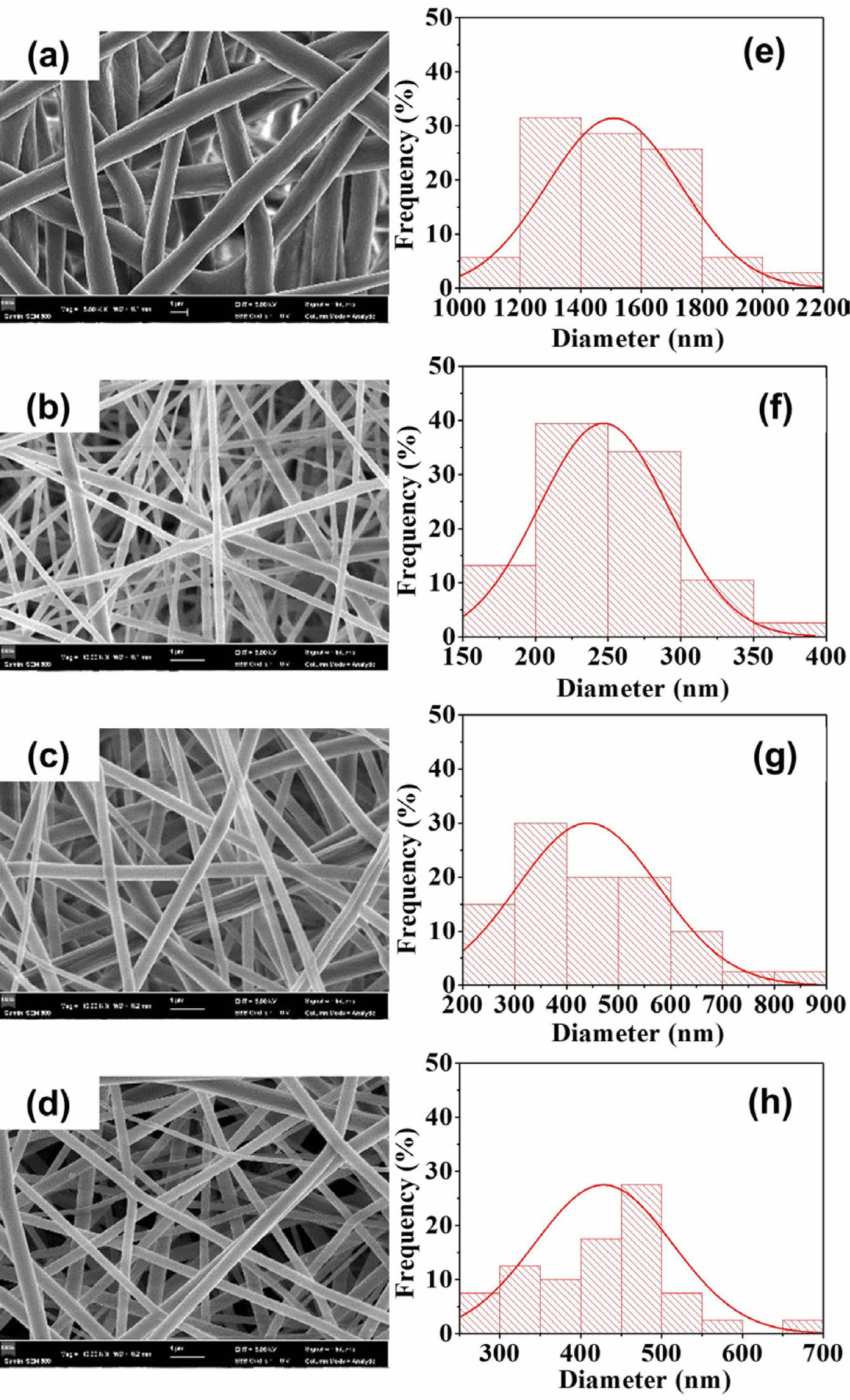
The surface morphology and the fiber diameter distribution of PCL, PCL/COL10, PCL/COL30 and PCL/COL50 nanofibrous membranes were analyzed by SEM observation (Figure 3). It can be seen that the fiber diameter distribution of the PCL is relatively uniform and smooth (Figure 3(a)). However, the fiber diameters of PCL/COL samples are significantly smaller by the addition of collagen owing to low viscosity of collagen compared to pure PCL. In addition, as the weight percent of collagen increases, the fiber diameter distribution becomes not uniform, especially for PCL/COL50 samples. Among them, PCL/COL10 and PCL/COL30 have relatively better surface morphology with suitable fiber diameters and porosity.
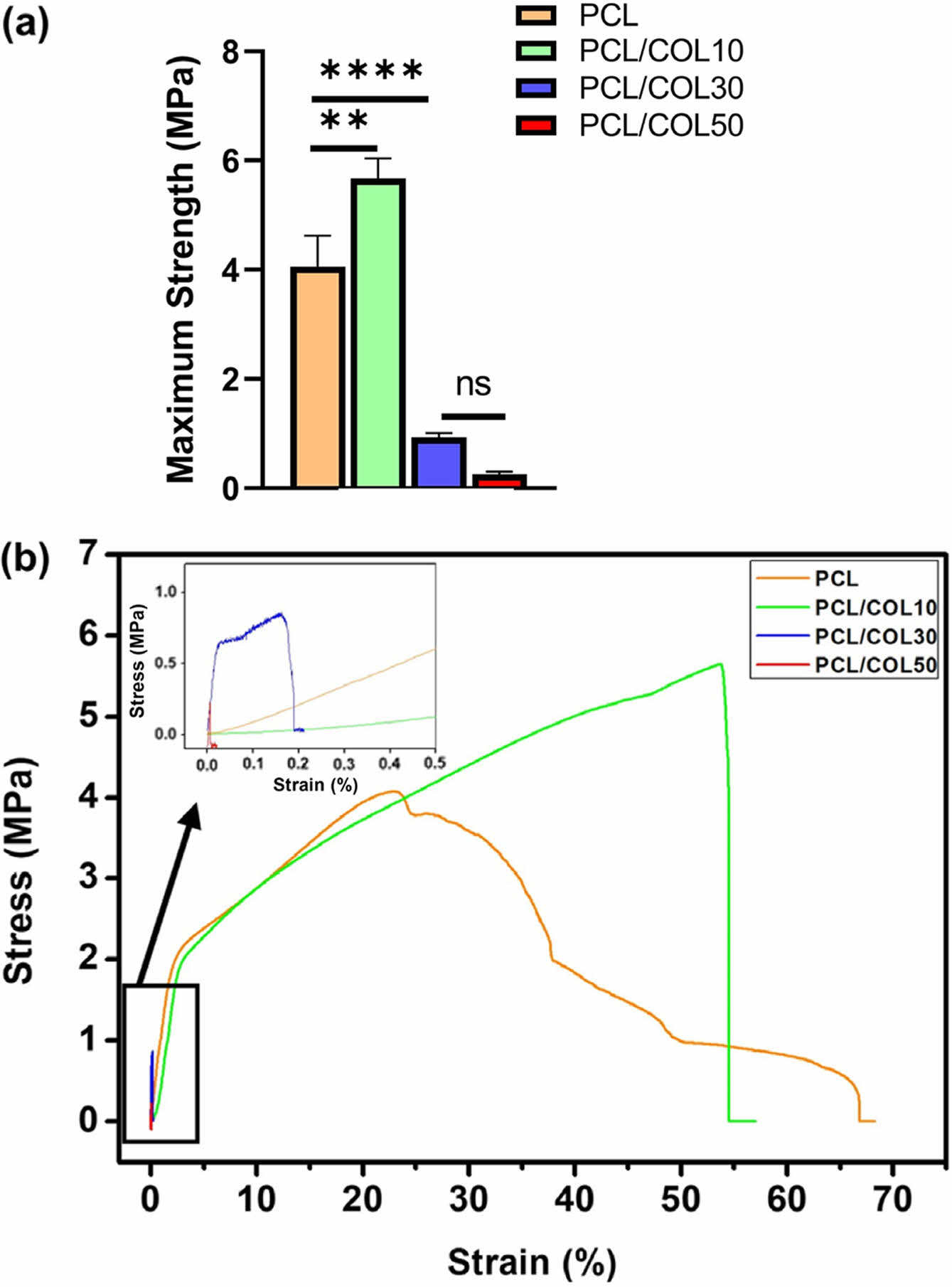
The mechanical performance of pure PCL and PCL/COL nanofibrous membranes are shown in Figure 4. The results show that the tensile strength of pure PCL, PCL/COL10, PCL/COL30, PCL/COL50 were 4.06 ± 0.56 MPa, 5.67 ± 0.37 MPa, 0.93 ± 0.08 MPa, 0.26 ± 0.04 MPa, respectively. Among all the samples, PCL/COL10 has the highest tensile strength, while that of PCL/COL30 and PCL/COL50 significantly decreases limiting their wider application. The reason may be related to their microstructures. As shown in Figure 3, nanofibers were randomly arrayed with porous structures; however, the average fiber diameters of PCL/COL10 are smaller than those of pure PCL, and spatial interlacing of PCL/COL10 is more tightly arranged compared with PCL leading to better tensile strength of PCL/COL10 than that of pure PCL, which are in accordance with the previous studies.25,26 However, as the amount of collagen increases, tensile strength of PCL/COL decreases due to relatively low viscosity of PCL/COL which is unfavorable to electrospinning and hinder fiber formation, causing uneven distribution of fiber diameters for PCL/COL30 and PCL/COL50.
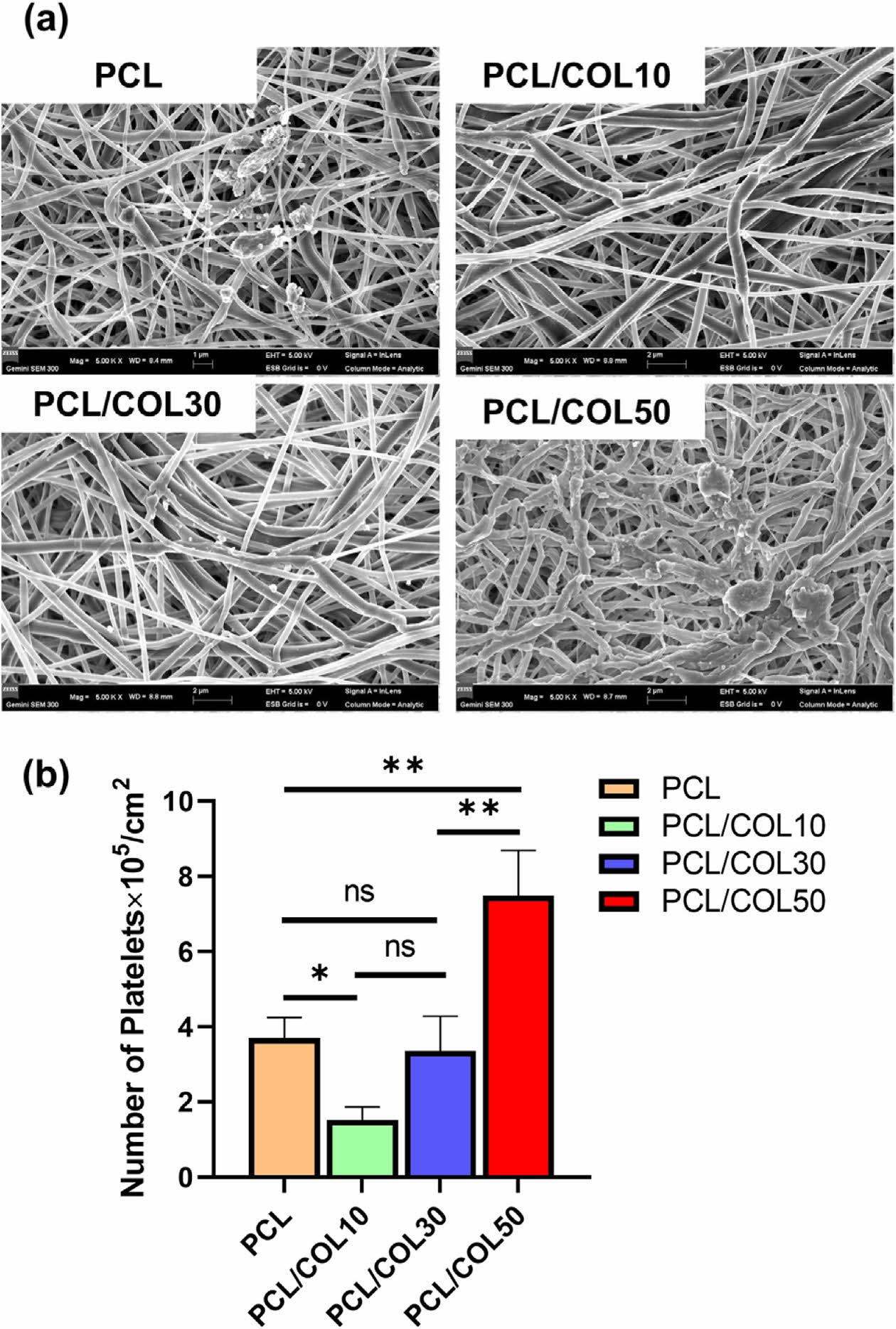
Platelet adhesion and activation is an important factor for blood-contacting materials. When fibrous membranes contact with blood, they will adsorb plasma proteins triggering the activation of platelets. In this process, platelets not only activate a variety of clotting factors, but also destroy red blood cells and white blood cells causing blood formation. In this study, in vitro platelet adhesion observed by SEM to assess the anti-platelet properties of PCL and PCL/COL membranes. As shown in Figure 5(a), a certain platelet is adhered on pure PCL membranes which is a hydrophobic polymer that is favorable for protein or platelet adhesion, whereas the number of platelet adhered on PCL/COL10 decreases. It may be the reason that the addition of collagen makes the PCL surface hydrophilic, and hydrophilic or superhydrophobic surface could reduce the platelet adhesion.27,28 However, as the content of collagen increases, the number of attached platelets decreases because the triple helix structure of collagen could induce platelet adhesion and activation.29,30
In addition, quantitative analysis was also carried out through LDH assay. Figure 5(b) shows that the number of attached platelets adhered on PCL/COL10 was less than half on that pure PCL which is consistent with the SEM images above.
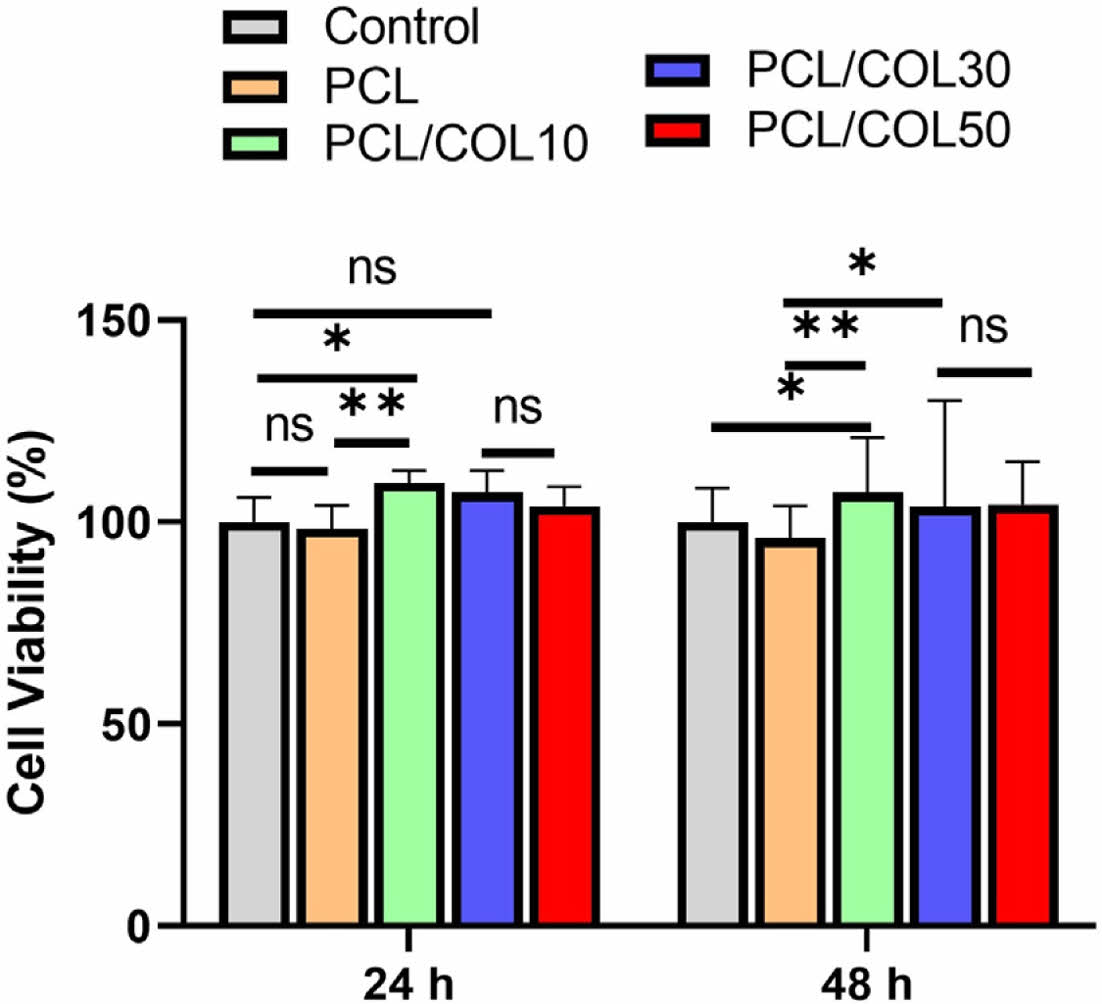
Generally, PCL is known as biocompatible materials, and they are widely used in biomedical application.31 Cytotoxicity of PCL and PCL/COL membranes with varying collagen contents ranging from 0, 10, 30, and 50% determines whether the PCL/COL samples will have toxic effects on L929 cells incubated for 24 h and 48 h.32,33 As shown in Figure 6, all the groups showed more than 95% cell viability and PCL with collagen exhibits higher cell viability in comparison with pure PCL, indicating that PCL itself as well as the collagen blended PCL samples are compatible with cells. Furthermore, the viability of L929 cells cultured on PCL/COL membranes for 48 h is higher than that of pure PCL, indicating good cytocompatibility of PCL/COL. In addition, the collagen has favorable effect on the initial cell adhesion and further cell growth due to its hydrophilicity.26 Moreover, the microstructure of PCL and PCL/COL is also an important factor for cell adhesion and cell proliferation, which providing a potential for tissue reconstruction.
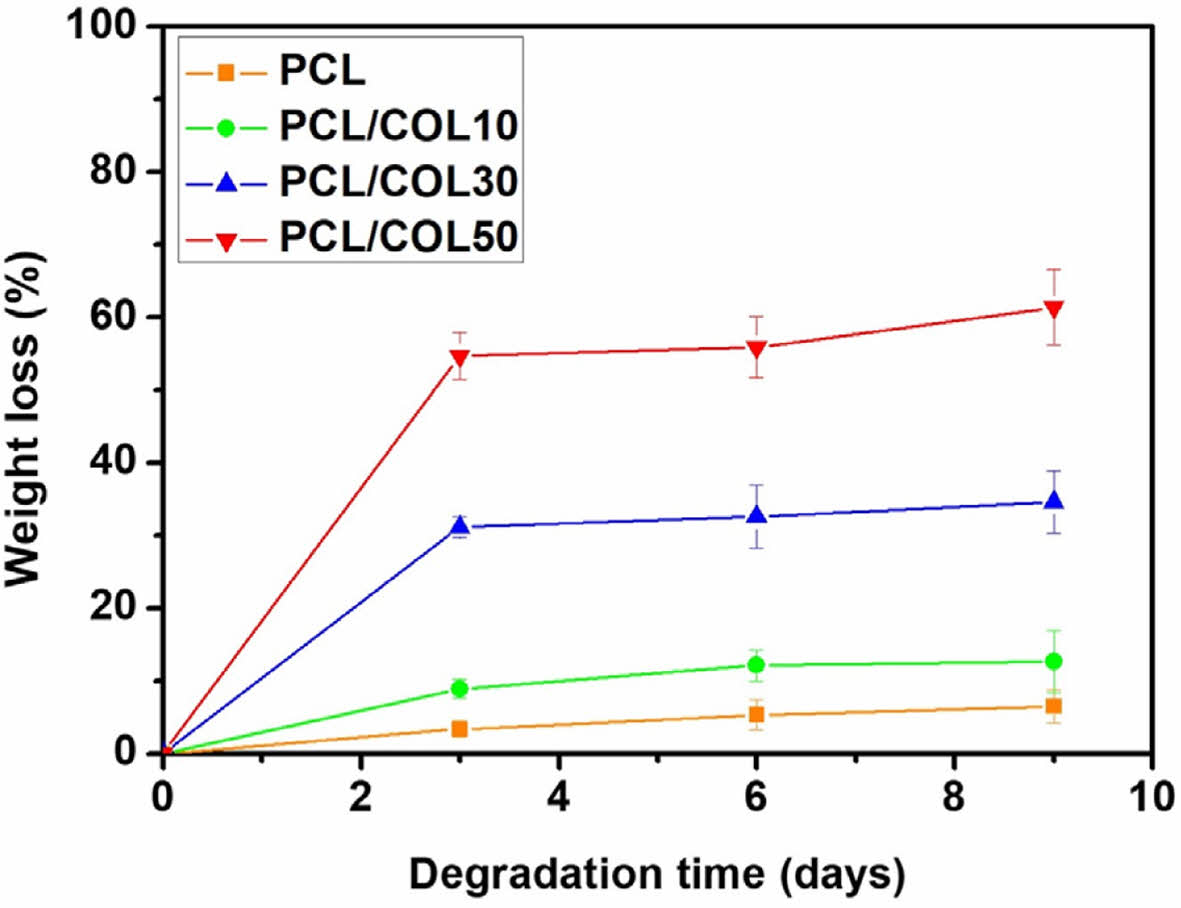
In vitro enzymic degradation test was carried out in the presence of lipase. Figure 7 shows the enzymatic degradation behavior of PCL and PCL/COL membranes. It can be found that PCL/COL membranes quickly degraded at the early stage, whereas it became slower after 3 days. However, pure PCL shows slow degradation behavior in lipase solution during the whole degradation process. The internal structure of nanofibrous membrane became looser in the beginning, subsequently the nanofibers began to break.34 The weight loss is 6.49%, 12.69%, 34.62%, and 61.41% after 9 days for PCL, PCL/COL10, PCL/COL30, and PCL/COL50, respectively, indicating that the degradation rate of PCL becomes faster as the collagen contents increases and it can be controlled by the addition appropriate amount of collagen.
|
Figure 2 Water contact angle images of (a) PCL; (b) PCL/COL10; (c) PCL/COL10 after 8 s; (d) PCL/COL30; (e) PCL/COL30 after 5 s; (f) PCL/COL50; (f) PCL/COL50 after 4 s. |
|
Figure 3 SEM images and diameter distributions of four groups of PCL/collagen composite fiber membranes: (a-d) microstructure of PCL, PCL/COL10, PCL/COL30, and PCL/COL50, respectively; (e-h) fiber diameter distribution of PCL, PCL/COL10, PCL/COL30, and PCL/COL50. |
|
Figure 4 Mechanical properties: (a) maximum stress; (b) stressstrain curves. |
|
Figure 5 (a) SEM images of the platelet adhesion; (b) number of platelets adhered on the PCL and PCL/COL. |
|
Figure 6 Cell viability of L929 cells after culturing 24 h and 48 h. |
|
Figure 7 Experimental diagram of degradation of PCL, PCL/COL10, PCL/COL30, and PCL/COL50. |
In this study, PCL, PCL/COL10, PCL/COL30, PCL/COL50 nanofibrous membranes were prepared via electrospinning, and their mechanical and biocompatibility were investigated. Fiber diameters of PCL/COL is significantly smaller than that of pure PCL, and the hydrophobic surface of PCL becomes hydrophilic after the addition of collagen, which affect platelet adhesion and cytocompatibility of nanofibrous membrane samples. In addition, tensile strength of PCL/COL10 is higher than that of others due to smaller fiber diameters and tight spatial interlacing of PCL/COL10 membranes. Furthermore, the addition of collagen could promote the cell viability as well as the degradation rate. Overall, PCL/COL10 has good morphology, the best mechanical properties, appropriate degradation rate, and the best antithrombotic effect as well as biocompatibility.
- 1. Baranwal, J.; Barse, B.; Fais, A.; Delogu, G. L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983.
-

- 2. Kovylin, R. S.; Aleynik, D. Y.; Fedushkin, I. L. Modern Porous Polymer Implants: Synthesis, Properties, and Application. Polym. Sci. Ser. C 2021, 63, 29-46.
-

- 3. Jurak, M.; Wiacek, A. E.; Ladniak, A.; Przykaza, K.; Szafran, K. What Affects the Biocompatibility of Polymers? Adv. Colloid Interface Sci. 2021, 294, 102451.
-

- 4. Xu, L. C.; Li, Z. J.; Tian, Z. C.; Chen, C.; Allcock, H. R.; Siedlecki, C. A. A New Textured Polyphosphazene Biomaterial with Improved Blood Coagulation and Microbial Infection Responses. Acta Biomater. 2018, 67, 87-98.
-

- 5. Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes-Different Cell Effects. Cytotechnology 2016, 68, 355-369.
-

- 6. Backes, E. H.; Harb, S. V.; Beatrice, C. A. G.; Shimomura, K. M. B.; Passador, F. R.; Costa, L. C.; Pessan, L. A. Polycaprolactone Usage in Additive Manufacturing Strategies for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1479-1503.
-

- 7. Jeong, Y.; Lee, D. Y. Mechanical Properties and Biocompatibility of Electrospun Poly(epsilon-caprolactone)/Gelatin Scaffolds Loaded with Cellulose Fiber. Polym. Korea 2022, 46, 837-842.
-

- 8. Majidnia, E.; Ahmadian, M.; Salehi, H.; Amirpour, N. Development of An Electrospun Poly(ε-caprolactone)/Collagen-Based Human Amniotic Membrane Powder Scaffold for Culturing Retinal Pigment Epithelial Cells. Sci. Rep. 2022, 12, 6469.
-

- 9. Yin, H. M.; Li, X.; Wang, P.; Ren, Y.; Liu, W.; Xu, J. Z.; Li, J. H.; Li, Z. M. Role of HA and BG in Engineering Poly(epsilon-caprolactone) Porous Scaffolds for Accelerating Cranial Bone Regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 654-662.
-

- 10. Shi, J.; Chen, S. Y.; Wang, L. N.; Zhang, X. Y.; Gao, J. C.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.; Kong, D. L. et al. Rapid Endothelialization and Controlled Smooth Muscle Regeneration by Electrospun Heparin-Loaded Polycaprolactone/Gelatin Hybrid Vascular Grafts. J. Biomed. Mater. Res. Part B 2019, 107, 2040-2049.
-

- 11. Nazeer, M. A.; Yilgor, E.; Yilgor, I. Electrospun Polycaprolactone/Silk Fibroin Nanofibrous Bioactive Scaffolds for Tissue Engineering Applications. Polymer 2019, 168, 86-94.
-

- 12. Baburaj, M. S.; Veeran, M. G.; Painuly, D.; Sreelekshmi, S.; Aprem, A. S. Fabrication and Characterisation of Polycaprolactone/Gelatin/Chitosan (PCL/GEL/CHI) Electrospun Nano-Membranes for Wastewater Purification. Desalination 2023, 563, 116709.
-

- 13. Jang, Y.; Jeong, Y.; Lee, D. Y. Double-Layer Wound Dressing Consisting of an Upper Layer of Robust Polyurethane/Polycaprolactone and a Lower Layer of Biodegradable Polycaprolactone/Gelatin/Cellulose. Polym. Korea 2023, 47, 151-156.
-

- 14. Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W. E.; Anderson, J. M.; van den Beucken, J.; Jansen, J. A. Biocompatibility and Degradation Characteristics of PLGA-Based Electrospun Nanofibrous Scaffolds with Nanoapatite Incorporation. Biomaterials 2012, 33, 6604-6614.
-

- 15. Shenoy, M.; Abdul, N. S.; Qamar, Z.; Bahri, B. M. A.; Al Ghalayini, K. Z. K.; Kakti, A. Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus 2022, 14, e24856.
-

- 16. Guo, Y. Y.; Cheng, N. Q.; Sun, H. X.; Hou, J. N.; Zhang, Y. C.; Wang, D.; Zhang, W.; Chen, Z. Y. Advances in the Development and Optimization Strategies of the Hemostatic Biomaterials. Front. Bioeng. Biotechnol. 2023, 10, 1062676.
-

- 17. Ghomi, E. R.; Nourbakhsh, N.; Kenari, M. A.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B 2021, 109, 1986-1999.
-

- 18. Lu, X. J.; Zou, H.; Liao, X. K.; Xiong, Y.; Hu, X. Y.; Cao, J.; Pan, J. Q.; Li, C. R.; Zheng, Y. Y. Construction of PCL-Collagen @PCL@PCL-Gelatin Three-Layer Small Diameter Artificial Vascular Grafts by Electrospinning. Biomed. Mater. 2023, 18, 015008.
-

- 19. Kim, M. S.; Kim, Y. J. Polycaprolactone Composite Membranes Containing Polyphenol-Loaded Hydroxyapatite Nanoparticles for Bone Regeneration. Polym. Korea 2019, 43, 337-345.
-

- 20. Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D. G. Electrospun Nanofibers for Periodontal Treatment: A Recent Progress. Int. J. Nanomed. 2022, 17, 4137-4162.
-

- 21. Hemamalini, T.; Dev, V. R. G. Comprehensive Review on Electrospinning of Starch Polymer for Biomedical Applications. Int. J. Biol. Macromol. 2018, 106, 712-718.
-

- 22. Kai, D.; Liow, S. S.; Loh, X. J. Biodegradable Polymers for Electrospinning: Towards Biomedical Applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 45, 659-670.
-

- 23. Li, Q.; Wang, X. L.; Lou, X. X.; Yuan, H. H.; Tu, H. B.; Li, B. Y.; Zhang, Y. Z. Genipin-crosslinked Electrospun Chitosan Nanofibers: Determination of Crosslinking Conditions and Evaluation of Cytocompatibility. Carbohydr. Polym. 2015, 130, 166-174.
-

- 24. Yang, Z. H.; Wu, G. F. Effects of Soft Segment Characteristics on the Properties of Biodegradable Amphiphilic Waterborne Polyurethane Prepared by A Green Process. J. Mater. Sci. 2020, 55, 3139-3156.
-

- 25. Gümüsderelioglu, M.; Dalkiranoglu, S.; Aydin, R. S. T.; Çakmak, S. A Novel Dermal Substitute Based on Biofunctionalized Electrospun PCL Nanofibrous Matrix. J. Biomed. Mater. Res. Part A 2011, 98A, 461-472.
-

- 26. Lee, J. J.; Yu, H. S.; Hong, S. J.; Jeong, I.; Jang, J. H.; Kim, H. W. Nanofibrous Membrane of Collagen-polycaprolactone for Cell Growth and Tissue Regeneration. J. Mater. Sci. - Mater. Med. 2009, 20, 1927-1935.
-

- 27. Jokinen, V.; Kankuri, E.; Hoshian, S.; Franssila, S.; Ras, R. H. A. Superhydrophobic Blood-Repellent Surfaces. Adv. Mater. 2018, 30, 1705104.
-

- 28. Lenz-Habijan, T.; Bhogal, P.; Peters, M.; Bufe, A.; Moreno, R. M.; Bannewitz, C.; Monstadt, H.; Henkes, H. Hydrophilic Stent Coating Inhibits Platelet Adhesion on Stent Surfaces: Initial Results In Vitro. Cardiovasc. Interv. Radiol. 2018, 41, 1779-1785.
-

- 29. Farndale, R. W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 2006, 36, 162-165.
-

- 30. Nuyttens, B. P.; Thijs, T.; Deckmyn, H.; Broos, K. Platelet Adhesion to Collagen. Thromb. Res. 2011, 127, S26-S29.
-

- 31. Malikmammadov, E.; Tanir, T. E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci.-Polym. Ed. 2018, 29, 863-893.
-

- 32. Kuppan, P.; Sethuraman, S.; Krishnan, U. M. PCL and PCL-Gelatin Nanofibers as Esophageal Tissue Scaffolds: Optimization, Characterization and Cell-Matrix Interactions. J. Biomed. Nanotechnol. 2013, 9, 1540-1555.
-

- 33. Son, S.; Choi, J. E.; Cho, H.; Kang, D.; Lee, D. Y.; Kim, J. T.; Jang, J. W. Synthesis and Characterization of Porous Poly(ε-caprolactone)/Silica Nanocomposites. Polym. Korea 2015, 39, 323-328.
-

- 34. Ma, W. X.; Wang, L. M.; Zhang, Q. L.; Dong, X. Y.; Zhu, T. H.; Lu, S. Y. Electrospun PCL/Collagen Hybrid Nanofibrous Tubular Graft Based on Post-Network Bond Processing for Vascular Substitute. Biomater. Adv. 2022, 139, 213031.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(1): 20-26
Published online Jan 25, 2024
- 10.7317/pk.2024.48.1.20
- Received on Aug 8, 2023
- Revised on Oct 24, 2023
- Accepted on Nov 13, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Wei Zhang*, ** , Yu Ren*, ** , and Mei-Xian Li*, **
-
*School of Textile and Clothing, Nantong University, Nantong 226019, China
**National and Local Joint Engineering Research Center of Technical Fiber Composites for Safety and Protection, Nantong University, Nantong 226019, China - E-mail: zhangwei@ntu.edu.cn, ren.y@ntu.edu.cn,
- ORCID:
0000-0002-3698-334X, 0009-0008-2096-3644, 0000-000









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.