- Adsorption Behaviors and Mechanism of Single/Multi-Metals by Ion Exchange Resins
Xinping Qian, Shaohui Lin, Xianshe Feng*, Garry L. Rempel*, Henry Grace*, and Qinmin Pan†

Green Polymer and Catalysis Technology Laboratory, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, Jiangsu Province, People’s Republic of China
*Department of Chemical Engineering, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada
- 이온 교환 수지에 대한 금속 이온의 흡착 거동 및 메카니즘 연구
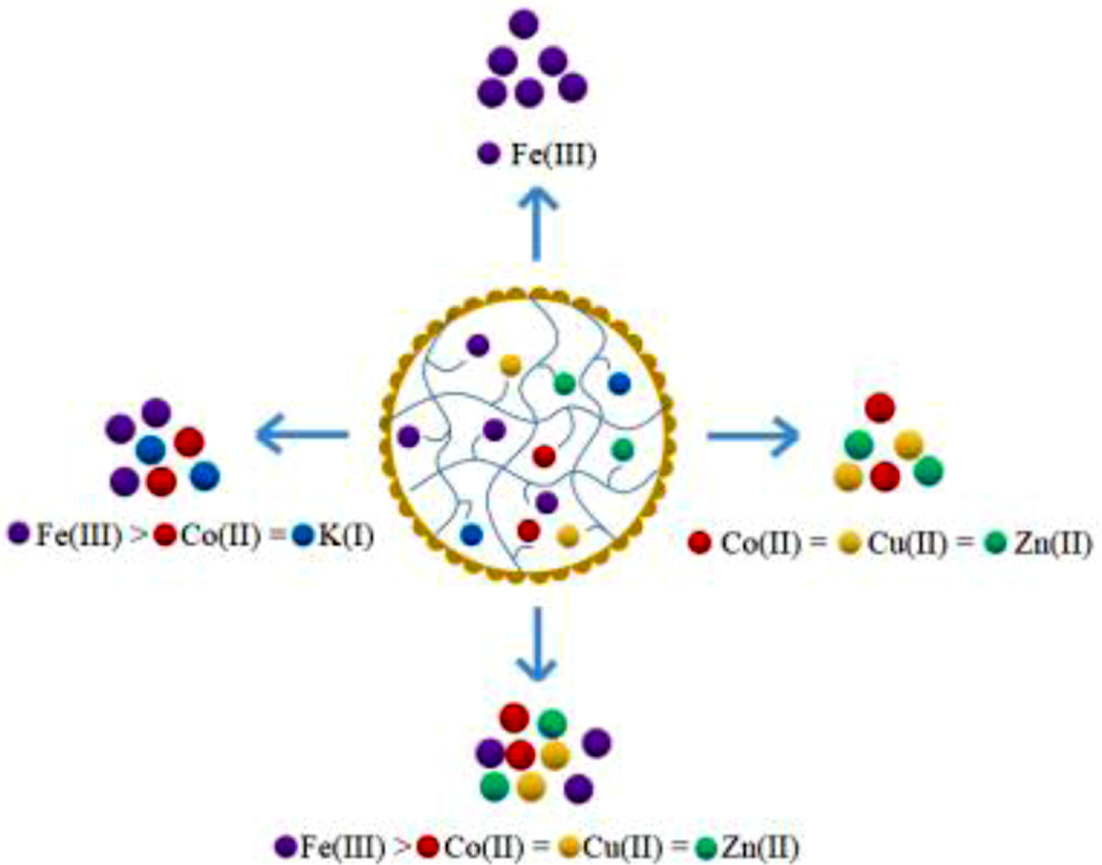
Adsorption of Fe(Ш), Co(II),
Cu(II), Zn(II) and K(I) by strong acid resins, Dowex 50WX2 200-400 (H) and
Amberlite IRP-69, was studied to investigate the adsorption behaviors and
mechanism in single-metal and multi-metal systems. The results showed that
99.9% Fe(III) can be removed by Dowex 50WX2 200-400 (H) in 100 min ([Fe(Ш)]
< 40 mg/L, resin amount = 50 mg, pH = 3.0, 60 ºC). Mechanism studies
revealed that Langmuir model and pseudo-second-order kinetic equation
illustrate better fit for the adsorption of Fe(Ш). Furthermore, the
investigations on multi-metal adsorption indicated that resins display a
similar adsorption ratio for the same-valence metal ions (Co(II) = Cu(II) =
Zn(II)), and a more selective adsorption for high-valence metal ions than
low-valence metal ions (Fe(III) > Co(II) > K(I)). The above results can
be used for the treatment of wastewater and the recovery of metals from the
spent catalysts.
The adsorption behaviors and mechanism of metal were
studied in single/multi-metal systems by Dowex 50WX2 200-400 (H) and Amberlite
IRP-69. The results showed that 99.9% Fe(III) can be removed by Dowex 50WX2
200-400 (H) in 100 min ([Fe(III)] < 40 mg/L, resin amount = 50 mg,
pH = 3.0, 60 ¨¬C), and both resins exhibited better selectivity
towards metal ions with high-valence than those with low-valence.
Keywords: adsorption, ion exchange, metal, kinetic, separation
Financial support from the National Natural Science Foundation of China
(No. 21176163; No. 21576174), Suzhou Industrial Park, the Priority Academic
Program Development of Jiangsu Higher Education Institutions and the Program of
Innovative Research Team of Soochow University are gratefully acknowledged.
In recent decades, metals have played an increasingly important role in
chemical production and human life, especially transition metals, which have
been widely used in catalysts,1 electroplating, batteries, etc..
However, the heavy metals2 (Fe, Co, Cu) in industry wastewater are
harmful to ecological environment, and discharging them directly pollutes the
water and soil, further endangers biological life and human health. In
addition, discarding the rare metals and precious metals (Pt, Pd, Rh) in spent
catalysts leads to the waste of resources.3 Therefore, separation
and recovery of transition metals from these wastes is particularly significant
for environmental protection and secondary use concern. At present, various
techniques have been employed to separate metals from solution such as
precipitation, solvent extraction, membrane filtration, and ion exchange.4
In the early research, precipitation was widely applied in separation of metals
from aqueous solution. Meanwhile, it has been found to be in efficient.5
Solvent extraction is another common method used in metals recovery, which can
improve the adsorption selectivity and efficiency. However, the use of organic
solvents has caused secondary pollution to the environment.6
Membrane separation was also discovered as a viable way to separate metals.4
The implementation of this method can reduce the use of organic solvents, but
the filter membrane is easily clogged. With the development of advanced
technology in separation areas, ion exchange was presented with high metal
selectivity and efficiency.7 It is generally employed in water
treatment, metal separation, and catalysis.8,9 These resins used
today are typically made of small beads of highly crosslinked polymers that
contain functional groups which facilitate the ion exchange.10,11
Consequently, metals can be separated from the solution by ion exchange when
their concentration is low. Moreover, ion exchange is environment friendly, and
the resins can be reused for many times after the adsorption-desorption
process.
Nowadays, many studies on ion exchanges have been devoted to the
separation of metals. Molybdenum can be separated from chloride leach liquors
in Nguyen’s survey which verified the possibility of Mo separation among Co and
Al with
AG1-x8 resin.12 Aboul-Magd found that Dowex HRWX2-Na exhibits great
adsorption for Fe(III), Cu(II), and Zn(II) in the presence of acid or organic
solute.13 Also, Duclos proposed to recover Pt from fuel cell
catalysts by combining extraction, ion exchange, and precipitation, resulting
in 76% Pt being recovered as solid (NH4)2PtCl6.14
Moreover, Sun published a literature about the recovery of Pt from chloride
leaching solution in which 99.6% Pt was obtained after the elution step.15
In the above work, ion exchange has been shown to be an efficient method for
separating metals, and the choice of resin is especially crucial.
Many factors affect the adsorption capacity of resins, the most
significant is the functional group of which the resin contains.16,17
A variety of resins were examined for the removal of metals from various
solutions. It was reported to have removed Cu(II) from aqueous solution by
using Dowex HCR S/S and Dowex Marathon C, strong acid cation resins with
functional group -SO3H, which resulted in a more than 98% Cu(II)
removal efficiency under optimum conditions.18 The adsorption of
Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) was surveyed using Amberlite IR-120
synthetic resin, whose functional group is also -SO3H.19
These bivalent metals could be removed completely from aqueous solution but the
marked selectivity of an individual ion by this resin was not shown. Diphonix,
a chelating resin, was used for Fe(Ш) removal from cobalt sulfate solution. Fe(Ш)
was able to be separated efficiently from the Fe(Ш)-Co(II) acid solution when
the acidity was controlled at pH = 1.20 A number of papers reported
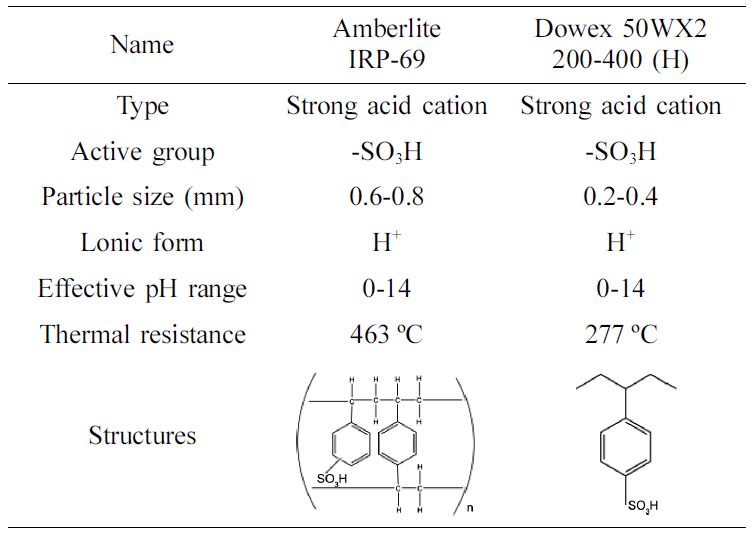
metal recovery from solutions by different types of resins. As shown in Table
1.
Pertinent data was obtained from Table 1, and it revealed that strong acid
cation resins with -SO3H group have a superior affinity for metal
cations, which provides a basis for the choice of resins in our research.
Amberlite IR-120 resin also showed similar adsorption capacity with metals of
the same valence but is not studied further. Also, most of the methods
previously mentioned require several adsorption and multiple cycle steps.
Therefore, the easily operable and efficient resins are needed to investigate
the separation of these metals.
Two strong acid cation resins,
Dowex 50WX2 200-400 (H) and Amberlite IRP-69, were typically chosen as for this
report. These two resins are easily obtained for laboratory and industry use
and are selected because of their low price. This research aims to test an
efficient resin to separate Fe(III) from aqueous solution in sing-metal system
and study the adsorption behaviors and rules of the resins for Co(II), Cu(II)
and Zn(II) ions in multi-metal system. The research investigated the effect of
contact time, pH, temperature, metal concentration, and resin dosage for
effective metal removal, and studied the influencing factors of the selective
adsorption for multiple ions. Furthermore, adsorption isotherm models and
kinetics were investigated.
Materials
and Instruments. All reagents were of analytical grade and all solutions
were prepared in deionized water. The Amberlite IRP-69 and Dowex 50WX2 200-400
(H) resins were purchased from Suzhou Ketong Biological Pharmaceutical
Technology Co. Ltd and Shanghai J&K Chemical Ltd. The properties of
Amberlite IRP-69 and Dowex 50WX2 200-400 (H) are showed in Table 2. Sulfuric
acid and sodium hydroxide were purchased from Chinasun Specialty Products Co.
Other chemicals, Fe2(SO4)3 (M=399.88 g/mol),
CoSO4· 7H2O (M=281.10 g/mol), CuSO4·5H2O
(M=249.69 g/mol), ZnSO4·7H2O (M=287.56 g/mol), and KCl
(M=74.5 g/mol) were purchased from Suzhou Industrial Park Instrument Co.
Ltd.
The pH of the aqueous solution was controlled by sulfuric acid (30 g/L)
and sodium hydroxide (30 g/L) and was tested by pH meter. The metal
concentration was measured by direct coupled plasma atomic emission
spectrometer (ICP-OES, 710-ES, Varian). The resins were dried in a vacuum
drying oven at 30 ℃.
Preparation
of Resins. Firstly, the resins need to be treated before the
adsorption experiments. 2.0 g resin was washed twice with 20 mL sulfuric
acid (30 g/L) to remove the possible metal complex residuals, then washed again
with deionized water to adjust the resins back to be neutral. The resins were
filtered and put in the vacuum drying oven at 30 ℃ until the weight was
constant. The treated resins were then ready for the experiments.
Single-metal
Adsorption. In a single-metal
system, 10 mg/L synthetic solution of Fe(III) was prepared with Fe2(SO4)3.
100 mL above synthetic solution and 20 mg Dowex 50WX2 200-400 (H) or Amberlite
IRP-69 resin were added into a 150 mL conical flask with the magneton. The
mixture was then stirred at 25 ℃ for 100 min in an oil bath (pH = 2). The
20 mL liquid after reaction was taken for concentration test. The resin was
then filtered and dried.21
The adsorption ratio a% was calculated by the following equation:
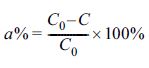 (1)
(1)
where C0 (mg/L) is the initial metal
concentration and C (mg/L) is the final metal concentration after the
reaction.
Multi-metal Adsorption. In multi-metal
systems, The Co(II)- Cu(II)-Zn(II) multi-metal system (2-MMS) was prepared with
CoSO4·7H2O, CuSO4·5H2O, and ZnSO4·7H2O
in aqueous solution. The Fe(III)-Co(II)-Cu(II)-Zn(II) multi-metal system (32-MMS)
was prepared with Fe2(SO4)3, CoSO4·7H2O,
CuSO4·5H2O and ZnSO4·7H2O in
aqueous solution. The Fe(III)-Co(II)-K(I) multi-metal system (321-MMS) was
prepared with Fe2(SO4)3, CoSO4·7H2O
and KCl in aqueous solution. The above adsorption processes in multi-metal
systems proceeded in 100 mL aqueous solution containing metal ions with
Dowex 50WX2 200-400 (H) or Amberlite IRP-69 (contact time = 100 min, pH = 3, T =
25 ℃, ions concentration is 10 mg/L for each metal). The ion
concentrations in the solution were analyzed by ICP-OES, and the adsorption
ratio a% was calculated by eq. (1).
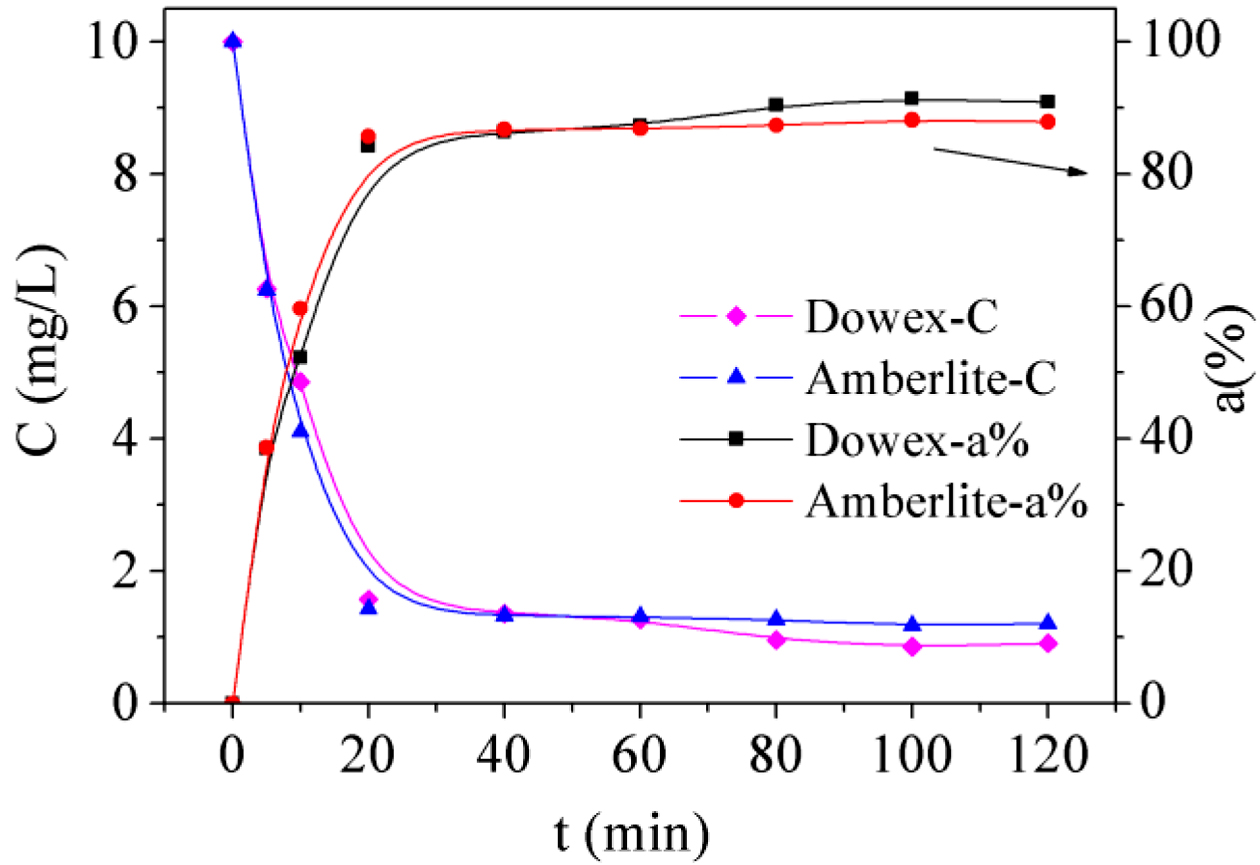
Fe(III) Adsorption in Single-metal System. Effect of Contact Time: The influence of
contact time was investigated regarding the adsorption reaction involving
20 mg Dowex 50WX2 200-400 (H) or Amberlite IRP-69 in 100 mL Fe(III)
solution (10 mg/L). As showed in Figure 1, with the increase of contact time,
the adsorption ratio of Fe(III) increased. The adsorption curve then tends to
level off. This can be described as attainment of adsorption equilibrium. It is
difficult to have more adsorption under these experimental conditions as most
active sites in the resins were already occupied by Fe(III).22 The
adsorption reaction was a fast process of ion exchange, consequently, the
adsorption ratio (a%) was over 80% for Dowex 50WX2 200-400 (H) in the first 20
min, and 91.4% in 100 min at which it achieved an equilibrium state.
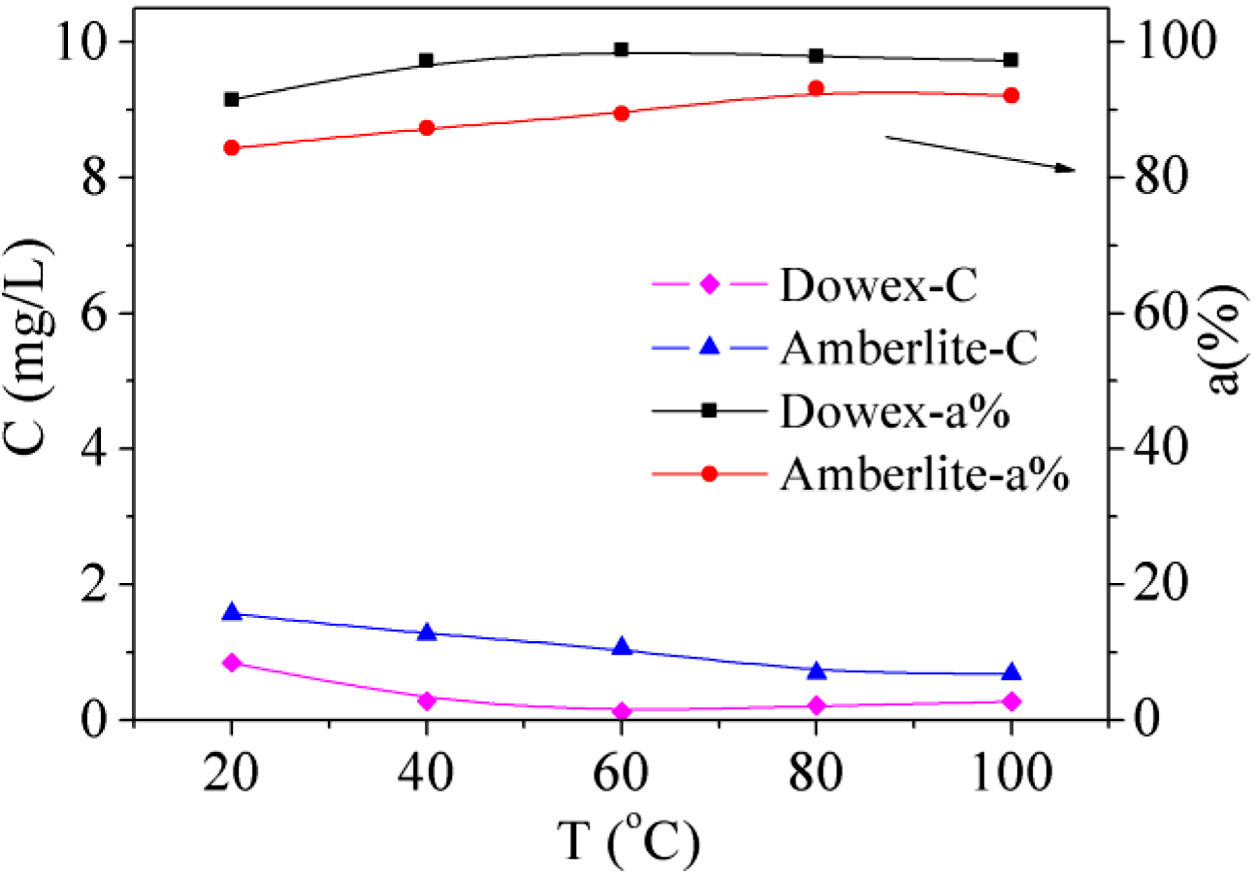
Effect
of Temperature: The effect of temperature on adsorption of Fe(III) by Dowex
50WX2 200-400 (H) and Amberlite IRP-69 was also studied in the experiments. The
temperatures were changed from 20 to 100 ℃ while all other conditions
were invariable. The remnant Fe(III) concentration and adsorption ratio are shown
in Figure 2. When the temperature was under 60 ℃ the adsorption ratio of Dowex
50WX2 200-400 (H) increased, which can be described by the fact that the
adsorption process was more favored than desorption at lower temperature when
the adsorption amount was within the maximum capacity of resin. While the
temperature was above 60 ℃ it decreased slightly because that some Fe(III) was
released from the resin with the increasing temperature. Consequently, the
optimum temperature was found to be 60 ℃ for Dowex 50WX2 200-400 (H) and 80 ℃
for Amberlite IRP-69, and Dowex 50WX2 200-400 (H) shows a better affinity for
Fe(III) with the effect of temperature.
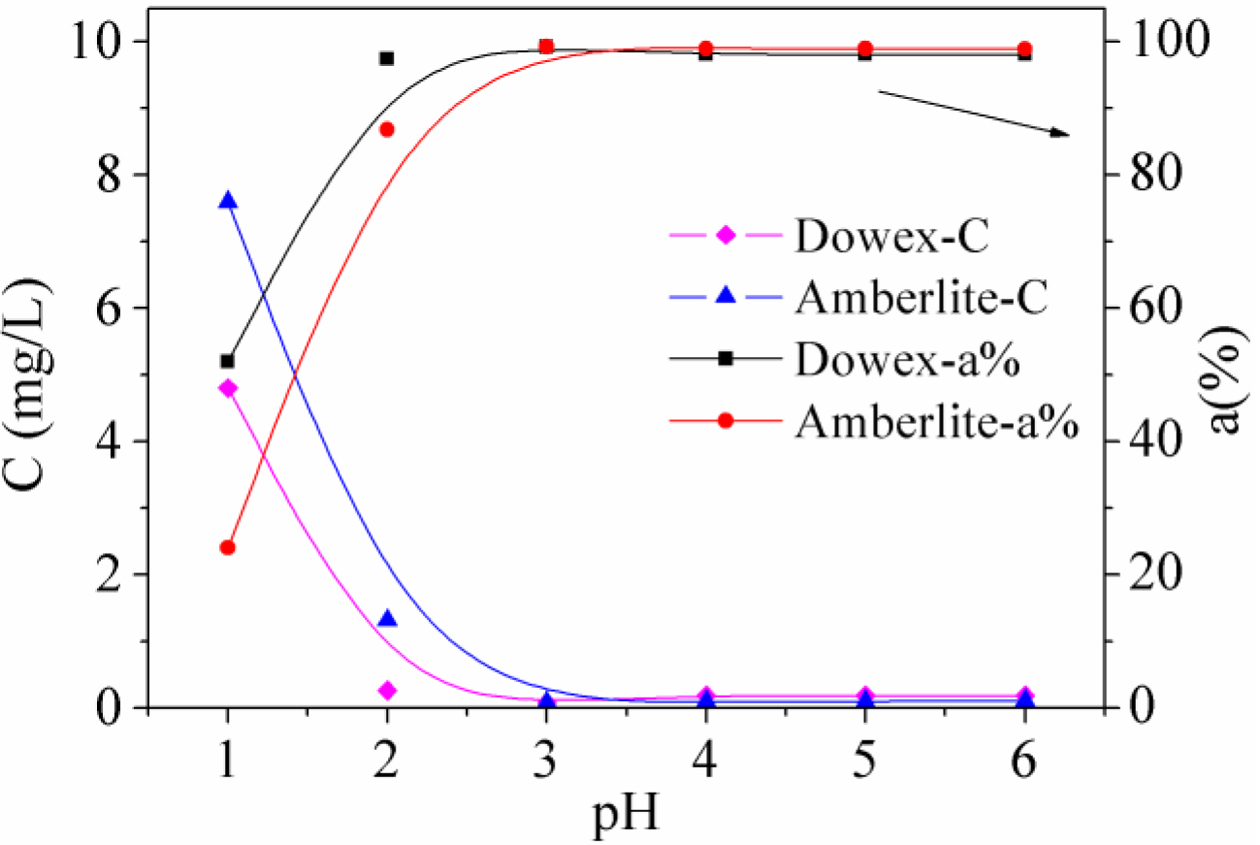
Effect
of pH: A series of experiments were performed using Dowex 50WX2
200-400 (H) and Amberlite IRP-69 to remove Fe(III) under different acidic
conditions. The impact of acidity on the adsorption was examined under the
optimum conditions obtained from the previous experiments. This study varied
the pH values in the range 1 - 6 and the Fe(III) concentration of the solution
were measured by ICP-OES. The results are shown in Figure 3. The investigation
in the effects of pH demonstrated a distinct influence on the adsorption. The
Fe(III) adsorption ratios of two resins were found to be quite low in the very
acidic conditions. Then the ratio increased rapidly until it plateaued with a
maximum recovery of Fe(III) at about 98% with a pH = 3. The resins in solutions
with lower pHs showed poor adsorption capacity because the extremely acidic
conditions inhibit the stripping process of protons from the -SO3H
group, thus reducing the ion exchange efficiency of the resins.
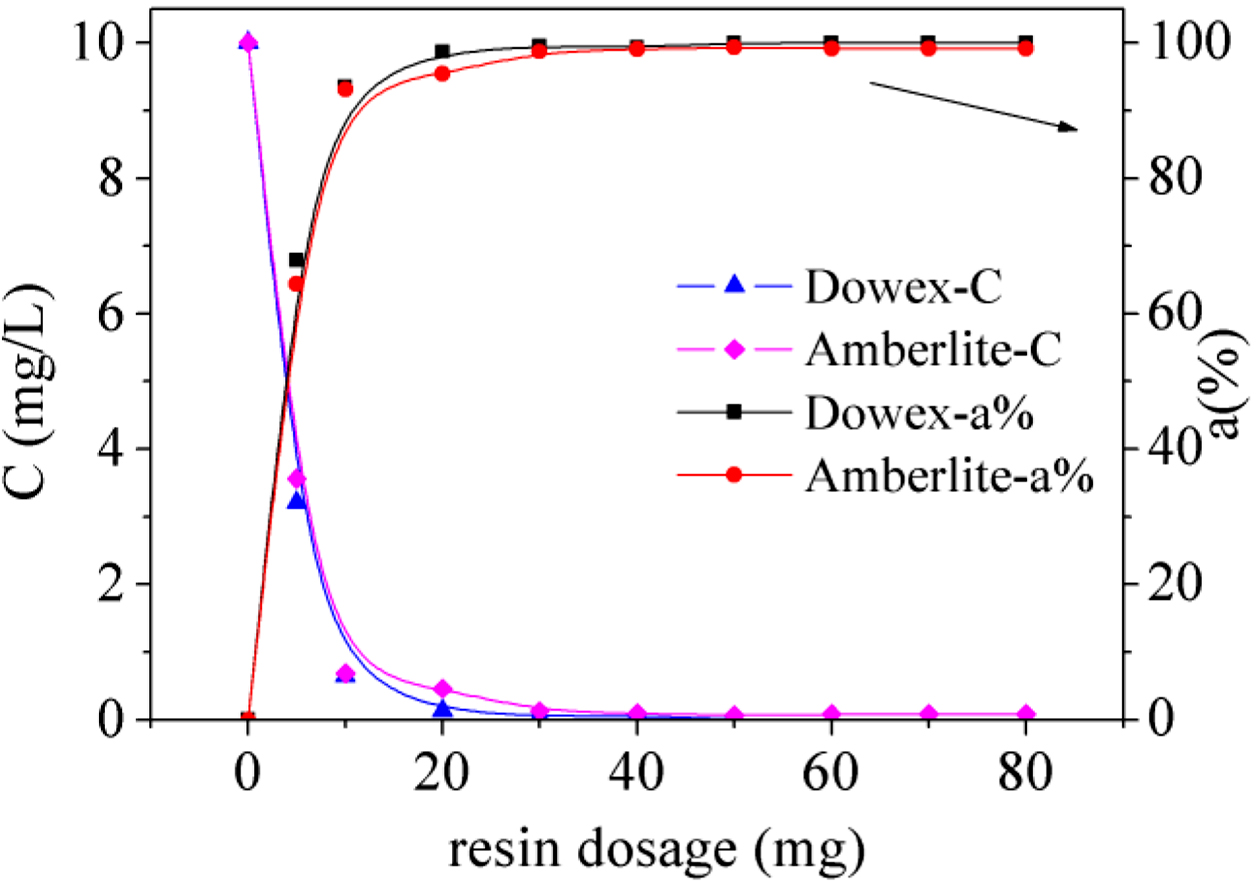
Effect
of Resin Dosage: The effects on the amount of resin have also been studied in this work.
The results are shown in Figure 4. It was apparent that the adsorption of
Fe(III) was affected greatly as the resin dosage increase. The adsorption ratio
increased along with the quantity of resin, which is because the larger surface
area and adsorption sites were provided by more resins, enhancing the removal
of Fe(III). When the resin dosage was 50 mg in the 100 mL aqueous solution, the
maximum adsorption of iron was 99.9% for Dowex 50WX2 200-400 (H) and 99.3% for
Amberlite IRP-69. Meanwhile, the Fe(III) was almost completely adsorbed with 50
mg Dowex 50WX2 200-400 (H).
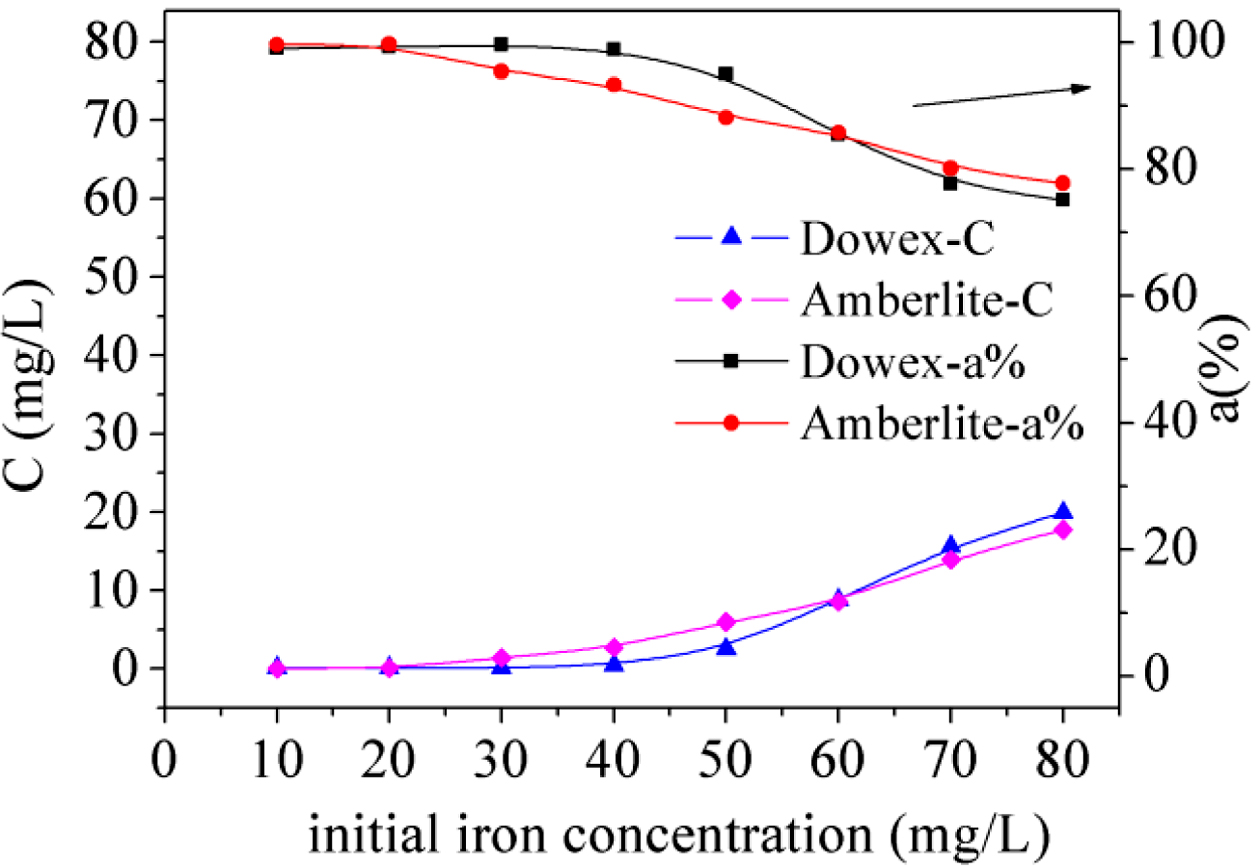
Effect
of Iron Concentration:
Different Fe(III) concentrations usually have different influences on the
adsorption ratio. Figure 5 demonstrates the effects of changing Fe(III)
concentration from 10 to 80 mg/L. The results show that the adsorption ratio
kept close to 99.9% as the Fe(III) concentration was below 40 mg/L for Dowex
50WX2 200-400 (H). A higher concentration is not favorable for Fe(III)
adsorption by the two resins. This is because with more Fe(III), the
availability of sites provided by the limited resins is not sufficient. Fe(III)
ions were residual in the solution, resulting in the lower adsorption ratio. It
can be seen that Fe(III) was easily removed from the aqueous solution by ion
exchange but cannot be separated efficiently by precipitation or membrane
filtration at lower concentrations.
Adsorption
Isotherms. Comparing the absorption ratio of Fe(III) with Dowex
50WX2 200-400 (H) and Amberlite IRP-69 in different conditions, it is found
that Dowex 50WX2 200-400 (H) showed better adsorption capacity for Fe(III) with
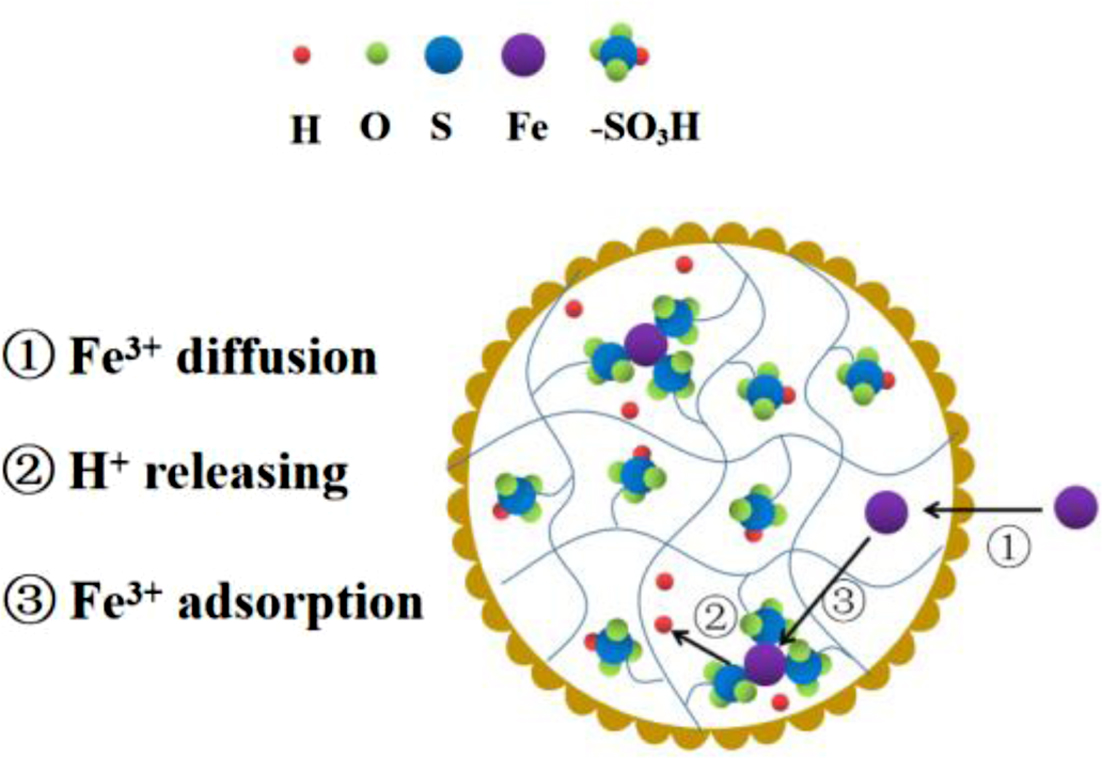
the optimum conditions. The adsorption mechanism of the Fe(III) with strong
acid cation resin can be represented as the formula (2), and a process of
Fe(III) adsorption is displayed in Figure 6.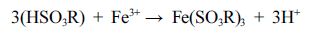 (2)
(2)
As shown in Figure 6, the Fe(III) is adsorbed by the functional group
with H+ being released when going through the resin. The Langmuir
and Freundlich isotherm models were introduced to understand the mechanism of
Fe(III) adsorption by Dowex 50WX2 200-400 (H) and Amberlite IR-120. The
adsorption capacity23 at equilibrium Qe (mg/g) was
calculated as follows:
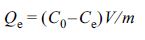 (3)
(3)
where V (mL) is the volume of the solution, m (mg) is the
weight of the resin dried, and Ce (mg/L) is the equilibrium
concentration.
The Langmuir model was described as eq. (4).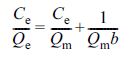 (4)
(4)
where b (L/mg) is Langmuir constant related to the affinity and Qm
(mg/g) is maximal adsorption capacity.
The Freundlich model was described as eq. (5).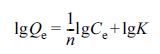 (5)
(5)
where n is Freundlich constant and K is Freundlich binding
constant.
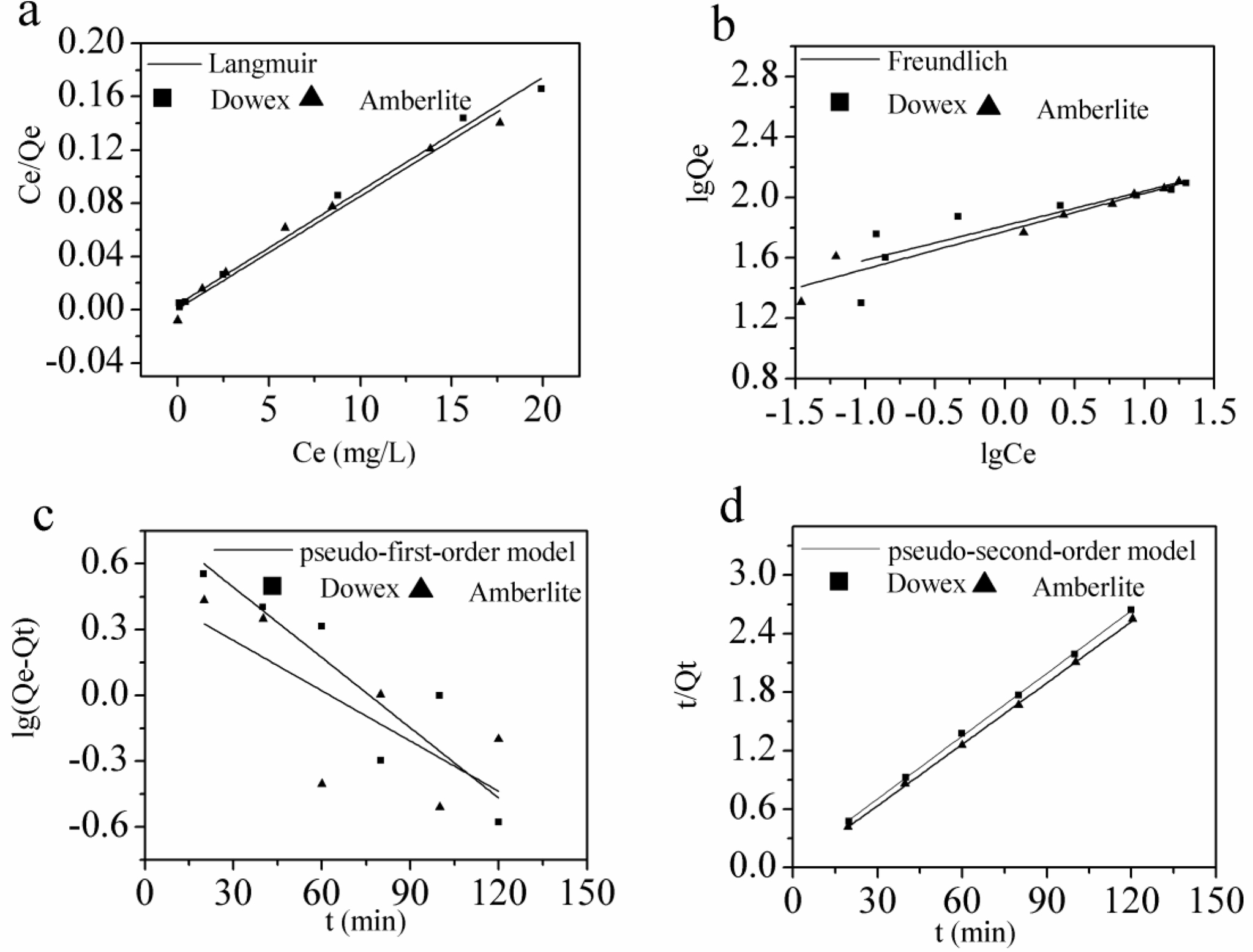
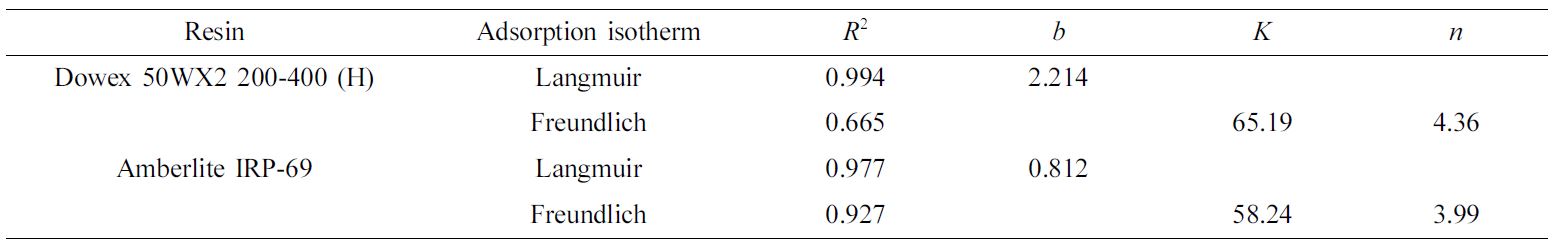
The adsorption data is shown with the model fit using the two isotherms
with resins adsorption of Fe(III) in Figure 7(a , b). The model parameters were
obtained by regression and indicated in Table 3. It was proven that Langmuir
isotherm represents a better fit of Fe(III) adsorption than the Freundlich
isotherm. Therefore, the Langmuir model was sufficient to describe the
adsorption of Fe(III) within the range of the errors permitted.
The Langmuir adsorption isotherm indicates that the adsorption is
monolayer and there is no other molecular coating, which is consistent with the
experimental results of Fe(III) concentration effects on adsorption ratio. More
ions cannot be adsorbed with the increasing of ion concentration because the
active sites are completely occupied. It also demonstrates that the probability
that an ion is absorbed at one site is independent of whether adjacent spaces
have been occupied by other ions. Therefore, the Fe(III) adsorption ratio would
increase with the increasing time until equilibrium state.
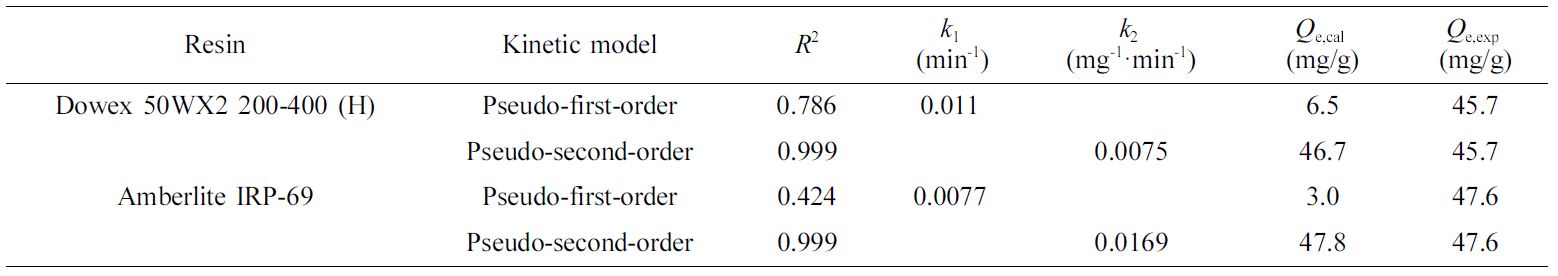
Adsorption
Kinetics. The adsorption
kinetic behavior of Fe(III) by Dowex 50WX2 200-400 (H) and Amberlite IRP-69 was
described with the pseudo-first-order model and the pseudo-second-order model.24,25
The pseudo-first-order model was described as eq. (6):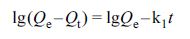 (6)
(6)
The pseudo-second-order model was described as eq. (7):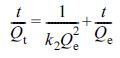 (7)
(7)
where Qe (mg/g) is adsorption capacity at equilibrium, Qt
(mg/g) is adsorption capacity at the time t, k1 (min-1)
is rate constant of the pseudo-first-order model, and k2
(g·mg-1·min-1) is rate constant of the
pseudo-second-order model.
The pseudo-first-order model and the pseudo-second-order model were
fitted in Figure 7(c, d) and the parameters are revealed in Table 4. Depending
on the experimental data, it was found that the correlation coefficient (R2>0.999)
of the pseudo-second-order model is higher than that of pseudo-first-order
model, and the equilibrium adsorption capacity calculated by
pseudo-second-order model (Qe,cal) is closer to the
experimental one (Qe,expl). These two points illustrate that
the pseudo-second-order model fits well the adsorption of Fe(III) by Dowex
50WX2 200-400 (H) and Amberlite IRP-69.
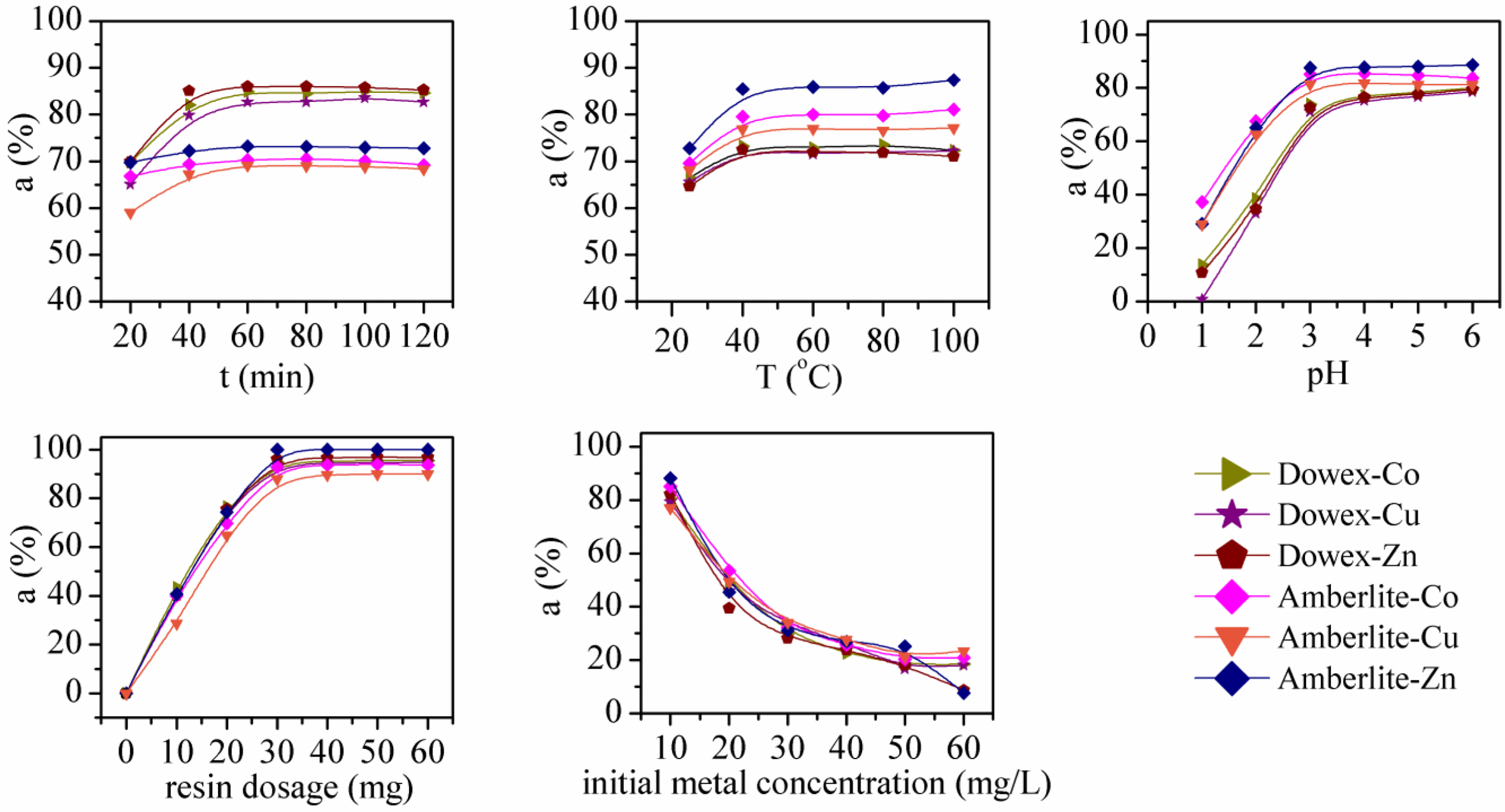
Selective Adsorption in Multi-metal Systems. Co(II), Cu(II), and Zn(II) Adsorption in 2-MMS: The adsorption of
Co(II), Cu(II), Zn(II) by Dowex 50WX2 200-400 (H) and Amberlite IRP-69 resins
from aqueous solution in different conditions was also explored in this study.
The aim was to evaluate the influencing factors and the adsorption behaviors of
resin for multiple metals. The results in 2-MMS are shown in Figure 8. It is
found that pH, resin dosage and initial concentration of metal ions have
greater effects on the adsorption ratios of the three ions. The adsorption
ratios of each metal increased with increase of pH and resin dosage but
decreased with increased initial metal concentration. This is coincident with
the conclusion obtained from Fe(III) adsorption in single-metal system. It is
also found that for each experimental factor changed, Dowex 50WX2 200-400 (H)
and Amberlite IRP-69 have similar adsorption ratios for each metal, and both
resins did not exhibit obvious selectivity for individual metal ions in 2-MMS.
This may be due to the same valence state of the three ions, which affects the
selectivity of the resins.
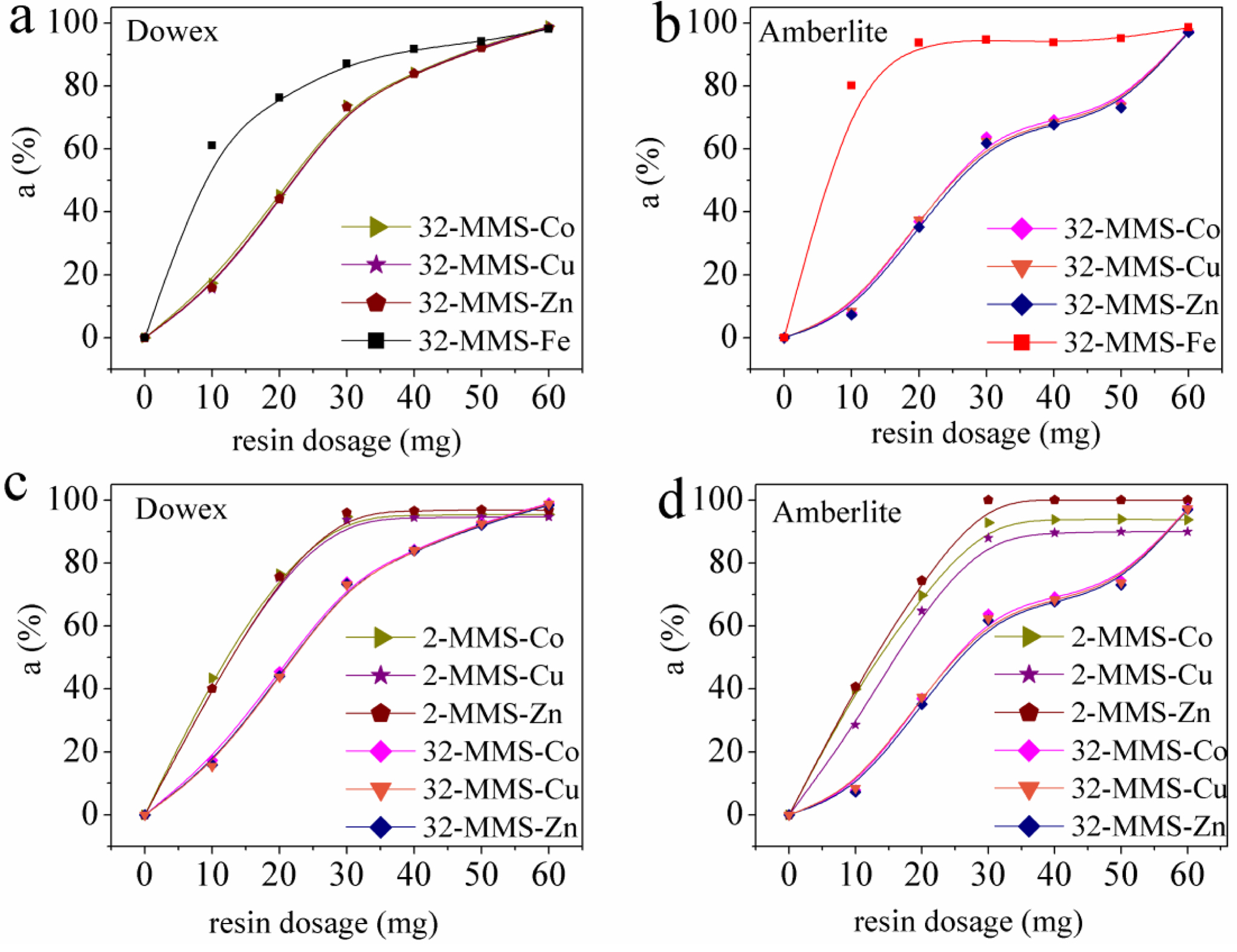
Fe(III),
Co(II), Cu(II), and Zn(II) Adsorption in 32-MMS. In order to
explore the adsorption behaviors for metals with different valence in multi-metal
systems, and to further verify that the selectivity of the resin is related to
the valence state, Fe(III), Co(II), Cu(II), and Zn(II) adsorption in 32-MMS was
carried out, and the respective adsorption ratio of four ions is shown in
Figure 9(a, b). The adsorption results described as: Fe(III) > Co(II) =
Cu(II) = Zn(II). Both resins exhibit the same adsorption selectivity for
Co(II), Cu(II), and Zn(II) in 32-MMS, which is consistent with the results of
previous studies. The resins showed a much better selectivity for Fe(III) in
the same conditions. It confirmed that the valence state of metals really
affects the selectivity of the resin. Metal ions of the same valence state lead
to the similar adsorption ratio, and metal ions of higher valence state are
more selectively adsorbed by resins. Therefore, the Fe(III) reached adsorption
equilibrium first with increasing of the resin dosages, after which Co(II),
Cu(II), and Zn(II) followed.
Comparing the results of Co(II), Cu(II), and Zn(II) adsorption in 2-MMS
and in 32-MMS by Dowex 50WX2 200-400 (H) and Amberlite IRP-69 in Figure 9(c, d). The results showed that the ratios of Co(II), Cu(II), and Zn(II) adsorption
in 2-MMS were higher than those in 32-MMS, and the adsorption ratio of each
metal in 32-MMS was reduced by about 20% than those in 2-MMS. The above results
can be explained as “competitive adsorption” when multiple metal ions coexist
in the solution, which is expressed that a negative effect on the adsorption
ratio of the metal in the original system happened when one or more metal ions
were added to the solution. This is because when two or more metal ions are
mixed in the solution, each metal ion competes for the functional groups on
resins at the same time, creating “competitive adsorption”. Therefore, the
adsorption ratio of each metal decreased with the limited amount of functional
group.
Fe(III),
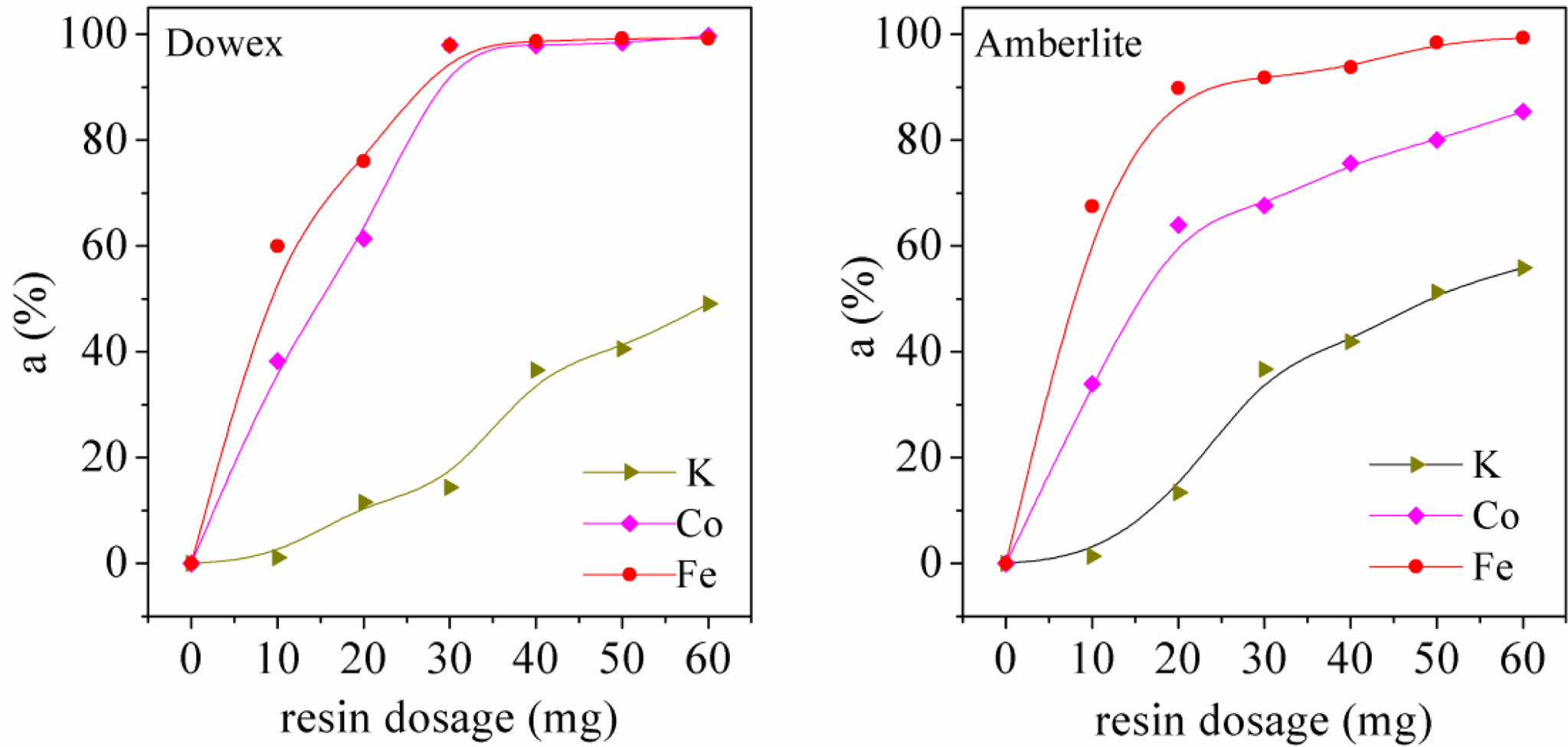
Co(II), and K(I) Adsorption in 321-MMS. The adsorption of
Fe(III), Co(II), and K(I) in 321-MMS by Dowex 50WX2 200-400 (H) and
Amberlite IRP-69 in 100 mL aqueous solution was further studied. The three metals
are in different valence states. The aim is to verify that the higher valence
state of metals, the higher adsorption ratio, and the better selectivity of
resins. The results are shown in Figure 10. The adsorption ratio of each metal
with the same amount of resins in the aqueous solution is: Fe(III) > Co(II)
> K(I). The selectivity of resin for ferric iron is greater than that of
divalent cobalt, and the adsorption of divalent cobalt is greater than that of
monovalent potassium, which is due to the different adsorption force by
functional groups. Three equivalents of H+ from -SO3H
group were consumed for exchange when one equivalents of Fe(III) was adsorbed,
and it is easy to form a stable structure of three chemical bonds. However, a
monovalent or divalent ion requires fewer functional groups when adsorbed, and
with a weak adsorption force, it is difficult to bond or easily to fall off
after being adsorbed. Combining the results from 32-MMS, it is confirmed that
ions with different valence states result in different adsorption ratios, and
the high-valence metal ions are selected preferentially than low-valence metal
ions.
|
Figure 1 Effect of contact time on adsorption of Fe(III) (iron concentration = 10 mg/L, 25 ℃, 20 mg/100 mL, pH = 2). |
|
Figure 2 Effect of temperature on adsorption of Fe(III) (iron concentration = 10 mg/L, 20 mg/100 mL, pH = 2, 100 min). |
|
Figure 3 Effect of pH on adsorption of Fe(III) (iron concentration = 10 mg/L, 20 mg/100 mL, 100 min, 60 ℃ for Dowex resin, 80 ºC for Amberlite resin). |
|
Figure 4 Effect of resin dosage on adsorption of Fe(III) (iron concentration = 10 mg/L, 100 min, pH = 3, 60 ℃ for Dowex resin, 80 ℃ for Amberlite resin). |
|
Figure 5 Effect of iron concentration on adsorption of Fe(III) (50 mg/100 mL, 100 min, pH = 3, 60 ℃ for Dowex resin, 80 ℃ for Amberlite resin). |
|
Figure 6 Process of Fe(III) adsorption with strong acid cation resin. |
|
Figure 7 Adsorption model (Langmuir-a, Freundlich-b); The kinetics (pseudo-first-order model-c, pseudo-second-order model-d) for Fe(III) adsorption. |
|
Figure 8 Adsorption ratio of Co(II), Cu(II), Zn(II) with different contact time, temperature, pH, resin dosage and initial concentration in 2-MMS. |
|
Figure 9 Comparison of adsorption ratios of Fe(III), Co(II), Cu(II), Zn(II) in multi-metal systems. |
|
Figure 10 Selective adsorption ratio of Fe(III), Co(II), K(I) in 321-MMS. |
|
Table 4 Parameters of Pseudo-first-order and
Pseudo-second-order Models for Fe(III) Adsorption |
Adsorption of Fe(III), Co(II), Cu(II), Zn(II), and K(I) was studied to
investigate the adsorption behaviors and mechanism in single-metal and
multi-metal systems. The influencing factors of contact time, temperature, pH,
resin dosage, and metals concentration were investigated to enhance the
adsorption ratio of resins. The results showed that Dowex 50WX2 200-400 (H) had
a stronger affinity and higher selectivity for Fe(III). This occurred when the
contact time was 100 min ([Fe(III)] < 40 mg/L, resin amount = 50 mg, pH
= 3.0, 60 ℃), with the maximum adsorption ratio for Fe(III) being over 99.9%.
Mechanism studies showed that Langmuir isotherms model and
pseudo-second-order-kinetic equation fit well in the Fe(III) adsorption of
single-metal system. Due to the simple operation, mild reaction conditions, and
high selectivity of Dowex 50WX2 200-400 (H) and Amberlite IRP-69, the
adsorption can be applied to the separation of Fe(III) from aqueous solution.
The results in multi-metal systems showed that Dowex 50WX2 200-400 (H)
and the Amberlite IRP-69 resins reveal a weak affinity for the three metals and
the similar adsorption ratios for the metal ions of the same valence state. It
is proved that the resins demonstrate higher selectivity for metal ions of
higher valence. The new findings and adsorption rules will benefit the treatment
of industrial wastewater and recovery of metals from spent catalysts.
- 1. K. Petya and R. Djingova, Anal. Chim. Acta, 464,7 (2002).
-

- 2. J. Yu, J. D. Zheng, Q. F. Lu, X. Wang, X. M. Zhang, Q.Z. Wang, and W. Yang, Polym. Korea, 41, 480 (2017).
-

- 3. A. N. Nikoloski, K. L. Ang, and D. Li, Hydrometallurgy,152, 20 (2015).
-

- 4. L.L. Tavlarides, J. H. Bae, and C. K. Lee, Sep. Sci. Technol., 22,581 (1987).
-

- 5. M.Amara and H. Kerdjoudj, Desalination, 168, 195 (2004).
-

- 6. K. Sarangi, P. K. Parhi, E. Padhan, A. K. Palai, K. C.Nathsarma, and K. H. Park, Sep. Purif. Technol., 55, 44 (2007).
-

- 7. Y. Zhang, Q. Liu, and L. Li, Hydrometallurgy, 164,154 (2016).
-

- 8. P. P. Sun, T. Y. Kim, B. J. Min, H. I. Song, and S. Y. Cho, Mater. Transact., 56, 1863 (2015).
-

- 9. M. D. Víctor-Ortega, J. M. Ochando-Pulido, and A.Martínez-Ferez, J. Ind. Eng. Chem., 36, 298 (2016).
-

- 10. R. Chiariza, E. P. Horwitz, S. D. Alexandrators, andM. J. Gula, Sep. Sci. Technol., 22, 581 (1997).
-

- 11. C. Lasanta, I. Caro, and L. Pérez, Chem. Eng. Sci.,60, 3477 (2005).
-

- 12. T. H. Nguyen, S. H. Sohn, and M. S. Lee, Mater. Transact., 54, 1750 (2013).
-

- 13. A.-A. S. Aboul-Magd, S. A.-R. Al-Husain, and S. A.Al-Zahrani, Arab. J. Chem., 9, S1 (2016).
-

- 14. L. Duclos, L. Svecova, V. Laforest, G. Mandil, and P.X. Thivel, Hydrometallurgy, 160, 79 (2016).
-

- 15. P. P. Sun, T. Y. Kim, B. J. Min, and S. Y. Cho, Mater.Transact., 56, 738 (2015).
-

- 16. A. Dabrowski, Z. Hubicki, P. Podkoscielny, and E.Robens, Chemosphere, 56, 91 (2004).
-

- 17. S. Edebali and E. Pehlivan, Powder Technol., 301,520 (2016).
-

- 18. S. Veli and B. Pekey, Fresenius Environ. Bull.,13, 244 (2004).
- 19. A. Demirbas, E. Pehlivan, F. Gode, T. Altun, and G.Arslan, J. Colloid Interface Sci., 282, 20 (2005).
-

- 20. M. S. Lee and M. J. Nicol, Hydrometallurgy, 86,6 (2007).
- 21. Y. Fan, X. Wang, and M. Wang, Hydrometallurgy,136, 31 (2013).
-

- 22. G. Karthikeyan, N. M. Andal, and K. Anbalagan, J.Chem. Sci., 117, 663 (2005).
-

- 23. M. Parijaee, M. Noaparast, K. Saberyan, and S. Z.Shafaie-Tonkaboni, Korean J. Chem. Eng., 31, 2237 (2014).
-

- 24. Q.T. Bui, Y. S. Jeon, D. Kim, and J. H. Kim, Polym.Korea, 40, 275 (2016).
-

- 25. H.-Y. Li, C. Li, M. Zhang, K. Wang, and B. Xie, Hydrometallurgy, 165, 381 (2016).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(6): 862-871
Published online Nov 25, 2019
- 10.7317/pk.2019.43.6.862
- Received on Jun 19, 2019
- Revised on Sep 2, 2019
- Accepted on Sep 9, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Qinmin Pan
-
Green Polymer and Catalysis Technology Laboratory, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, Jiangsu Province, People’s Republic of China
- E-mail: qpan@suda.edu.cn
- ORCID:
0000-0001-5410-6845









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.