- Natural Evaporation Rate of Toluene in Aqueous Solution for Correct Estimation of Adsorption Performance
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- 흡착 성능평가를 위한 수용액 내 톨루엔 자연증발 속도연구
안양대학교 환경공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this work, we examine the natural evaporation rate of toluene in aqueous solutions as a function of concentration, time, and temperature, with the aim of refining our understanding of its behavior and increasing the precision of adsorption performance evaluations. To this end, the well-established Antoine equation is utilized to calculate the evaporation rate of toluene in the investigated systems. Our findings reveal a marked dependence of toluene evaporation on both time and temperature, with a discernable increase in the mass of evaporated toluene observed as exposure durations are extended and temperatures are elevated. This underscores the importance of scrupulous control over experimental conditions in order to obtain reliable results. By incorporating the natural evaporation rate into the experimental design, one can effectively minimize the influence of evaporation on the outcomes. This may contribute significantly to a comprehensive understanding of toluene evaporation in aqueous environments, offering a valuable reference for future endeavors in toluene removal, be it through adsorption or alternative techniques. Moreover, our findings serve to inform the optimization of experimental designs in future studies, drawing attention to the critical role that both time and temperature play in the evaporation of toluene, thereby facilitating more accurate and reproducible results in this field of inquiry.
본 연구에서는 수용액 중 톨루엔의 자연 증발 속도를 농도, 시간, 온도의 함수로서 조사하고 거동에 대한 이해와 흡착 성능 평가의 정밀도를 높이는 것을 목적으로 한다. 이러한 목적을 위하여 조사되는 시스템에서 톨루엔의 증발 속도를 확립된 앙투안 방정식을 활용하여 계산하였다. 조사 결과 톨루엔의 증발은 시간과 온도 모두에 크게 의존하고 있으며, 노출 시간이 길어지고 온도가 상승하면 증발한 톨루엔의 질량이 증가하는 것으로 나타났다. 연구 결과와 같이 신뢰할 수 있는 결과를 얻기 위하여 실험 조건을 면밀히 관리하는 중요할 것으로 판단되며, 이를 통해 자연 증발 속도를 실험 설계에 통합하면 결과에 대한 증발의 영향을 효과적으로 최소화할 수 있을 것으로 보여진다. 또한 수성 환경에서의 톨루엔의 증발에 대한 포괄적인 이해에 크게 기여하며, 흡착 또는 대체 기술에 의한 톨루엔 제거의 향후 연구에 귀중한 참고 자료가 될 수 있을 것으로 판단되며, 본 연구 결과는 향후 연구에서 실험 설계를 최적화하는 데 많은 도움이 될 것으로 보여진다. 따라서 톨루엔 증발에서 시간과 온도가 중요한 역할을 한다는 점에 주목함으로써 해당 연구 분야에서 정확하고 재현 가능한 결과를 얻을 수 있다.
In this study, to improve the understanding of toluene behavior and improve the accuracy of adsorption performance evaluation, the evaporation rate was calculated using Antoine's equation and the natural evaporation rate of toluene from aqueous solution according to concentration, time, and temperature was calculated. As a result, it was confirmed that time and temperature had a significant effect on toluene evaporation.
Keywords: toluene, vapor pressure, evaporation rate, adsorption.
This research was funded Korea Evaluation Institute of Industrial Technology (KEIT), grant number 00155462.
The authors state no conflict of interest.
Toluene, a compound consisting of a benzene ring bonded to a methyl group, is a versatile organic solvent due to its capacity to dissolve a wide range of chemicals. Its widespread use in industrial and commercial settings can be attributed to its specific gravity of 0.87 and molecular weight of 92.14 g/mol, rendering it less dense than water and immiscible with it. Toluene is found in crude oil and can be synthesized through petroleum refining and coal tar processes.1 It is a prevalent constituent in gasoline, contributing to enhanced octane ratings and fuel efficiency. Additionally, toluene serves as a precursor for the synthesis of various chemicals and is employed in the fabrication of synthetic materials, including fibers and plastics.2-4 However, toluene evaporates at 20 ℃, which can have negative effects on human health and the environment.5-6 Consequently, numerous methodologies have been devised to eliminate toluene from aqueous solutions, with adsorption frequently utilized for this purpose.7-8 Carbonaceous adsorbents, characterized by their high surface area and porous structure, are capable of capturing significant quantities of molecules via adsorption.9-10 Among carbonaceous adsorbents, activated carbon fiber (ACF) has uniform pore formation and high specific surface area, which has excellent adsorption efficiency for fine dust, viruses, VOCs and more. This technique can be applied to a diverse array of water matrices, including groundwater, surface water, and wastewater, rendering adsorption a more sustainable and cost-effective approach compared to other technologies. Despite the extensive research on toluene adsorption, few studies have accounted for the natural evaporation rate of toluene in aqueous solutions to correct adsorbent performance. Ignoring evaporation may result in an overestimation of adsorption efficiency, as the observed decrease in toluene concentration within the aqueous phase could be partially attributed to evaporation rather than adsorption. Furthermore, understanding the natural evaporation rate aids operators in evaluating risks associated with toluene release into the environment or workspace, thereby informing the implementation of appropriate safety measures, such as ventilation systems, protective equipment, and handling procedures.
We determine the natural evaporation rate of toluene in aqueous solutions over varying toluene concentrations, time intervals, and temperatures.11 Here, we used 99.5% toluene acquired from Deoksan Reagent (Republic of Korea). The adsorption environment was simulated using toluene in a 1 L beaker filled with water under typical room conditions. The paper is structured as follows: Natural evaporation rate of toluene by various temperatures presents the calculation of the natural evaporation rate of toluene over time intervals ranging from 15 to 60 min, while corrected adsorption trends of toluene using carbonaceous materials explore the rate across various temperature ranges. In all instances, the toluene concentration was varied from 10 to 30 mg/L. Employing the calculated values, we provide a corrected estimation of the adsorption phenomenon of toluene in aqueous solutions using ACF as a representative carbonaceous adsorbent sample. Here, the residual toluene and the physical properties of ACF samples were analyzed by inductively coupled plasma optical emission spectrometry (OPTIMA 7300DV, Perkin Elmer, USA) and Brunauer-Emmett-Teller (BET, ASAP-2460, Micromeritics, Republic of Korea), respectively.
Natural Evaporation Rate of Toluene by Time Intervals. Prior to engaging in detailed calculations, it is imperative to outline the general context of the environment. Throughout this article, we shall consider a prototypical laboratory setting in which the majority of adsorption experiments are conducted. To streamline our discussion, we shall not incorporate potential complicating factors such as fluctuations in humidity, water flow, or air flow. Additionally, we shall assume that the evaporation of toluene proceeds linearly. The vapor pressure of toluene at 25 ℃ is approximately 28.4 mmHg using the Antoine Equation.12
Vapor pressure of toluene = 10 × (A – (B/(T + C))) (1)
Here, A (6.95), B (1344.80), and C (-53.10) are the Antoine constants for toluene. T is the temperature in Celsius. In order to compute the molar evaporation rate (ER), we employ the following Eq. (2) as:
ER = (Vapor pressure of toluene / (R × T)) × Exposed surface area (2)
Here, R represents the ideal gas constant (8.314 J/mol·K), and T denotes the temperature in Kelvin. Considering a 1 L beaker with a diameter of 8 cm, we approximate the exposed surface area to be 0.005027 m2. Consequently, the ER calculation proceeds as follows:
R = 8.314 J/mol·K × (1 Pa·m³/J) × (1 atm/101325 Pa) × (760 mmHg/atm) × (1 L/0.001 m3)
= 62.364 L·mmHg/mol·K (4)
ER = (28.4 mmHg / (62.364 L·mmHg/mol·K × 298.15 K)) × 0.005027 m2 (5)
which is
ER » 0.00000774 mol/min (6)
Subsequently, we determine the mass evaporation rate (MER) using the molecular weight of toluene:
MER = ER × Molecular weight of toluene (7)
Thus, Eq. (6) becomes
MER » 000000774 mol/min × 92.14 g/mol » 0.0007127 g/min (8)
For the sake of convenience, we convert the toluene concentrations from mg/L to mol/L:
10 mg/L = 10 mg/L × (1 g/1000 mg) × (1 mol/92.14 g) » 0.0001085 mol/L (9)
20 mg/L = 20 mg/L × (1 g/1000 mg) × (1 mol/92.14 g) » 0.000217 mol/L (10)
30 mg/L = 30 mg/L × (1 g/1000 mg) × (1 mol/92.14 g) » 0.0003255 mol/L (11)
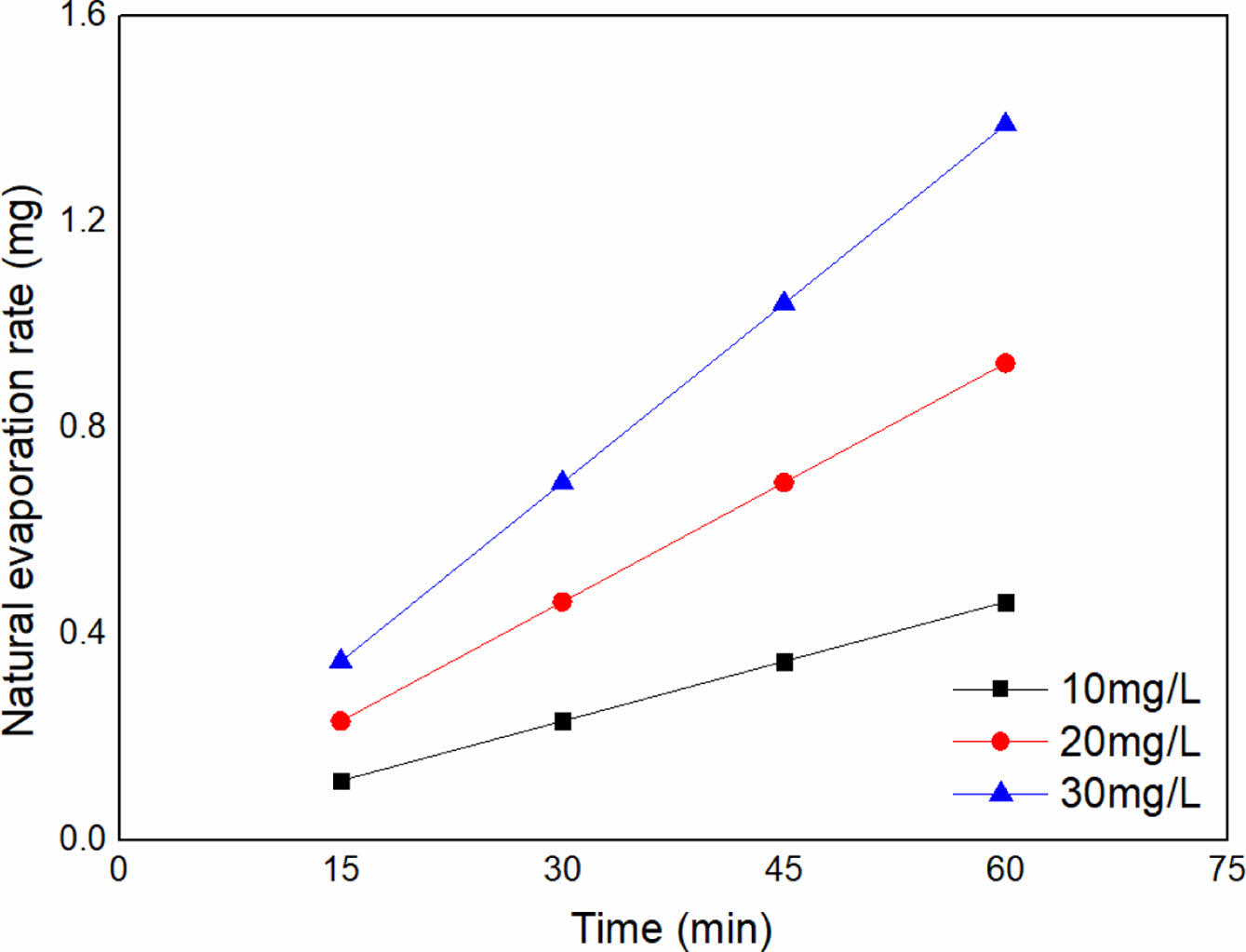
Consequently, we are able to calculate the mass of evaporated toluene at 15 min for 10 mg/L of toluene concentration as shown in Eq. (11) whilst others are plotted in Figure 1.
0.0007127 g/min × 15 min × 0.0001085 mol/L » 0.1158 mg (12)
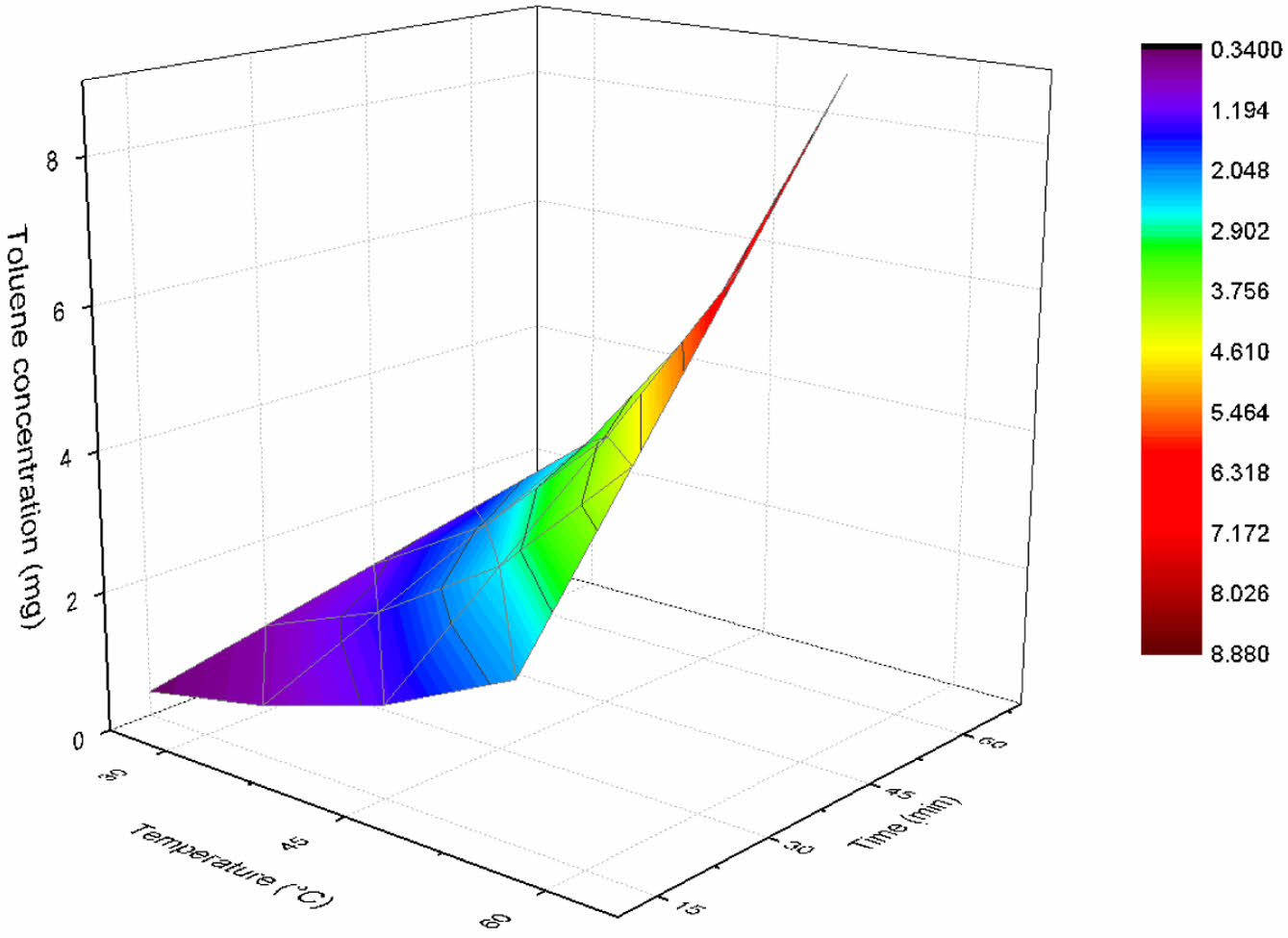
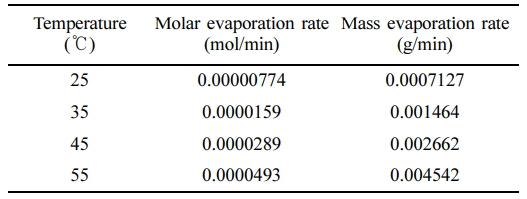
Natural Evaporation Rate of Toluene by Various Temperatures. We investigate the evaporation dynamics of toluene at a fixed concentration of 30 mg/L and varying temperatures of 25, 35, 45, and 55 ℃. It is imperative to account for the vapor pressure of toluene at the specified temperatures. The vapor pressure of toluene at 25 ℃ has been previously computed in Section 2, and analogous calculations were performed for the remaining temperatures, yielding values of 60.3 mmHg at 35 ℃, 117.7 mmHg at 45 ℃, and 213.8 mmHg at 55 ℃. For the given concentration of 30 mg/L, we have already determined the molar concentration to be 0.0003255 mol/L. In Table 1, we present the ER and MER corresponding to the different temperatures. Subsequently, we proceed to calculate the mass of evaporated toluene as a function of time for each temperature, as delineated in Figure 2. Our analysis reveals that the mass of evaporated toluene exhibits an increasing trend with respect to time at each individual temperature. Moreover, we observe an augmentation in the mass of evaporated toluene as the temperature escalates. This observation can be attributed to the enhancement in toluene's vapor pressure at elevated temperatures, which consequently promotes a greater mass transfer between the liquid and gaseous phases. As a result, an increase in temperature induces a more pronounced evaporation of toluene from the solution.
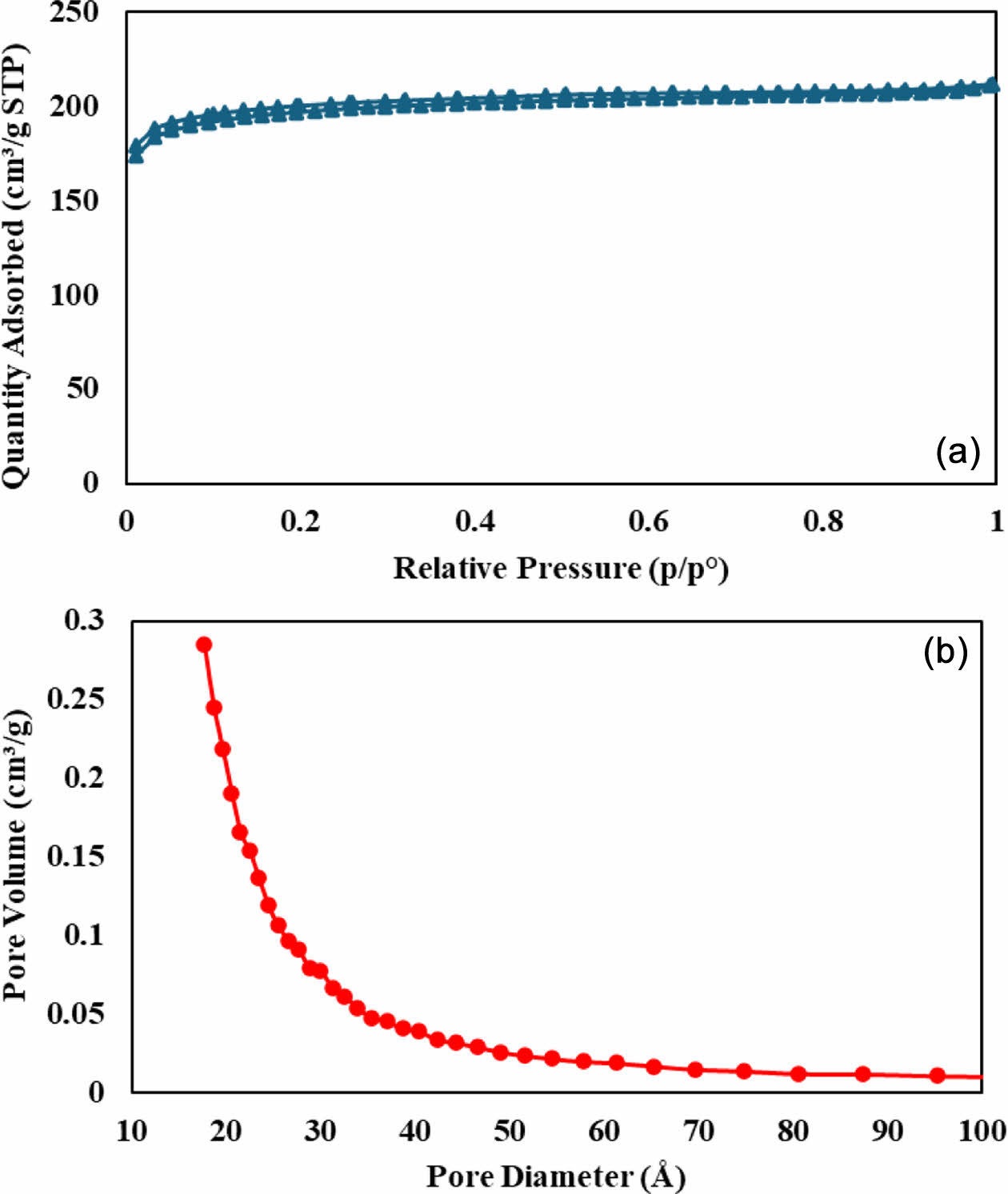
Corrected Adsorption Trends of Toluene Using Carbonaceous Materials. We investigate the adsorption performance of toluene in aqueous solutions employing commercially available ACF as a carbon-based adsorbent. The ACF under consideration exhibits a significant Brunauer-Emmett-Teller surface area of 821 m2/g, accompanied by a micropore surface area of 663 m2/g, as shown in Figure 3. The elevated surface area implies an increased number of adsorption sites for toluene molecules, potentially resulting in an enhanced adsorption capacity.13-15 The presence of micropores further contributes to the augmented adsorption capacity due to their extensive internal surface area and robust adsorbate-adsorbent interactions.16-17
To evaluate the adsorption performance of toluene, we utilized the Langmuir isotherm model for initial concentrations of 30 mg/L, analyzing the maximum adsorption capacity as a function of time (0 to 60 min) and temperature (25 to 55 ℃). The Langmuir equation is suitable since toluene is a nonpolar chemical and tends to adsorb as a monolayer on the adsorbent surface. The Langmuir isotherm can be represented by:
qe = (Q0 × KL × Ce) / (1 + KL × Ce) (12)
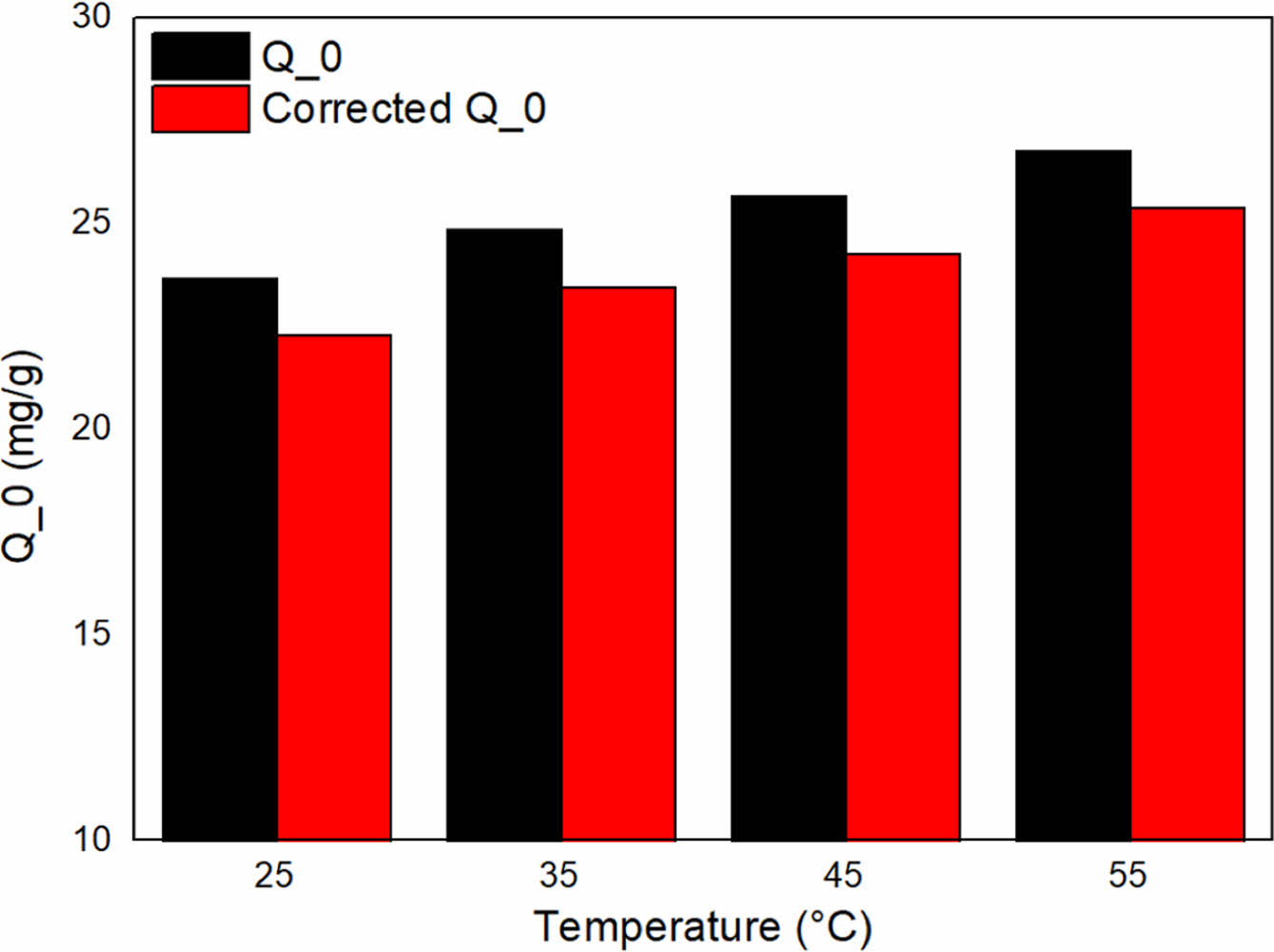
Here, qe denotes the equilibrium adsorbed amount of toluene (mg/g), Ce represents the equilibrium concentration of toluene in the solution (mg/L), Q0 signifies the maximum adsorption capacity (mg/g), and KL stands for the Langmuir constant (L/mg). We present the results for Q0 and the adjusted adsorption performance, accounting for the natural evaporation rate of toluene in Figure 4. The discrepancies between Q0 and the corrected Q0 values for samples at varying temperatures are as follows: 5.86% at 25 ℃, 5.60% at 35 ℃, 5.42% at 45 ℃, and 5.19% at 55 ℃. This adjustment facilitates a more precise depiction of the actual adsorption efficiency of ACF in eliminating toluene from the solution. The decreasing percentages indicate that a considerable fraction of the detected toluene removal may be ascribed to natural evaporation, rather than the adsorption process. This observation implies that the natural evaporation phenomenon plays a non-trivial role in the overall toluene removal mechanism. This insight may prove valuable in optimizing adsorption processes for the maximization of toluene removal from contaminated water.
|
Figure 1 The mass of toluene evaporated at the designated time intervals. |
|
Figure 2 The mass of toluene evaporated at the designated time intervals and temperatures. |
|
Figure 3 (a) BET surface area; (b) pore size distribution curves. |
|
Figure 4 The corrected adsorption performance of ACF by applying the natural evaporation rate of toluene. |
|
Table 1 The Molar Evaporation Rates and the Mass Evaporation Rates of Toluene at Designated Temperatures |
In the pursuit of a comprehensive understanding of the behavior of toluene in aqueous systems, and to enhance the accuracy of adsorption performance assessments of adsorbents, it is imperative to accurately quantify its natural evaporation rate. The current investigation delves into the natural evaporation rate of toluene in aqueous solutions under varying conditions, encompassing diverse concentrations, time intervals, and temperatures. Initially, we employed the Antoine equation to determine the evaporation rate of toluene. Our findings underscore the substantial influence of both temporal and thermal factors on the evaporation of toluene from aqueous solutions. Specifically, we observed an increase in the mass of evaporated toluene concomitant with extended exposure durations and elevated temperatures. These observations emphasize the necessity of meticulous control of experimental conditions when conducting research involving toluene. By integrating the natural evaporation rate into the experimental design, researchers can refine their methodologies, thereby mitigating the repercussions of evaporation on their results. In summation, the insights gleaned from our investigation elucidate the intricacies of toluene evaporation in aqueous systems, furnishing a valuable reference for future endeavors focusing on toluene removal via adsorption or alternative techniques. The implications of this study are manifold, not only augmenting our knowledge of toluene's behavior in aqueous environments but also informing the optimization of experimental designs pertaining to toluene removal. Additionally, our findings serve to heighten awareness of the critical role played by time and temperature in toluene evaporation, further emphasizing the need for stringent control of these parameters in related experiments. Ultimately, this investigation paves the way for future research on toluene remediation and offers a valuable resource for the relevant methodologies.
- 1. White, K. L.; Knutson, B. L.; Kimber, G. Extraction of Coal Tar Pitch Using a Mixture of Compressed CO2 and Toluene. Ind. Eng. Chem. Res. 1999, 38, 3360-3366.
-

- 2. Kim, S.; Seoung, Y. H. Fabrication and Characterization of 3D Printed Polyvinyl Toluene Based Plastic Scintillator. J. Korean Phys. Soc. 2019,75, 953-956.
-

- 3. Monfared, Y. E.; Liang, C.; Khosravi, R.; Kacerovska, B.; Yang, S. Selectively Toluene-filled Photonic Crystal Fiber Sagnac Interferometer with High Sensitivity for Temperature Sensing Applications. Results Phys 2019,13, 102297.
-

- 4. Sun, H.; Yu, X.; Yang, X.; Ma, X.; Lin, M.; Shao, C. Au/Rod-like MNO2 Catalyst via Thermal Decomposition of Manganite Precursor for the Catalytic Oxidation of Toluene. Catal. Today 2019,332, 153-159.
-

- 5. Lee, J. H.; Jeon, E.; Song, J. K.; Son, Y.; Choi, J.; Khim, S. Adsorption Phenomenon of Vocs Released from the Fiber-reinforced Plastic Production Onto Carbonaceous Surface. Polymers 2023,15, 1640.
-

- 6. Maryiantari, E. S.; Keman, S. Analysis of Health Risk and Respiratory Complaints on Footwear Craftsman Exposed to Toluene Vapour. J. Public. Health Res. 2020,9, 1818.
-

- 7. Wei, X.; Li, K.; Zhang, X.; Tong, Q.; Ji, J.; Cai, Y. CEO2 Nanosheets with Anion-induced Oxygen Vacancies for Promoting Photocatalytic Toluene Mineralization: Toluene Adsorption and Reactive Oxygen Species. Appl. Catal. B 2022, 317, 121694.
-

- 8. Meng, X.; Yang, L.; Jiang, W.; Yao, L. Adsorption of Acetone and Toluene by N-functionalized Porous Carbon Derived from Zif-8. J. Ind. Eng. Chem. 2022, 111, 137-146.
-

- 9. Lee, J. H.; Sim, S. J.; Kang, J. H.; Choi, S. S. Isotherm and Thermodynamic Modelling of Malachite Green on CO2-activated Carbon Fibers. Chem. Phys. Lett. 2021, 780, 138962.
-

- 10. Choi, S. S.; Lee, J. H.; Jin, Y. M.; Lee, S. H. Adsorption Characteristics of Volatile Organic Compounds Onto Lyocell-based Activated Carbon Fibers. Carbon Lett. 2019, 29, 633-642.
-

- 11. Kim, K. D.; Park, E. J.; Seo, H. O.; Jeong, M. G.; Kim, Y. D.; Lim, D. C. Effect of Thin Hydrophobic Films for Toluene Adsorption and Desorption Behavior on Activated Carbon Fiber Under Dry and Humid Conditions. J. Chem. Eng. 2012, 200, 133-139.
-

- 12. Chae, Y.; Kim, L.; Lee, J.; Kim, D.; Cui, R.; An, Y. J. Estimation of Hazardous Concentration of Toluene in the Terrestrial Ecosystem Through the Species Sensitivity Distribution Approach. Environ. Pollut. 2021, 289, 117836.
-

- 13. Bardestani, R.; Patience, G. S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—bet, BJH, and DFT. The Canadian. J. Chem. Eng. 2019,97, 2781-2791.
-

- 14. Lee, J. H.; Lee, S. H.; Suh, D. H. High Micropore Number and Specific Surface of Carbon Fibers Pretreated with a Swarm of CO2 Micro–nanobubbles. Environ. Chem. Lett. 2021,19, 3565-3571.
-

- 15. Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of High Surface Area Sludge-based Activated Hydrochar via Hydrothermal Carbonization and Application in the Removal of Basic Dye. Environ. Res. 2019, 175, 457-467.
-

- 16. Yu, S. A.; Cho, J. H.; Park, J. Y.; Rhee, Y. W. Adsorption and Desorption Characteristics of Binary-component Volatile Organic compounds (Toluene-MEK) on Activated Carbon. Clean Technol. 2017, 23, 421-428.
-

- 17. Lillo-Ródenas, M. A.; Cazorla-Amorós, D.; Linares-Solano, A. Benzene and Toluene Adsorption at Low Concentration on Activated Carbon Fibres. Adsorption 2011, 17, 473-481.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(4): 390-394
Published online Jul 25, 2024
- 10.7317/pk.2024.48.4.390
- Received on Jan 26, 2024
- Revised on Apr 2, 2024
- Accepted on Apr 4, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sang Sun Choi
-
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- E-mail: choiss@anyang.ac.kr
- ORCID:
0000-0002-3133-7808










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.